High altitude sickness (hypobaric hypoxia) is a form of cellular hypoxia similar to that suffered by critically ill patients.
The study of mountaineers exposed to extreme hypoxia offers the advantage of involving a relatively homogeneous and healthy population compared to those typically found in Intensive Care Units (ICUs), which are heterogeneous and generally less healthy.
Knowledge of altitude physiology and pathology allows us to understanding how hypoxia affects critical patients.
Comparable changes in mitochondrial biogenesis between both groups may reflect similar adaptive responses and suggest therapeutic interventions based on the protection or stimulation of such mitochondrial biogenesis.
Predominance of the homozygous insertion (II) allele of the angiotensin-converting enzyme gene is present in both individuals who perform successful ascensions without oxygen above 8000m and in critical patients who overcome certain disease conditions.
La hipoxia de la altitud (hipoxia hipobárica) no deja de ser una hipoxia celular similar a la que presentan los enfermos críticos. Estudiar a los alpinistas expuestos a la hipoxia extrema ofrece la ventaja de que es una población relativamente homogénea y sana, en contraste con la población heterogénea y generalmente menos saludable que suele observarse en las Unidades de Cuidados Críticos. El conocimiento de la fisiología y la enfermedad de la altitud abren caminos para comprender en qué medida afecta la hipoxia a los pacientes críticos. Los cambios comparables en la biogénesis mitocondrial entre ambos grupos pueden reflejar respuestas adaptativas similares y sugieren intervenciones terapéuticas basadas en la protección o estimulación de la biogénesis mitocondrial.
El predominio del alelo homocigótico de inserción (II) de la enzima de conversión de la angiotensina está presente tanto en las ascensiones exitosas sin oxígeno por encima de los 8.000m como en la supervivencia de algunas enfermedades de los enfermos críticos.
Humans have been able to climb mountains to a height of over 8000 meters without the need for supplementary oxygen. The first “eight thousand” to be conquered was the Annapurna (8091m) in the year 1950: this was achieved by breathing atmospheric air, and there had been no plans to use supplementary oxygen. The summit of Mount Everest (8848m) was reached two years later, though this time with the help of supplementary oxygen, and in 1978 the peak of this highest of all mountains was reached breathing only environmental air. The climbers Reinhold Messner and Peter Habeler proved wrong the physiological theories predicting that humans could not reach the top of this mythical mountain breathing only atmospheric air. These theories, shared by both physiologists and high altitude mountaineers, were fundamented upon the principle that a maximum inspired oxygen partial pressure of 43mmHg precluded human tolerance of hypoxia.1
An expedition led by West, the American Medical Research Expedition to Everest (AMREE), which reached the summit of Mount Everest in 1981 without oxygen, marked a turning point in this physiological challenge.2 Important respiratory physiologists and leading mountain climbers, with Hackett and Milledge, participated in the expedition.3 In a recent article, West recalled this investigation into human tolerance of extreme hypoxia.4 In the year 2007, a British expedition known as the Caudwell Xtreme Everest Expedition (CXEE) further contributed new knowledge in this field.5
These studies, which have helped to improve our knowledge of high altitude physiology and disease, have also opened ways to understand how hypoxia affects critically ill patients and in general all individuals exposed to hypoxemia and cellular hypoxia.6 It is no exaggeration to affirm that the hypoxemia-adapting mechanisms induced by high altitude play a role similar to that of the hypoxia response observed in critical patients, and that knowledge acquired in mountain medicine may be transferred to the critical care setting.
The mechanisms underlying adaptation to hypoxia are still little known, and investigation in this field among critical patients is difficult. Mountaineers exposed to extreme hypoxia offer the advantage of constituting a relatively homogeneous and healthy study population, in contrast to the heterogeneous and generally much less healthy individuals seen in the Intensive Care Unit (ICU). The main research question of the present review is: What can the healthy mountain climber model of hypoxia teach us about critical patient management?
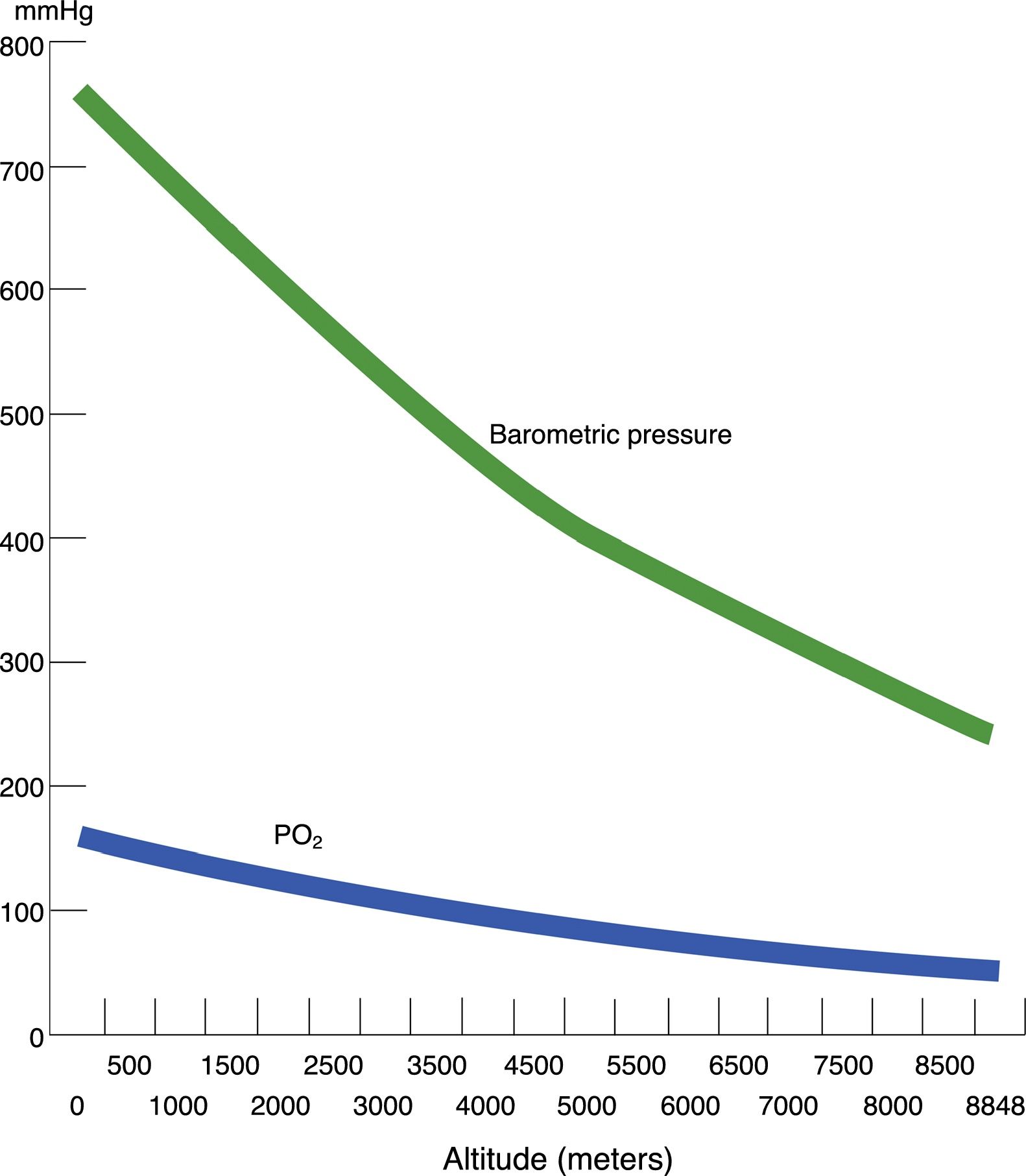
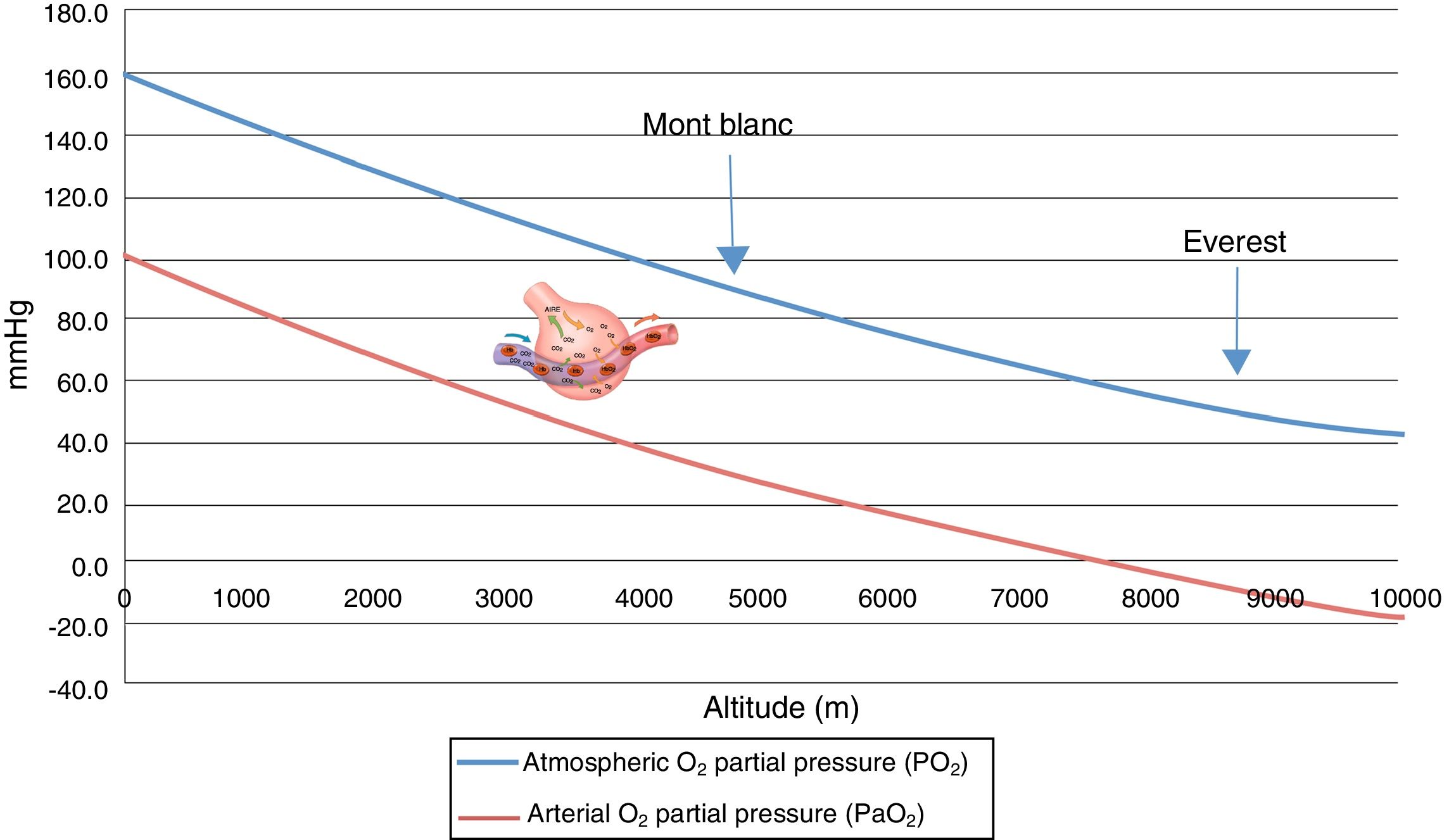
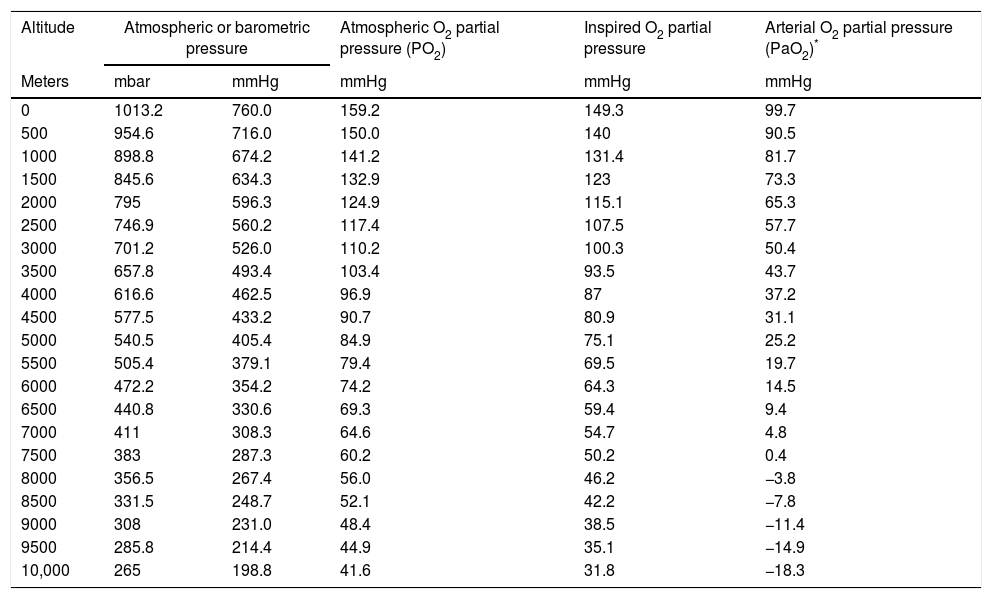
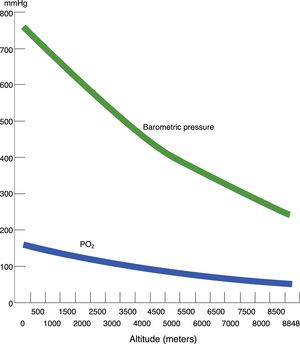
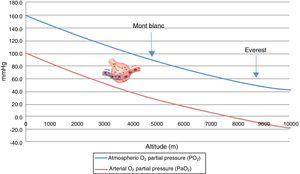
Hypobaric hypoxia and tissue hypoxiaHypobaric hypoxia (HH) refers to high altitude hypoxia, i.e., a lowering of atmospheric or barometric pressure (BP). The oxygen partial pressure in atmospheric air (PO2) remains constant above 11,000m, and the gas is always in a proportion of 21%. At sea level, BP is 760mmHg and PO2 is 159.2mmHg. However, as we rise in altitude, BP decreases, and consequently although oxygen remains present in the same proportion as at sea level, PO2 also decreases. In other words, increasing altitude is associated to lesser BP, lower PO2 and, consequently, lower inspired oxygen pressure. Considering that BP is about 405mmHg at the top of Mont Blanc (4810m), the PO2 is 84mmHg. Likewise, at the summit of Mount Everest, the theoretical BP is 236.3mmHg, with a PO2 of 49.5mmHg (Table 1 and Fig. 1).
Correlation among altitude, barometric pressure, atmospheric oxygen partial pressure (dry environment) and inspired oxygen partial pressure (with water vapor), and arterial oxygen partial pressure.
| Altitude | Atmospheric or barometric pressure | Atmospheric O2 partial pressure (PO2) | Inspired O2 partial pressure | Arterial O2 partial pressure (PaO2)* | |
|---|---|---|---|---|---|
| Meters | mbar | mmHg | mmHg | mmHg | mmHg |
| 0 | 1013.2 | 760.0 | 159.2 | 149.3 | 99.7 |
| 500 | 954.6 | 716.0 | 150.0 | 140 | 90.5 |
| 1000 | 898.8 | 674.2 | 141.2 | 131.4 | 81.7 |
| 1500 | 845.6 | 634.3 | 132.9 | 123 | 73.3 |
| 2000 | 795 | 596.3 | 124.9 | 115.1 | 65.3 |
| 2500 | 746.9 | 560.2 | 117.4 | 107.5 | 57.7 |
| 3000 | 701.2 | 526.0 | 110.2 | 100.3 | 50.4 |
| 3500 | 657.8 | 493.4 | 103.4 | 93.5 | 43.7 |
| 4000 | 616.6 | 462.5 | 96.9 | 87 | 37.2 |
| 4500 | 577.5 | 433.2 | 90.7 | 80.9 | 31.1 |
| 5000 | 540.5 | 405.4 | 84.9 | 75.1 | 25.2 |
| 5500 | 505.4 | 379.1 | 79.4 | 69.5 | 19.7 |
| 6000 | 472.2 | 354.2 | 74.2 | 64.3 | 14.5 |
| 6500 | 440.8 | 330.6 | 69.3 | 59.4 | 9.4 |
| 7000 | 411 | 308.3 | 64.6 | 54.7 | 4.8 |
| 7500 | 383 | 287.3 | 60.2 | 50.2 | 0.4 |
| 8000 | 356.5 | 267.4 | 56.0 | 46.2 | −3.8 |
| 8500 | 331.5 | 248.7 | 52.1 | 42.2 | −7.8 |
| 9000 | 308 | 231.0 | 48.4 | 38.5 | −11.4 |
| 9500 | 285.8 | 214.4 | 44.9 | 35.1 | −14.9 |
| 10,000 | 265 | 198.8 | 41.6 | 31.8 | −18.3 |
The consequence of HH is tissue hypoxia. In turn, high altitude cellular hypoxia is the same as that observed in critical patients when oxygen delivery to the tissues is impaired as a result or cause of well known disease processes.
In most body tissues the primary mechanism producing energy in the form of adenosine triphosphate (ATP) is oxidative phosphorylation, which takes place in the internal membrane of the mitochondrion. Under conditions of hypoxia, energy homeostasis and metabolic adaptation face the challenge of maintaining cell functions and energy.7
Many physicians were surprised to discover that mountain climbers at the top of Mont Blanc have a partial oxygen pressure in arterial blood (PaO2) of about 40mmHg, versus about 25mmHg at the summit of Mount Everest, and that they moreover perform very strenuous exercise with these PaO2 values.2,8
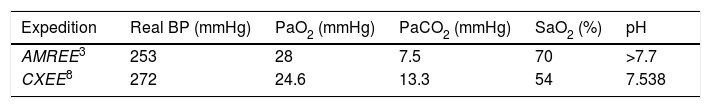
Two expeditions have been able to estimate alveolar gas at the summit of Mount Everest, along with the corresponding PaO2 values (Table 2). The first expedition was the AMREE led by West.3 In this expedition, the data were estimated from samples of venous blood and expired alveolar gas up to an altitude of 8050m, while only alveolar gas samples were collected at the summit. The next expedition was the CXEE.5,8 In contrast to the previous expedition, on this occasion supplementary oxygen was used above 7100m, and blood sampling was performed using a femoral artery catheter. The data at the top of the mountain were extrapolated, since the samples were collected at 8400m, after starting the descent and following 20min without supplementary oxygen.
Estimated arterial gas values at the summit of Mount Everest (see text).
| Expedition | Real BP (mmHg) | PaO2 (mmHg) | PaCO2 (mmHg) | SaO2 (%) | pH |
|---|---|---|---|---|---|
| AMREE3 | 253 | 28 | 7.5 | 70 | >7.7 |
| CXEE8 | 272 | 24.6 | 13.3 | 54 | 7.538 |
AMREE: American Medical Research Expedition to Everest; CXEE: Caudwell Xtreme Everest Expedition; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; BP: barometric pressure.
One of the first investigators of human tolerance of hypoxia was Paul Bert, who in 1874 conducted the “Operation Everest”, in which a number of volunteers remained at a BP of 248mmHg for one hour and 29min. He published these and other investigations in 1878 in his book La presion barométrique. Recherches de physiologie experimentales. Encouraged by his studies, this author was one of the sponsors of the Zenit balloon, which reached an altitude of 8000m - though at the price of the death of two of the aeronauts.
Acute exposure to altitudes above 6000m results in loss of consciousness within about 10min, followed by death, since gas exchange within the lungs is not possible at such altitudes without prior acclimatization (Fig. 2).
In the case of gradual exposure to HH, the human body is able to adapt through a process known as acclimatization. A series of physiological adjustments ensure improved oxygen supply and utilization at cell level. Such adjustments take place at different levels: respiratory, cardiac, endocrinological, hematological, muscular and microcirculatory. The fastest and most important element in acclimatization is the increase in respiratory frequency and depth, i.e., hyperventilation capacity. The rest of the changes develop over the subsequent days, including increased hematocrit and hemoglobin concentration. In the AMREE, the four mountaineers that reached the summit were those exhibiting the best ventilatory response, and they moreover reached the summit in order of greater to lesser ventilatory response.9
Table 2 shows the estimation of arterial gases on the summit of Mount Everest. In addition to the hyperventilation of the mountaineers, it is interesting to note the difference in oxygen saturation recorded by the two expeditions. The explanation for this may be possible inhibition of the ventilatory response in the CXEE, which used supplementary oxygen and collected the blood samples after only 20min without oxygen use.
Permanent exposure to HH can give rise to generational adaptation. In this regard, the native Tibetan populations, which have spent over 25,000 years living at altitudes of 4000m on average, exhibit normal aerobic metabolism, reduced hemoglobin concentration, increased ventilation under both resting conditions and during exercise, an increase in nitric oxide (NO) synthesis, and the absence of pulmonary hypertensive response. In contrast, native populations in the Andes, which have been living at high altitude for less time (a little over 12,000 years) continue to present elevated hemoglobin and persistent pulmonary hypertension.10
Hypoxia, acute mountain sickness and inflammationA lack of acclimatization to high altitude gives rise to “acute mountain sickness”, which in its most severe and acute form is characterized by lung or brain edema. Hypoxia acts as a causal agent in this regard.11 The incidence and severity of the condition are related to the speed of ascent and the maximum altitude reached – this evidencing a dose–response relationship conditioned to individual susceptibility.12 On the other hand, a number of studies indicate that inflammation can also contribute to the pathogenesis of mountain sickness, because people with a history of inflammatory disorders such as infectious diarrhea or upper airway infection are more susceptible to acute mountain sickness.11,13–15
Bronchoalveolar lavage (BAL) in people with high altitude pulmonary edema (APE) reveals a marked increase in the total number of cells, with a predominance of macrophages, along with high cytokine levels—including interleukins 6 and 8, and tumor necrosis factor α.16 It can be affirmed that the inflammatory process is of a magnitude and characteristics similar to those seen in patients with adult respiratory distress syndrome (ARDS) admitted to the Intensive Care Unit.17
Studies in healthy volunteers ascending to great altitudes have improved our understanding of several disease conditions related to hypoxia by demonstrating the importance of sodium transport for eliminating alveolar fluid in the pathogenesis of APE. In individuals susceptible to APE, respiratory transepithelial sodium transport is defective, and this dysfunction worsens during hypoxia.18 The pharmacological stimulus produced by inhaled salmeterol, a β-adrenergic agonist used for the prevention of APE, reduced the incidence of the disorder by up to 50% in susceptible individuals.18 Based on these studies, intravenous salbutamol has been used to accelerate the resolution of alveolar edema in hypoxic patients with acute lung injury and ARDS, since in most such individuals’ alveolar fluid clearance capacity is altered,19 and the drug was seen to reduce the amount of extravascular lung water and improve the efficacy of mechanical ventilation.20 Nevertheless, a subsequent multicenter study involving ARDS patients was suspended after an intermediate analysis because intravenous salbutamol was seen to be poorly tolerated and increased mortality after 28 days.21 However, despite this setback, measures for stimulating transepithelial sodium transport continue to generate interest, and it remains to be determined whether such stimulation through alternative interventions (e.g., drugs other than salbutamol) may improve the resolution of lung edema, ARDS and other disease characterized by the presence of lung edema.
Maggiorini et al.22 used a pulmonary artery catheter to study mountain climbers susceptible to APE at an altitude of 4559m. The conclusion was that an acute increase in pulmonary capillary pressure of ≥20mmHg, due to an excessive pulmonary vasoconstrictive response to hypoxia, was the core hemodynamic factor in the physiopathology of lung edema. A recent study has shown that the isolated increase in pulmonary artery pressure induced by high altitude does not justify the formation of edema in a population susceptible to APE.23 When inflammation is present, as in ARDS, the increase in permeability of the alveolar-capillary barrier lowers the edema-forming threshold, and lung fluid extravasation occurs at physiologically normal capillary pressure values.
Recent studies underscore the importance of endothelial dysfunction induced by hypoxia in both the systemic and the pulmonary circulation, with an imbalance between vasoconstrictors (e.g., endothelin 1) and vasodilators (e.g., NO), and a greater production of reactive oxygen species (ROS).24–26 These substances, which are also implicated in other forms of pulmonary arterial hypertension, suggest that investigation of the molecular pathways underlying the disorders found in healthy individuals exposed to hypoxic environments may offer promising new information.
Oxygen consumption and hypobaric hypoxiaBotella and Compte27 showed populations in mountainous regions to have oxygen saturation (SaO2) values as determined by pulsioximetry higher than those of non-acclimatized mountain climbers evaluated at the same altitude. Furthermore, SaO2 was seen to increase early during the acclimatization period, and the already acclimatized individuals had SaO2 values similar to those living permanently at the same altitude.
A Spanish study28 with calculations based on the data of the AMREE3 indicated that SaO2 would gradually decrease with increasing altitude until reaching 65% at 7500m, though above this altitude SaO2 would rise as a consequence of hyperventilation – thereby facilitating an increase in alveolar oxygen partial pressure. These same authors were able to confirm this personally in the Gasherbrum II expedition (8035m).29
In contrast to SaO2, maximum oxygen consumption does not recover with acclimatization even in acclimatized individuals breathing pure oxygen, where the values would be expected to exceed those recorded at sea level.30
These surprising findings show that oxygen transport is not a limiting factor for oxygen consumption at high altitude (hemoglobin levels rise with acclimatization); that SaO2 recovers breathing oxygen; and that cardiac output increases secondary to a rise in heart rate and systolic volume. As a result, in recent years, the focus of interest has shifted from the adaptive processes of the cardiorespiratory and hematological systems toward studies on the peripheral microcirculation and the ATP production mechanisms within the mitochondria.
Hypoxia inducible factor-1The drop in PO2 from inspired air to the interior of the mitochondrion is known as the oxygen cascade, due to its similarity with a river flowing down a gradient. The oxygen cascade exists independently of the altitude level. In the mitochondria, the oxygen pressure may be close to zero, but cannot be suppressed at any time: it is imperceptible but also essential.
Hypoxia inducible factor-1 (HIF-1) plays a key role in oxygen homeostasis by facilitating oxygen supply to the tissues under hypoxic conditions, as during acclimatization to HH31 or in the hypoxemia/inflammation molecular response prevalent in sepsis.32
Hypoxia inducible factor-1 is found in almost all body tissues. Under normoxic conditions it is degraded through hydroxylation, but does not undergo degradation in the presence of hypoxia. In fact, its levels increase exponentially under such conditions. The presence of HIF-1 activates many genes that mediate in cytoprotective effects, facilitating increased oxygen supply to the tissues and improved oxygen use.
Hyperoxia is well known as a cellular oxidative stress factor. Hypoxia is paradoxically also a cell stressor from both the oxidative and metabolic perspective, and can induce alterations in mitochondrial structure and function, oxidative metabolic deficiencies, and increased ROS production.
The carotid body is the most specialized peripheral blood oxygen monitoring tissue in the body, and is located bilaterally at the bifurcation of the common carotid artery. The sensory discharge rate of the carotid body is low under conditions of normoxia (PaO2 100mmHg), but increases when the arterial O2 levels drop (PaO2 50–60mmHg). When hypoxia develops, a series of neurotransmitters are quickly released.33 The acute and chronic response to hypoxia depends on this oxygen sensing capacity.34 In order to maintain oxygen supply to the tissues, the carotid body modulates the respiratory, vascular and cardiac responses, and increases ventilation and arterial pressure. Two interesting recent articles have again demonstrated the relationship between high altitude hypoxia and arterial hypertension.35,36 The sensing activity of the carotid body is crucial for ventilatory and cardiovascular acclimatization, and its dysfunction is associated with disease.37
Hypoxia inducible factor-1 maintains functional antagonism between HIF-1α and HIF-2α. The expression of enzymes dependent on HIF-1α contributes to a pro-oxidizing state, while the expression of enzymes dependent on HIF-2α contributes to an antioxidant state.38 In situations of hypoxia, an alteration in HIF-1α/HIF-2α balance increases oxidative stress and triggers respiratory alterations such as apnea or cardiovascular disturbances such as arterial hypertension and arrhythmias.39 Martínez-Ferrer40 conduced Holter electrocardiographic recordings at high altitude and found that complete atrioventricular block episodes with pauses of over three seconds and sinus arrest reaching a duration of 8.3s occurred during sleep. Duran et al.,41 in a sleep polysomnographic study on Mount Everest, found that exposure to high altitude triggered alterations in SaO2, heart rate and ventilation consistent with a diagnosis of sleep apnea syndrome. In future, manipulation of the changes in intracellular reduction-oxidation (redox) status through selective modulation of the expression of HIF-1α or HIF-2α might become an important tool for the treatment of sleep apnea syndrome.38
Hypoxia inducible factor-1 and its genetic expressionAdaptation to hypoxia induces many reactive mechanisms, and HIF-1 is the key regulator of the expression of genes implicated in these mechanisms.31,39,42 As can be seen in Table 3, it is involved in many regulatory processes.
Products of some of the genes regulated by hypoxia inducible factor-1 (HIF-1) and their physiological function.
| Vascular biology |
| Angiogenesis: vascular endothelial growth factor (VEGF), transforming growth factor β3, plasminogen activator inhibitor 1 |
| Vascular tone regulator: endotelina-1, nitric oxide synthase, hemoxygenase 1, adrenomedullin, α1B adrenergic receptor |
| Iron/erythropoiesis |
| Erythropoietin |
| Ceruloplasmin |
| Transferrin |
| Transferrin receptor |
| Metabolism |
| Carbonic anhydrase-9 (pH regulator), adenylate kinase 3 (nucleotide metabolism) |
| Glucose metabolism: glucose transporter 1, glucose transporter 3 |
| Glycolytic enzymes: phosphofructokinase, enolase 1, phosphoglycerate kinase 1, pyruvate kinase, gliceraldehyde-3-P-dehydrogenase, lactate dehydrogenase A |
| Proliferation/survival |
| Adrenomedullin |
| Erythropoietin |
| Hemoxygenase 1 |
| Nitric oxide synthase 2 |
| IGF 1 binding protein |
| IGF 2 binding protein |
| IGF 3 binding protein |
| p21 |
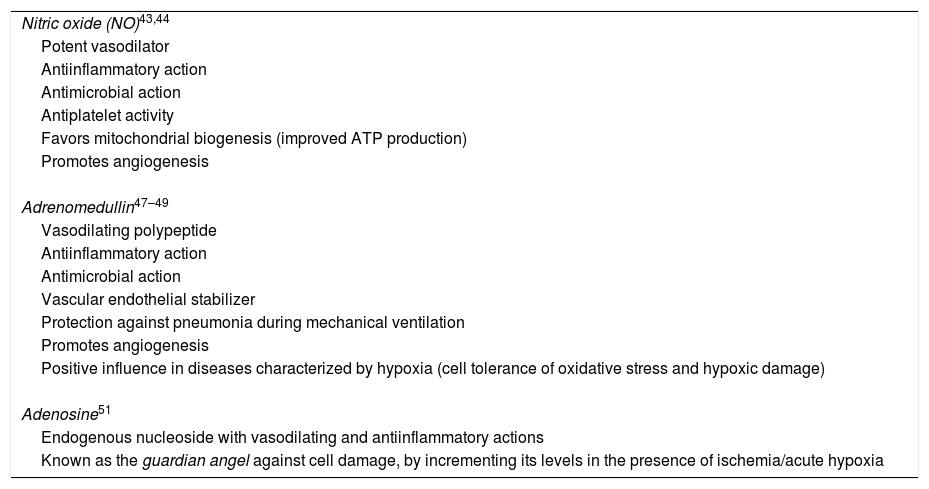
There are three important vasodilators related to hypoxia and HIF-1: NO, adrenomedullin and adenosine. Their main actions are summarized in Table 4.
Principal effects and actions of nitric oxide, adrenomedullin and adenosine.
| Nitric oxide (NO)43,44 |
| Potent vasodilator |
| Antiinflammatory action |
| Antimicrobial action |
| Antiplatelet activity |
| Favors mitochondrial biogenesis (improved ATP production) |
| Promotes angiogenesis |
| Adrenomedullin47–49 |
| Vasodilating polypeptide |
| Antiinflammatory action |
| Antimicrobial action |
| Vascular endothelial stabilizer |
| Protection against pneumonia during mechanical ventilation |
| Promotes angiogenesis |
| Positive influence in diseases characterized by hypoxia (cell tolerance of oxidative stress and hypoxic damage) |
| Adenosine51 |
| Endogenous nucleoside with vasodilating and antiinflammatory actions |
| Known as the guardian angel against cell damage, by incrementing its levels in the presence of ischemia/acute hypoxia |
Nitric oxide is an ROS that exerts a cytotoxic effect when rapidly combined with oxygen, producing free radicals and exerting potent vasodilatory action. Because of this vasodilatory effect, particularly at pulmonary level, NO is used in the treatment of ARDS.
Nitric oxide is not only elevated among individuals living at high altitudes10; it is also seen to increase in people living on low ground when attempting to adjust to high altitudes.45 For the authors of this investigation, these findings are not only relevant to healthy subjects exposed to HH, where oxygen availability is low, but also to critical patients with diseases in which oxygen availability is limited. In future, the management of such patients could experience a change in which the tolerance of hypoxia would be incremented by treatments that increase NO production.45 Perhaps for this reason a trial involving an inhibitor of NO synthesis in septic patients with under 24h of clinical evolution was suspended due to the recording of increased mortality after 28 days.46
Recently, mid-regional proadrenomedullin (precursor of adrenomedullin) has been proposed as new biomarker for predicting mortality in sepsis.50
New studies on the role of adenosine in the adaptation to HH have opened important possibilities for new therapeutic applications capable of countering the tissue damage induced by hypoxia. These studies52,53 are based on the role of erythrocyte adenosine in countering the effects of poor adaptation to HH, which has been considered responsible for so-called “hypoxic memory”. It has always been considered that humans acclimatize to HH better the more often they are exposed to it (high altitude memory). This memory had no scientific basis until the two abovementioned studies demonstrated that repeated exposure to high altitude promotes faster adaptation to HH, and that the molecular bases of this “hypoxic memory” are located in the red blood cells, which moreover increase in number during acclimatization.
Skeletal muscle in acclimatization to high altitudeFor many years it has been known that capillary density increases with acclimatization and is even higher among humans that live at high altitudes.54 This observation based on muscle biopsies has recently been confirmed by videomicroscopic study of the lip capillary network.55
In addition to increased capillary density, studies in mountain climbers returning from high altitude expeditions have demonstrated a decrease in body weight, muscle mass, muscle fibers and mitochondrial density.56
The behavior of mitochondria in response to high altitudes is opposite that seen in relation to capillary density. Upon returning from the expeditions, a decrease in mitochondrial density was observed—this decrease being even greater among individuals living at high altitudes54 – together with an increase in lipofuscin deposits in the subsarcolemmal mitochondria of the muscle fibers (as an expression of mitochondrial loss).57
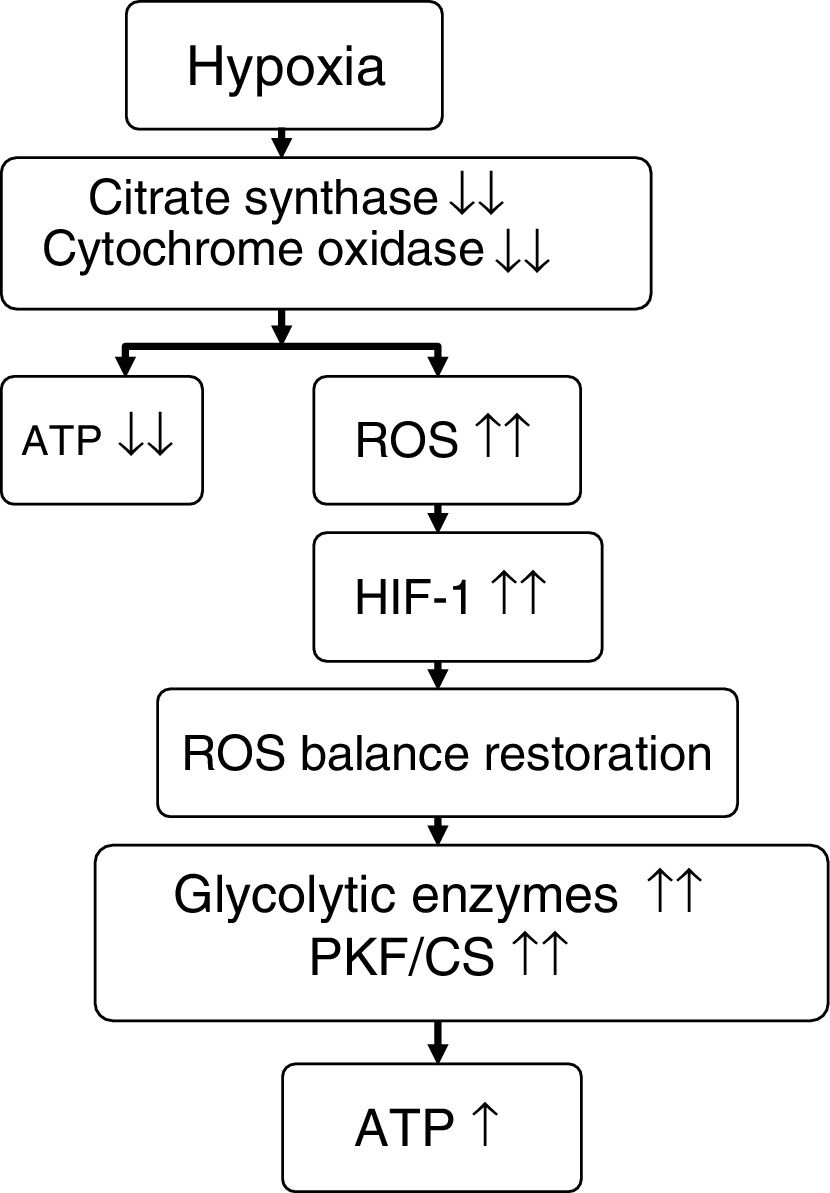
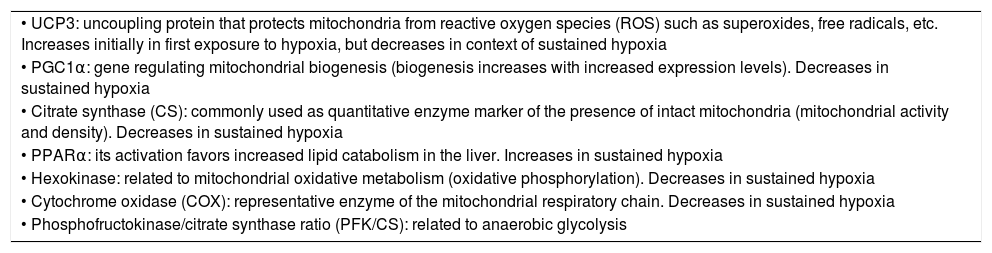
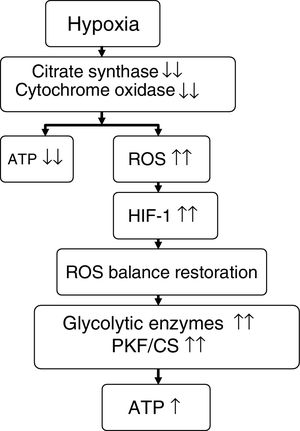
On comparing climbers that remained for 6 days (initial hypoxia) at Mount Everest base camp at 5300m versus those that remained for 66 days (sustained hypoxia) at an altitude of between 5300 and 8848m, the former individuals showed no mitochondrial loss, while the latter presented a 21% decrease in mitochondrial density and a 73% loss of subsarcolemmal mitochondria.58 In these same groups of individuals, studies were made of skeletal muscle mitochondrial acclimatization based on the comparison of a series of genes and proteins (Table 5). It was seen that in the former group, mitochondria could be protected against oxidative stress (increase in UCP3), though in the latter group such protection not only disappeared but a suppression of mitochondrial biogenesis (decrease in PGC1α and citrate synthase) and of oxidative metabolism (decrease in hexokinase and cytochrome oxidase, with an increase in peroxisome PPARα and phosphofructokinase/citrate synthase ratio) was noted.7,56,58 It seemed as if mitochondria under conditions of hypoxia induced their own destruction (autophagia), with the purpose of ensuring oxygen supply to the remaining mitochondria for the generation of ATP, modification of oxidative metabolism, and minimization of ROS production58,59 (Fig. 3).
Genes, proteins and enzymes related to skeletal muscle mitochondrial acclimatization to high altitude hypoxia.
| • UCP3: uncoupling protein that protects mitochondria from reactive oxygen species (ROS) such as superoxides, free radicals, etc. Increases initially in first exposure to hypoxia, but decreases in context of sustained hypoxia |
| • PGC1α: gene regulating mitochondrial biogenesis (biogenesis increases with increased expression levels). Decreases in sustained hypoxia |
| • Citrate synthase (CS): commonly used as quantitative enzyme marker of the presence of intact mitochondria (mitochondrial activity and density). Decreases in sustained hypoxia |
| • PPARα: its activation favors increased lipid catabolism in the liver. Increases in sustained hypoxia |
| • Hexokinase: related to mitochondrial oxidative metabolism (oxidative phosphorylation). Decreases in sustained hypoxia |
| • Cytochrome oxidase (COX): representative enzyme of the mitochondrial respiratory chain. Decreases in sustained hypoxia |
| • Phosphofructokinase/citrate synthase ratio (PFK/CS): related to anaerobic glycolysis |
Proposed effect of hypoxia upon energy metabolism.
Source: adapted from Raguso et al.7,42
ATP: adenosine triphosphate; HIF-1: hypoxia inducible factor-1; PKF/CS: phosphofructokinase/citrate synthase ratio; ROS: reactive oxygen species.
The key factor responsible for the mentioned changes is HIF-1, the levels of which progressively increase with altitude.58 These same changes are also noted in native populations living under conditions of chronic hypoxia, such as Tibetans, Sherpa and Incas,57 as well as in patients with chronic obstructive pulmonary disease (COPD). However, in contrast to the previous population groups, patients with COPD do not present increased capillary density—a fact that may explain their lesser tolerance of physical exercise.7
Brain circulation and microcirculation in adaptation to high altitudeIn relation to brain circulation at high altitude, transcranial Doppler ultrasound study of the middle cerebral artery has revealed a gradual increase in mean arterial diameter from a height of 5300m (5.30mm at sea level; 5.23mm at 5300m; 6.66mm at 6400m; and 9.34mm at 7950m). Similar behavior has also been observed for other cerebral parameters such as perfusion velocity, blood flow and oxygen delivery. However, these changes have been seen to quickly revert on administering oxygen at an altitude of 7950m. The same authors performed a magnetic resonance imaging study at sea level breathing hypoxic air for three hours (O2 fraction 12%), and obtained similar results.60 Vasodilatation is possibly the regulating mechanism for countering the increase in blood viscosity caused by acclimatization, thereby favoring oxygen delivery.
The study of the sublingual microcirculation has shown the increase in capillary density to occur at the expense of vessels measuring up to 50μm in diameter. Vessels with a diameter of under 25μm were seen to increase from an altitude of 5300m, while those measuring 25–50μm in diameter increased from 3500m. These changes in turn were accompanied by a decrease in sublingual microcirculatory flow index (MFI), giving rise to very evident abnormal microcirculation from an altitude of 4900m, and which appeared to become interrupted, with out of focus images of the red cells.61,62 At high altitude, the hemoglobin dissociation curve is shifted to the left (increased saturation but less oxygen release). This slowing of flow, together with the blood viscosity and increased density, could represent a beneficial adaptation to hypoxia and facilitate oxygen delivery to the mitochondria. In the same way as in the brain circulation, the administration of oxygen at 7950m normalized saturation but produced an important decrease in MFI, with deviation of the circulation from vessels measuring under 25μm in diameter toward vessels between 25 and 50μm in diameter.62 Possibly the production of NO—a potent vasodilator dependent upon HIF-1—could become sharply reduced with the administration of oxygen. This reversal of hypoxemia predicts that hypoxia produces vasodilatation, while hyperoxia causes vasoconstriction61 and a decrease in microvascular perfusion.63 Such findings may have implications referred to the chronic administration of high concentrations of oxygen in critical patients.
Genes, hypoxia and performanceIt is now known that genetic factors influence high altitude tolerance and performance. One of the possible implicated factors is polymorphism of the gene encoding for angiotensin converting enzyme. Montgomery et al.64 compared a control population versus mountain climbers experienced in ascents to over 7000m without supplementary oxygen, and found the latter to have a predominance of homozygosity for insertion allele (II) of the angiotensin converting enzyme gene versus the control group. The same findings were obtained on comparing climbers with successful ascents to over 8000m without supplementary oxygen versus those with unsuccessful ascents.65
This predominance is not only associated to high altitude performance but also to the survival of critical patients with ARDS,66 pediatric meningococcal sepsis67 or severe trauma,68 or the incidence of high altitude pulmonary edema (APE).69
Healthy mountain climber and critical patient modelsIn the course of this review we have already commented some of the coincident elements between the two models. Hypoxia inducible factor-1 (HIF-1) plays a key role in oxygen homeostasis both in acclimatization to high altitude hypoxia31 and in the context of the hypoxemia/inflammation molecular response found in sepsis.32 The genetic expression of HIF-1 (NO, adrenomedullin and adenosine) facilitates oxygen supply to the tissues.
The expression of mRNA encoding for HIF-1α is inversely correlated to the severity of sepsis. The correlation is greater among patients with septic shock than in septic patients without shock—a fact that may imply poor adaptation to hypoxia or depression of the immune response. In vitro, the acute administration of lipopolysaccharide raises the HIF-1α expression levels, while prolonged stimulation results in suppression, reaching expression levels even below basal. Furthermore, the administration of dimethyloxalylglycine (an inhibitor of the catabolism of certain factors induced by hypoxia) in septic patients with a clinical evolution of several hours does not increase the HIF-1 levels, and in these patients HIF-1 expression is greatly reduced upon admission to the Intensive Care Unit.33 This means that when septic shock patients are admitted to the Intensive Care Unit they have already suffered the inflammatory response for many hours, and their response to continuous stimulation with the endotoxin has been exhausted.70,71
There are coincident aspects between the abnormal sublingual microcirculation seen at altitudes above 4900m61,62 and that observed in critical patients. Boerma et al.72 studied the decrease in sublingual microcirculatory flow index (MFI) in critical patients with abdominal sepsis between the first day following diagnosis and the third day of active treatment, and found an association between the severity of sepsis and an altered sublingual MFI.
The comparable changes in mitochondrial biogenesis between acclimatized mountain climbers and critical patients may reflect similar adaptive responses. In both groups the initial response is characterized by a hyperdynamic state with increased cardiac output that facilitates oxygen delivery to the tissues, along with mitochondrial protection against oxidative stress, in order to ensure improved oxygen use.59 Posteriorly, mitochondrial biogenesis is seen to decrease, though accompanied by improved oxygen use.6,58,59 In sepsis, this decrease in mitochondrial activity and in oxidative phosphorylation is conditioned by an acquired cellular respiration defect known as “cell hibernation” or “cytopathic hypoxia”, which initially can be linked to cell survival but subsequently may trigger multiorgan failure.73,74
Bioenergetic dysfunction is of increasing relevance in explaining the paradox between clinical and biochemical organ failure in sepsis and survival characterized by minimum cell death (the cells are able to enter a “hibernation” state in the face of overwhelming inflammation), the maintenance of cell oxygenation and mitochondrial function, and elevation of the biogenesis markers.74 Although no clear cause–effect relationship can be established, these observations point to a new therapeutic intervention strategy focused on mitochondrial protection or acceleration of the recovery process through the stimulation of mitochondrial biogenesis—this being particularly pertinent in view of the multiple failures recorded with experimental immune modulating therapies.74
Metformin is an important activator of AMP kinase, regarded as a cell energy detector that promotes mitochondrial activity and biogenesis, and which in addition to elevating lactate concentration also exerts anti-endotoxemic and antiinflammatory effects.73 A recent study carried out by Park et al.75 on the impact of metformin in patients with severe sepsis or septic shock that were previously using the drug has shown that despite the high lactate levels, survival increased almost 30%—though this result should be viewed with caution because of the limited number of cases involved. On the other hand, it must be considered that there are routine interventions in intensive care that affect mitochondrial biogenesis or function, such as the use of bacteriostatic agents, catecholamines, corticosteroids or thyroid therapies, the type of nutrition, or evolutive situations such as hyperglycemia, immobility or mechanical ventilation.76
Permissive hypoxiaHumans possess potential hypoxia tolerance due to the experiencing of severe and sustained hypoxia in fetal life. It is important to underscore that the fetal physiological mechanisms that maintain cellular oxidative metabolism operate through the modification of oxygen consumption.77 The mechanisms that differentiate mountain climbers who acclimatize to HH from those who fail to acclimatize, or inhabitants adapted to chronic hypoxia, and survivors from non-survivors of a critical illness, may at least in part involve cellular control of oxygen consumption through the abovementioned signaling pathways.6 It is not a matter of increasing the oxygen supply but of improving its use. It is in this therapeutic approach where the basis of permissive hypoxia may be found. Having accepted the concept of permissive hypercapnia, we now should also accept the concept of permissive hypoxia, as apparently advised by recent studies.78–80
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Avellanas Chavala ML. Un viaje entre la hipoxia de la gran altitud y la hipoxia del enfermo crítico: ¿qué puede enseñarnos en la compresión y manejo de las enfermedades críticas? Med Intensiva. 2018;42:380–390.