Proportional assist ventilation plus (PAV+) applies pressure depending on the patient's inspiratory effort, automatically adjusting flow and volume assist to changes in respiratory mechanics. We aimed to assess the clinical factors associated with the success of PAV+ as first-line support in the acute phase of critical illness.
MethodsA prospective cohort study was carried out. Mechanically ventilated patients >24h were switched from assist-control ventilation to PAV+ as soon as they regained spontaneous breathing activity. PAV+ was set to deliver the highest assistance. We compared patients in whom PAV+ succeeded vs those in whom it failed.
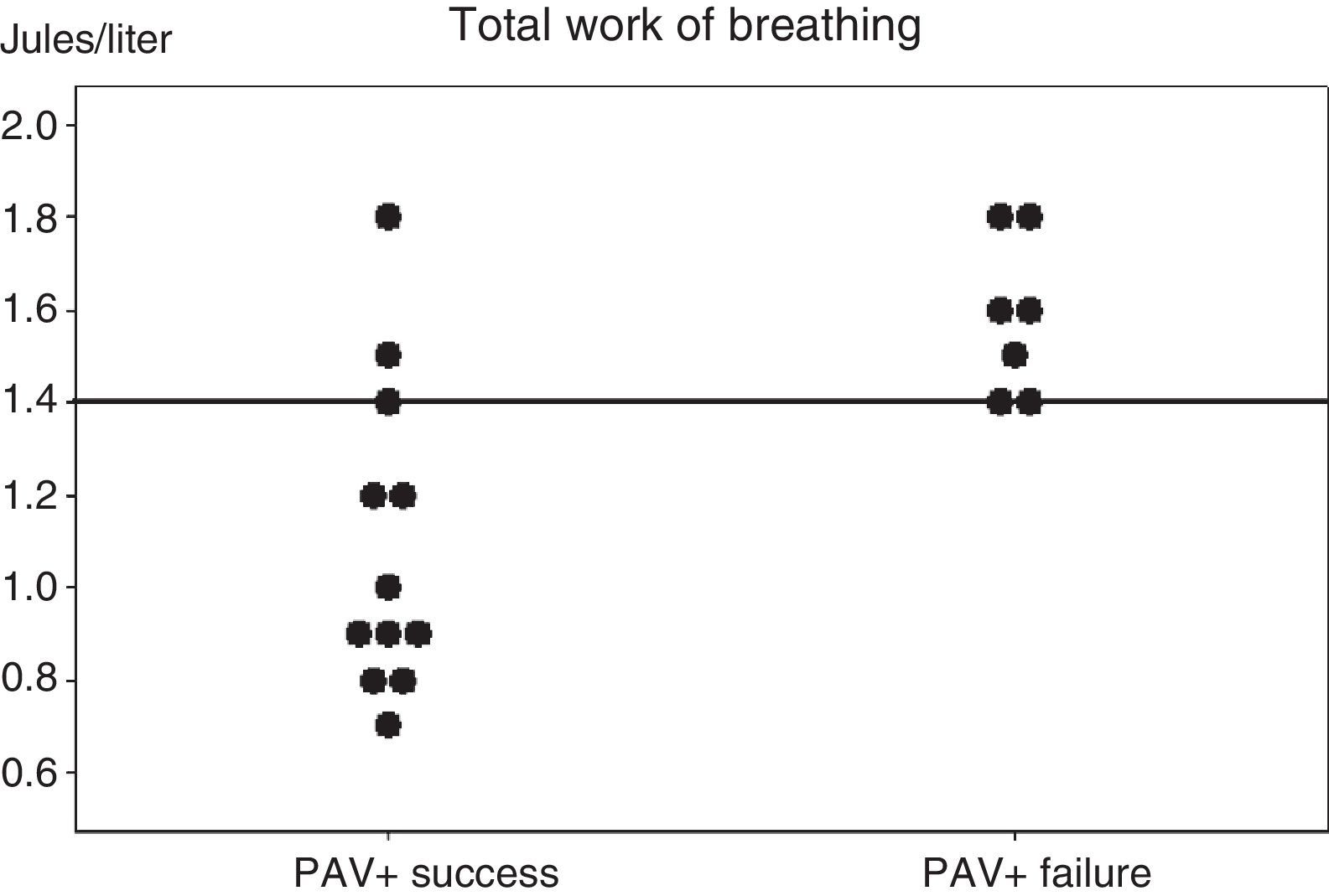
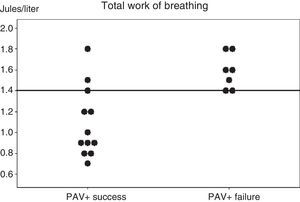
ResultsPAV+ succeeded in 12 (63%) patients, but failed in 7 (37%) due to tachypnea (n=4), hypercapnia (n=2), and metabolic acidosis (n=1), but without statistical significance. Both groups had similar clinical parameters. On the day of inclusion, total work of breathing per breath was lower in the successful PAV+ group (WOBTOT: 0.95 [0.8–1.35] vs 1.6 [1.4–1.8]J/l; P<.007). The area under the ROC curve was 0.89±0.08 for WOBTOT. The best cut-off for predicting PAV+ success was WOBTOT<1.4J/l (sensitivity: 1 [0.7–1], specificity: 0.6 [0.4–0.6], PPV: 0.7 [0.5–0.7], and NPV: 1 [0.6–1]).
ConclusionPAV+ proved feasible as first-line ventilatory support in 63% of the patients, mostly in individuals without extreme derangements in WOBTOT. Tachypnea and hypercapnia were the clinical factors associated with failure, though statistical significance was not reached.
La ventilación asistida proporcional (PAV) + genera una presión en la vía aérea que depende del esfuerzo inspiratorio del paciente, ajustando automáticamente el flujo y volumen generados según los cambios en la mecánica respiratoria. Pretendimos analizar los factores clínicos asociados al éxito de la PAV+ como primera línea de tratamiento en la fase aguda del paciente crítico.
MétodosEstudio prospectivo, de cohortes. En todo paciente con ventilación mecánica estimada>24h se sustituía la ventilación asistida controlada por PAV+ en cuanto recuperaban la actividad respiratoria espontánea. La PAV+ se programó para generar una asistencia elevada. Se compararon los pacientes en que la PAV+ se aplicó con éxito frente a aquellos en los que fracasó.
ResultadosPAV+ fue un éxito en 12 pacientes (63%) y fracasó en 7 (37%) debido a taquipnea (n=4), hipercapnia (n=2) y acidosis metabólica (n=1), aunque no llegó a demostrarse estadísticamente. Los parámetros clínicos fueron similares para ambos grupos. El día de ingreso, el trabajo total respiratorio (WOBTOT) fue inferior en el grupo de éxito (WOBTOT: 0,95 [0,8–1,35] vs. 1.6 [1,4–1,8]J/L; p<0,007). El área bajo la curva ROC fue 0,89±0,08 para WOBTOT. El mejor punto de corte para predecir el éxito de la PAV+ fue un WOBTOT<1,4J/L (sensibilidad: 1 [0,7–1], especificidad: 0,6 [0,4–0,6], VPP: 0,7 [0,5–0,7], y VPN: 1 [0,6–1]).
ConclusiónPAV+ fue una técnica aplicable como primera línea de tratamiento en el 63% de los pacientes, fundamentalmente en aquellos sin deterioro excesivo del trabajo respiratorio. Las variables clínicas asociadas al fracaso fueron la taquipnea y la hipercapnia, aunque sin significación estadística.
Proportional assist ventilation (PAV) is an assisted ventilation mode developed and described by Younes in 1992.1,2 It was initially conceived as a tool for the investigation of the respiratory center that compensated respiratory muscle activity. However, PAV was also shown to be able to largely replace the excess muscle work observed during acute respiratory failure.
In PAV, the ventilator generates inspiratory pressure directly proportional to the flow and volume generated by the muscle work of the patient.3,4 The patient spontaneously maintains the respiratory pattern best suited for controlling internal homeostasis, while the professional determines the level of ventilatory support (gain) considered most appropriate for helping the patient. In contrast to other ventilatory modalities, PAV establishes positive feedback between the patient and the ventilator. Rather than substituting the muscle work of the patient, PAV multiplies the results of such work in a predefined proportion (gain).
Although PAV originally proved difficult to apply in the clinical setting, new software has been developed (proportional assist ventilation plus, PAV+) that continuously reflects the changes occurring in respiratory system mechanics. The system is based on the noninvasive determination of resistance and elastance values. Such a software makes it easier to use this ventilation mode, since the predetermined proportion of ventilatory assist (gain) is kept stable during the usual physiological changes in respiratory mechanics.5,6
In recent years, different studies have compared PAV with pressure-support ventilation (PSV).7–14 In general terms, PAV has been shown to offer an advantage in terms of patient–ventilator interaction, comfort, lessened work of breathing (WOB), and patient capacity to adapt to variations in homeostasis and respiratory mechanics.7,10–13 Two of these studies have analyzed the early application of PAV+ in critical patients. In this respect, Kondili et al.13 compared the respiratory pattern and energy cost between PAV and PSV, with and without the addition of loading, during a short period of time in critical patients. They demonstrated superior compensation capacity on the part of PAV+ in the face of added loading, in terms of energy consumption and neuroventilatory coupling. On the other hand, Xirouchaki et al.14 compared the application of PAV+ and PSV during 48h in critical patients, and recorded a greater incidence of ventilatory failure and asynchrony in the patients subjected to PSV. These results show that although PSV is an easily applicable routine ventilation mode, PAV+ is probably better in terms of compensation in the face of added clinical derangement and patient–ventilator synchrony. On the other hand, PAV+ allows us to decide the degree of muscle compensation in the patient in order to overcome deteriorated respiratory mechanics through gain. Ruiz-Ferron et al.15 analyzed the WOB among patients with different levels of ventilatory support, confirming the inverse relationship between gain and WOB. In this context, a gain of 80% corresponded to a patient WOB level of 0.1–0.2J/l, which is equivalent to maximum muscle compensation.
Considering the limited experience with PAV+ in the acute phase of critical illness and the benefits of spontaneous breathing,16–18 the present study was designed to analyze the clinical factors (respiratory mechanics, hemodynamics and blood gas characteristics) associated to PAV success when used on a routine basis in the acute phase of critical illness under high-assist conditions, once the patient has regained a spontaneous breathing rhythm that suffices to regulate internal homeostasis.
Materials and methodsPatientsThe study was carried out in three medical-surgical Intensive Care Units (ICUs) following approval of the corresponding local Ethics Committees. The patient relatives or representatives gave written informed consent in each case (Appendix A). As an inclusion criterion, the patients were expected to undergo mechanical ventilation (MV) during at least 24h, and screening was carried out in the first 48h of admission to the ICU. We excluded patients under 18 and over 85 years of age, subjects with FiO2≥0.8, ventilation in the prone position, pregnancy, cardiogenic shock, serious cardiac arrhythmias, important vasoactive drug needs (dopamine>15μg/kg/min or noradrenalin>0.5μg/kg/min), high sedation requirements capable of influencing spontaneous ventilation, cases characterized by treatment withdrawal or limitation instructions, hypothermia protocols, contraindication to PAV (air leakage, neurological or neuromuscular disease), and lack of availability of a ventilator system with PAV.
The inclusion period comprised the first 24h of admission, in which the patients were initially ventilated in volume assist-control ventilation (V-ACV). As soon as sufficient hemodynamic, respiratory and sedation stability to activate the ventilatory trigger was achieved, the patients were switched to PAV+ mode. The aim was to reach a sedation level of 3–4 according to the Ramsay scale.19 The management of positive end-expiratory pressure (PEEP) and FiO2 was established according to routine practice, and the patients were kept in a semiseated position (between 30° and 40°).
ProtocolArterial pressure monitoring (invasive and noninvasive) was performed on a continuous basis, along with oxygen saturation and heart rate (electrocardiogram). Mechanical ventilation was carried out with Puritan-Bennett® 840 plus ventilators (Nellcor Puritan Bennett LLC, Gosport, UK).
The aim was to achieve maximum release from muscle work from the start of PAV+ and during the entire study period (acute phase), this corresponding to a gain of 90%. If the patient developed high tidal volumes (VT) or high peak airway pressures (Paw), the gain was lowered in fractions of 10% to a minimum assistance of 60%.
Successful PAV+ was regarded as its application from the start and until critical criteria for weaning were met (FiO2≤0.4 in the supine position, systolic blood pressure>100mmHg without vasopressor drugs, heart rate<140l/min, adequate level of consciousness, ability to cough). The criteria indicating PAV+ failure and its replacement by VAC ventilation were: VT≤3ml/kg or ≥10ml/kg, respiratory frequency≤10min−1 or ≥40min−1, peak pressure (Paw)>45cmH2O, important accessory muscle use, perspiration, paradoxical abdominal movements, or hemodynamic instability (severe arrhythmias, progressive increase in vasoactive drug needs, and incapacity to maintain a systolic blood pressure of >100mmHg).
Once the weaning criteria were met, a T-tube was directly positioned or ventilatory support with PSV or PAV+ was gradually reduced. This process was carried out according to the criterion of the supervising physician, since the study was focused on assessing the critical phase, not the weaning process.
During the inclusion period and before applying PAV+, we recorded information on the cause of respiratory failure, patient age, gender, mortality risk (SAPS3 score) and PaO2/FiO2. Data collection under PAV+ was carried out on the day of inclusion, during the first hour after switching to this ventilation mode, and after achieving a stable respiratory pattern. On the subsequent days, data collection was performed early in the morning. The respiratory parameters were selected from the ventilator screen: VT, respiratory frequency; Paw, respiratory system compliance (CPAV), respiratory system resistance (RPAV=Raw, corresponding to the airway resistance estimated by the ventilator after eliminating the calculated artificial airway resistance), and the total work of breathing per breath (WOBTOT=WOBpatient+WOBventilator). WOBpatient was calculated through electronic integration of the estimated muscle pressure and inspiratory volume–the former being obtained from the movement equation based on the values of resistance and compliance continuously measured by the ventilator. The precision of this method was validated by Lotti et al.20, comparing it with the esophageal pressure. Blood gases were determined daily at the same time as collection of the respiratory data.
Statistical analysisThe demographic data are reported as the median and interquartile range. The Mann–Whitney U-test was used to compare the PAV+ success and failure groups, given the limited number of patients involved. Receiver operating characteristic (ROC) curves were plotted to determine the predictive value of each of the variable associated with PAV+ success, with calculation of the sensitivity, specificity and positive (PPV) and negative predictive values (NPV). The SPSS® version 14.0 statistical package was used throughout.
ResultsThe study was carried out between September 2008 and March 2009. During this period we analyzed a total of 108 patients among the three hospitals; of these subjects, only 19 met the inclusion criteria. The patients were enrolled in the first 24h after intubation. The remaining 89 patients were excluded due to an estimated duration of mechanical ventilation of under 24h (postoperative cases, n=36; 40%), hemodynamic or respiratory instability (n=12; 13%), neurological disorders (n=21; 24%), an expected fatal outcome (n=9; 10%), and others (n=11; 12%).
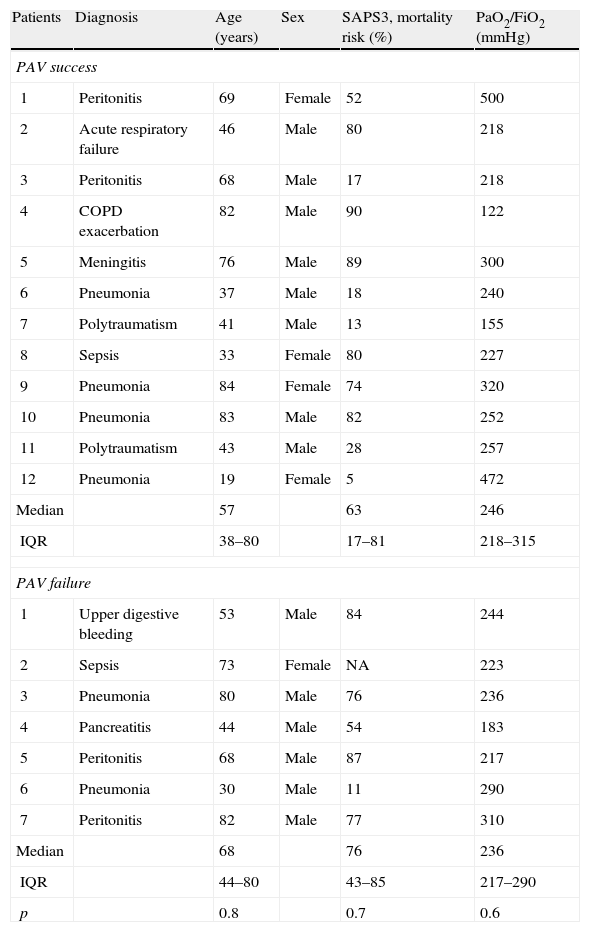
PAV+ was successfully applied in 12 patients (63%) and failed in 7 (37%). According to the supervising physician, failure was attributable to tachypnea in four cases (57%) (one with Paw>50cmH2O), hypercapnia in two cases (29%) and metabolic acidosis in one (14%). There were no differences between the two groups in terms of age, severity score or oxygenation ratio (Table 1).
Baseline characteristics of the patients at the time of inclusion.
| Patients | Diagnosis | Age (years) | Sex | SAPS3, mortality risk (%) | PaO2/FiO2 (mmHg) |
| PAV success | |||||
| 1 | Peritonitis | 69 | Female | 52 | 500 |
| 2 | Acute respiratory failure | 46 | Male | 80 | 218 |
| 3 | Peritonitis | 68 | Male | 17 | 218 |
| 4 | COPD exacerbation | 82 | Male | 90 | 122 |
| 5 | Meningitis | 76 | Male | 89 | 300 |
| 6 | Pneumonia | 37 | Male | 18 | 240 |
| 7 | Polytraumatism | 41 | Male | 13 | 155 |
| 8 | Sepsis | 33 | Female | 80 | 227 |
| 9 | Pneumonia | 84 | Female | 74 | 320 |
| 10 | Pneumonia | 83 | Male | 82 | 252 |
| 11 | Polytraumatism | 43 | Male | 28 | 257 |
| 12 | Pneumonia | 19 | Female | 5 | 472 |
| Median | 57 | 63 | 246 | ||
| IQR | 38–80 | 17–81 | 218–315 | ||
| PAV failure | |||||
| 1 | Upper digestive bleeding | 53 | Male | 84 | 244 |
| 2 | Sepsis | 73 | Female | NA | 223 |
| 3 | Pneumonia | 80 | Male | 76 | 236 |
| 4 | Pancreatitis | 44 | Male | 54 | 183 |
| 5 | Peritonitis | 68 | Male | 87 | 217 |
| 6 | Pneumonia | 30 | Male | 11 | 290 |
| 7 | Peritonitis | 82 | Male | 77 | 310 |
| Median | 68 | 76 | 236 | ||
| IQR | 44–80 | 43–85 | 217–290 | ||
| p | 0.8 | 0.7 | 0.6 | ||
COPD: chronic obstructive pulmonary disease; IQR: interquartile range; NA: not available; PaO2/FiO2: arterial pressure of oxygen/fraction of inspired oxygen; PAV: proportional assist ventilation.
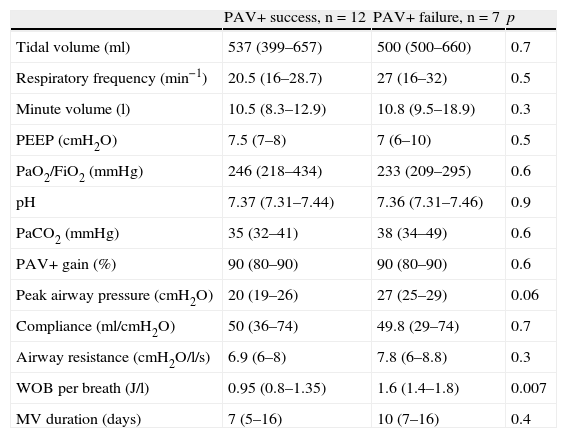
The analysis of the parameters predicting PAV+ success or failure during the first day of inclusion showed no differences except for WOBTOT, which was seen to be significantly higher in the PAV+ failure group (WOBTOT: 1.6 [1.4–1.8] vs 0.95 [0.8–1.35]J/l; p<0.007) (Fig. 1). There were no significant differences in respiratory frequency, PaCO2 or pH in the overall assessment of the two groups, despite the fact that these parameters were a cause of suspension of the ventilation mode in the PAV+ failure group according to the clinical evaluation of the professionals (Table 2 and Fig. 1).
Individual work of breathing (WOB) values of the two groups of patients, recorded in the first hour after starting proportional assist ventilation plus (PAV+) and in the clinical and respiratory stability phase. A horizontal line shows the best cut-off value for predicting the results of PAV+.
Variables on the first day of proportional assist ventilation plus (PAV+) in the patients in which the technique proved successful vs those in which it failed.
| PAV+ success, n=12 | PAV+ failure, n=7 | p | |
| Tidal volume (ml) | 537 (399–657) | 500 (500–660) | 0.7 |
| Respiratory frequency (min−1) | 20.5 (16–28.7) | 27 (16–32) | 0.5 |
| Minute volume (l) | 10.5 (8.3–12.9) | 10.8 (9.5–18.9) | 0.3 |
| PEEP (cmH2O) | 7.5 (7–8) | 7 (6–10) | 0.5 |
| PaO2/FiO2 (mmHg) | 246 (218–434) | 233 (209–295) | 0.6 |
| pH | 7.37 (7.31–7.44) | 7.36 (7.31–7.46) | 0.9 |
| PaCO2 (mmHg) | 35 (32–41) | 38 (34–49) | 0.6 |
| PAV+ gain (%) | 90 (80–90) | 90 (80–90) | 0.6 |
| Peak airway pressure (cmH2O) | 20 (19–26) | 27 (25–29) | 0.06 |
| Compliance (ml/cmH2O) | 50 (36–74) | 49.8 (29–74) | 0.7 |
| Airway resistance (cmH2O/l/s) | 6.9 (6–8) | 7.8 (6–8.8) | 0.3 |
| WOB per breath (J/l) | 0.95 (0.8–1.35) | 1.6 (1.4–1.8) | 0.007 |
| MV duration (days) | 7 (5–16) | 10 (7–16) | 0.4 |
MV: mechanical ventilation; PaCO2: arterial partial pressure of CO2; PaO2/FiO2: arterial pressure of oxygen/fraction of inspired oxygen; PAV: proportional assist ventilation; PEEP: positive end-expiratory pressure; WOB: work of breathing.
Data expressed as the mean (interquartile range).
Fig. 1 shows the individual values of WOBTOT in the first hour of PAV+ for each group. The area under the ROC curve was 0.89±0.08 for WOBTOT. The best cut-off value for predicting PAV+ success was WOBTOT<1.4J/l (sensitivity: 1 [0.7–1], specificity: 0.6 [0.4–0.6], positive predictive value: 0.7 [0.5–0.7], and negative predictive value: 1 [0.6–1]).
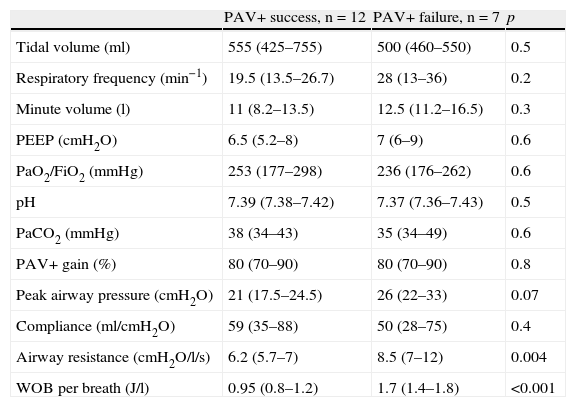
The second day of application of PAV+ (Table 3) likewise showed no differences in the respiratory parameters, referred to both breathing pattern and gas exchange. A significant difference in WOBTOT was seen to persist (WOBTOT: 1.7 [1.4–1.8] vs 0.95 [0.8–1.2]J/l; p<0.001), and an increased airway resistance was observed in the PAV+ failure group, which did not reach significance on the first day (Raw 7.8 vs 6.9cmH2O/l/s; p=0.3), but became more intense on the second day (Raw 8.5 [7–12] vs 6.2 [5.7–7]cmH2O/l/s; p=0.004). Nevertheless, no such a difference was seen on the third day.
Variables on the second day of proportional assist ventilation plus (PAV+) in the patients in which the technique proved successful vs those in which it failed.
| PAV+ success, n=12 | PAV+ failure, n=7 | p | |
| Tidal volume (ml) | 555 (425–755) | 500 (460–550) | 0.5 |
| Respiratory frequency (min−1) | 19.5 (13.5–26.7) | 28 (13–36) | 0.2 |
| Minute volume (l) | 11 (8.2–13.5) | 12.5 (11.2–16.5) | 0.3 |
| PEEP (cmH2O) | 6.5 (5.2–8) | 7 (6–9) | 0.6 |
| PaO2/FiO2 (mmHg) | 253 (177–298) | 236 (176–262) | 0.6 |
| pH | 7.39 (7.38–7.42) | 7.37 (7.36–7.43) | 0.5 |
| PaCO2 (mmHg) | 38 (34–43) | 35 (34–49) | 0.6 |
| PAV+ gain (%) | 80 (70–90) | 80 (70–90) | 0.8 |
| Peak airway pressure (cmH2O) | 21 (17.5–24.5) | 26 (22–33) | 0.07 |
| Compliance (ml/cmH2O) | 59 (35–88) | 50 (28–75) | 0.4 |
| Airway resistance (cmH2O/l/s) | 6.2 (5.7–7) | 8.5 (7–12) | 0.004 |
| WOB per breath (J/l) | 0.95 (0.8–1.2) | 1.7 (1.4–1.8) | <0.001 |
PaCO2: arterial partial pressure of CO2; PaO2/FiO2: arterial pressure of oxygen/fraction of inspired oxygen; PAV: proportional assist ventilation; PEEP: positive end-expiratory pressure; WOB: work of breathing.
Data expressed as the mean (interquartile range).
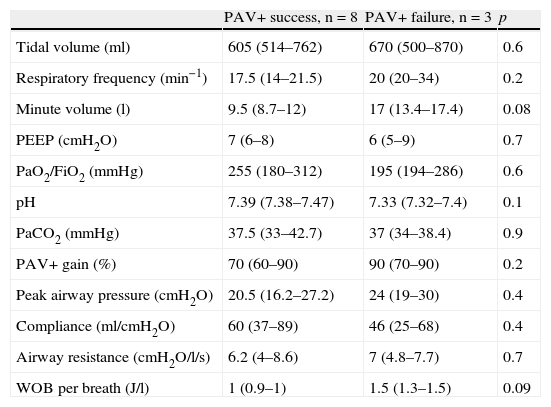
On the third day of ventilation, with fewer patients in each group (PAV+ success n=8 and PAV+ failure=3), no differences were seen for any of the parameters analyzed (Table 4).
Variables on the third day of proportional assist ventilation plus (PAV+) in the patients in which the technique proved successful vs those in which it failed.
| PAV+ success, n=8 | PAV+ failure, n=3 | p | |
| Tidal volume (ml) | 605 (514–762) | 670 (500–870) | 0.6 |
| Respiratory frequency (min−1) | 17.5 (14–21.5) | 20 (20–34) | 0.2 |
| Minute volume (l) | 9.5 (8.7–12) | 17 (13.4–17.4) | 0.08 |
| PEEP (cmH2O) | 7 (6–8) | 6 (5–9) | 0.7 |
| PaO2/FiO2 (mmHg) | 255 (180–312) | 195 (194–286) | 0.6 |
| pH | 7.39 (7.38–7.47) | 7.33 (7.32–7.4) | 0.1 |
| PaCO2 (mmHg) | 37.5 (33–42.7) | 37 (34–38.4) | 0.9 |
| PAV+ gain (%) | 70 (60–90) | 90 (70–90) | 0.2 |
| Peak airway pressure (cmH2O) | 20.5 (16.2–27.2) | 24 (19–30) | 0.4 |
| Compliance (ml/cmH2O) | 60 (37–89) | 46 (25–68) | 0.4 |
| Airway resistance (cmH2O/l/s) | 6.2 (4–8.6) | 7 (4.8–7.7) | 0.7 |
| WOB per breath (J/l) | 1 (0.9–1) | 1.5 (1.3–1.5) | 0.09 |
PaCO2: arterial partial pressure of CO2; PaO2/FiO2: arterial pressure of oxygen/fraction of inspired oxygen; PAV: proportional assist ventilation; PEEP: positive end-expiratory pressure; WOB: work of breathing.
Data expressed as the mean (interquartile range).
The degree of assist corresponded to a gain of 90%. During the study we applied a gain of between 60 and 90%, with an average of 80%–no differences in the analyzed data being observed between the two groups (Tables 2–4).
PAV+ failure showed a insignificant tendency to increase the days of mechanical ventilation (10 days [7–16] vs 7 days [5–16]; p=0.4) (Table 2). Lastly, two patients in each group died during admission to the ICU.
DiscussionProportional assist ventilation plus (PAV+), as a first ventilation option in acute patients, was successfully applied in two-thirds of our cases. On the other hand, WOBTOT was seen to be a good predictor for the application of this technique. These findings could prove useful in future for improving patient selection.
Previous studies have shown that in comparison with PSV, the application of PAV offers advantages in terms of patient–ventilator interaction in different clinical situations.8,10,11,21 Most of these publications focus on the application of PAV during the weaning phase or for short periods of time in the context of physiological studies. The results generally show PAV to offer safe support for critically ill patients. The main contribution of our study has been the identification of the clinical factors associated to success or failure with the continuous application of this ventilation mode.
An obligate first comment on our study is the low proportion (<20%) of patients selected. This was probably a consequence of our conservative approach, with the exclusion those patients expected to require less than 24h of ventilation, together with postoperative subjects or patients with hemodynamic and/or respiratory instability. Likewise, neurological damage, deep sedation or muscle paralysis are intrinsic contraindications in relation to PAV.
Clinical failure of PAV+ was always associated to elevated WOBTOT, despite the theoretical capacity of this ventilation mode to compensate important derangements in respiratory mechanics. We consider that patients with important respiratory muscle problems were unable to cope with this clinical situation, even when generating minimum WOB under high gain conditions with PAV+. The fact that direct measurement of muscle function was not previously included in the protocol precludes us from drawing further conclusions in this respect. On the other hand, the absence of significant differences in minute volume, respiratory frequency or compliance could be related to the small sample size involved.
The PAV+ failure group showed a progressive increase in airway resistances, which reached statistical significance by day two. This finding was not recorded over the subsequent days, and was therefore not considered relevant. The fact that there were fewer patients in each PAV+ group on day three does not allow us to draw further conclusions. On the other hand, it must be taken into account that the aim of the study was to estimate predictive values of failure on the first day of inclusion.
The clinical criterion most often used by the clinicians in diagnosing PAV+ failure was tachypnea (>40rpm). This circumstance could be related to the high WOBTOT, despite the fact that the differences in mean respiratory frequency between the two groups failed to reach statistical significance (Table 2) because the ventilation data were collected at a certain time of day, and the episode causing PAV+ failure occurred in the course of the day. Different hypotheses could be proposed to explain the tachypnea observed by the clinicians. Firstly, the ventilatory pattern adopted in PAV+ varies widely due to the intrinsic capacity of this ventilation mode to reflect the inherent physiological changes of the patient, designed to adapt internal homeostasis in different clinical situations, and which in many cases are masked when other ventilation modes are used.22,23 Secondly, when tachypnea evidences patient discomfort (use of accessory muscles, hemodynamic instability), it may be reflecting patient–ventilator asynchrony. Du et al.24 analyzed patient–ventilator synchrony under ventilation with PAV, using software simulation and healthy volunteers. Significant asynchrony could be observed at the end of inspiration, consisting of a delayed decrease in the inspiratory flow of the ventilator with respect to the cessation of muscle work on the part of the patient. This phenomenon was correlated to three factors: the intrinsic delay of the ventilator processor, gain, and the respiratory time constant. This asynchrony phenomenon developed from the combination of high Raw and high programmed gain values. This may have been the case in our study, where the programmed gain values were always high and there was a significant increase in airway resistance in the PAV+ failure group on day two (Table 3). This is only a hypothetical explanation, however.
Of the patients with tachypnea, only one showed high Paw. Georgopoulos et al.22 attributed high Paw to excessive pressure or volume release (the so-called runaway phenomenon) and the lack of linearity in the pressure–volume and pressure–flow relationships.25 The authors likewise found this phenomenon to be extremely infrequent with gains of <85%. In our study, although the initial target gain was 90%, the applied average was 80% in both groups. Likewise, in the study published by Xirouchaki et al.14, also with high gain, Pplateau was <30cmH2O in 98% of the cases and <26cmH2O in 94%. These results are consistent with the observations of Younes et al.25, who found PAV to activate the Hering–Breuer reflex, inhibiting the inspiratory muscles when the lungs reach total capacity–thereby reducing the risk of hyperinsufflation.
Elevated PaCO2 was interpreted by the clinicians as constituting hypercapnia in one-third of the patients in which PAV+ failed, and was clinically attributed to excess sedation. In this case, we likewise observed no significant differences in the values on comparing PAV+ success vs failure (Table 2). The absence of differences could also be explained by the fact that the data analyzed were those contemplated in the protocol as information collected on a daily basis, and that PaCO2 was not documented at the time when the technique was considered to have failed (i.e., measurement corresponded to any routine blood gas control made in the course of the day). Elevated PaCO2 is one of the critical issues of PAV+, since the levels are particularly sensitive to excess sedation–inhibiting central control of the ventilation pattern and lessening feedback capacity. Xirouchaki et al.14 also described this circumstance as a result of sedation. They considered sedation control to be one of the key elements in the PAV learning curve, along with the adoption of protocols designed to minimize the risk of inappropriate sedation. Although a sedation target level of 3–4 on the Ramsay scale was established in our study, theoretically allowing patients to autoregulate their internal homeostasis, we noted inter-individual variation characterized by a narrow margin between deep sedation (Ramsay score 5–6), superficial sedation and agitation (Ramsay score 1)–this making it difficult in some cases to reach the mentioned sedation target. We thus agree with the abovementioned opinion of Xirouchaki et al.
No significant gas exchange differences (PaO2/FiO2, PaCO2 and pH) were observed in either of the two groups, in agreement with the observations of other authors,10,13,14 thus reflecting the capacity of PAV to maintain blood homeostasis. However, in our study we recorded a case of metabolic acidosis and two cases of hypercapnia that were interpreted as PAV+ failure due to excess sedation, but which were not recorded among the analyzed data.
In sum, the clinical causes of PAV+ suspension were not statistically defined. The diagnoses of treatment failure represented individual clinical decisions in concrete moments and which were considered to be sufficiently relevant to require a switch in ventilation mode. A larger patient sample possibly could reflect differences with the PAV+ success group.
We largely reached our goal of achieving maximum ventilatory support in the acute phase with high assist PAV+. Although the initial objective was a gain of 90%, a value of 80% was finally reached for both groups–this likewise representing ventilator assistance of most of the work of breathing (WOB). Delaere et al.26 showed a PAV gain of 80% to represent high ventilation assist in terms of respiratory muscle work compared with a gain of 50%. Xirouchaki et al.14 compared the effectiveness of PAV+ vs PSV in the acute phase of the critical patient, though only during the first 48h. PAV+ was successfully applied in 89% of the cases vs PSV in terms of asynchrony. The authors concluded that PAV+ increases the probability of remaining under spontaneous respiration thanks to better patient–ventilator interaction. The causes of PAV+ failure were associated to dyssynchrony (particularly ineffective effort) and clinical derangements (hemodynamic instability, respiratory distress and hypercapnia). However, the investigators conducted no in-depth exploration of the factors underlying failure of the technique. Our low PAV+ success rate (60%) compared with the aforementioned study may be due to the fact that patient inclusion was made earlier, with a longer application time (>48h).
Study limitationsGiven the lack of clinical studies on the efficacy of this ventilation mode in the early phase of critical illness, patient screening was very cautious, avoiding those subjects with very serious clinical conditions requiring additional aggressive therapies, and patients in which prompt extubation was expected. This explains the small number of included patients compared with the total number of initially analyzed individuals, and the fact that the patients were subjected to volume assist-control (VAC) ventilation in the first hours of admission (always within the first 24h), until sufficient spontaneous breathing was achieved to allow the adjustment of internal homeostasis, after the optimization of sedation. This study was carried out during routine clinical activity, and the data were obtained in each patient only once a day. Therefore, since the PAV+ respiratory pattern can vary constantly depending on the clinical condition of the patient (fever, pain, anxiety, etc.), our results may have been influenced simply by the timing of data collection.
In the present study, although sedation was not protocolized, the three participating hospital centers used the Ramsay sedation scale. Nevertheless, the use of different types of sedatives could affect the degree and duration of sedation, and thus the preservation of central control. The lack of a unified weaning protocol could have influenced physician decision to reduce the high ventilatory gain level and proceed with the weaning process.
To summarize, it must be taken into account that this is a pilot study with a very small and heterogeneous sample, offering few chances of identifying all the possible factors associated to ventilation failure, and thus limiting broader interpretation of the results. Future studies are needed to establish comparisons with ventilation modalities routinely used in the acute phase of critical illness.
Lastly, we conclude that high assist PAV+ is applicable as a ventilatory alternative in the acute phase of critical illness in a substantial number of patients, particularly those without important worsening of WOBTOT. In this context, WOB can be used as a predictor of success or failure of the technique.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Hospital Sant Joan de Déu, Fundación Althaia; Hospital Clínic de Barcelona; Hospital General de Cataluña.
Ethics Committee and project approval number: Comité Ètic d’Investigació Clínica Fundació Unió Catalana d’Hospitals. Approval number: none. Approval date: 4 September 2008.
Please cite this article as: Delgado M, Zavala E, Tomás R, Fernandez R. Factores clínicos asociados al éxito de la ventilación asistida proporcional en la fase aguda del paciente crítico: estudio piloto. Med Intensiva. 2014;38:65–72.