This study aims to assess the prescription profile and license status of drugs used in a neonatal and pediatric intensive care unit (NPICU).
MethodsA prospective observational study was conducted on a dynamic cohort of children admitted to an NPICU (N=81) in a tertiary hospital (Granada, Spain). All prescriptions were classified as off-label or unlicensed based on the Summary of Product Characteristics (SPC).
ResultsOf a total of 601 prescriptions, the patients received a mean of 7.4±6 drugs each. The most commonly prescribed drugs corresponded to classes J (anti-infectious, systemic use) N (nervous system) and C (cardiovascular). A little over one-half of the prescriptions were off-label (52%), usually due to dosages differing from the SPC recommendations (79%), followed by different indications (13.5%), age (5%) and administration route (2.5%). In this NPICU, unlicensed usage represented only 5% of all prescriptions.
ConclusionsThis study contributes data on prescription of this kind in a Spanish NPICU, revealing at least one off-label prescription in 89% of the children and at least one unlicensed use in 22.3%. These are high figures, but are to be expected given the inclusion of newborn infants and the critical care setting. Even though such usage follows clinical protocols, we underscore the dual need to base treatment on the best available evidence, and to upgrade the SPC accordingly.
Evaluar los usos off-label (fuera de ficha técnica [FT]) y unlicensed (medicamentos no autorizados específicamente para niños) en cuidados intensivos neonatales y pediátricos.
MetodologíaSe realizó un estudio transversal en la UCINP (Unidad de Cuidados Intensivos Neonatales y Pediátricos) de un hospital público de tercer nivel de Granada, incluyéndose a todos los niños en los que se indicara al menos un tratamiento farmacológico, mediante reclutamiento consecutivo, y durante un periodo de 5 meses (N=81). Las variables recogidas fueron sociodemográficas, clínicas, y medicación. Todas las prescripciones fueron clasificadas a partir de la información contenida en FT sobre uso en niños.
ResultadosHubo un total de 601 prescripciones, con una media de 7,4±6 medicamentos por niño. Los fármacos más empleados pertenecían a los grupos J (antiinfecciosos), N (sistema nervioso) y C (cardiovascular). Algo más de la mitad de las prescripciones fueron off-label (52%), fundamentalmente por emplear una dosificación distinta de la recomendada en FT (79%), seguida de diferente indicación (13,5%), edad (5%) y vía de administración (2,5%). El uso de medicamentos no específicamente autorizados en niños solo supuso el 5% de las prescripciones.
ConclusionesEl presente estudio aporta datos sobre este tipo de prescripciones en una UCINP española. Pone de manifiesto que el 89% de los niños tiene al menos una prescripción fuera de FT y un 22,3% al menos un uso de fármaco no autorizado para niños. Cifras elevadas, pero justificables dentro del ámbito de unos cuidados intensivos que, además, incluyen neonatos. Pero aunque muchos de los tratamientos estén protocolizados, sería deseable mejorar la evidencia disponible, así como actualizar las FT.
It has been estimated that less than 50% of the drugs used in children have been investigated in the pediatric population. Because of this, the treatment of such patients traditionally has been based on extrapolations referred to drugs that have been developed for adults. However, we know that the conduction of adequate clinical research is the best guarantee for safe, effective and quality treatment. The scarcity of clinical trials in children is due on one hand to the limited interest of the drug industry in this concrete patient population, and on the other to the difficulties inherent to pediatric research, which involves a number of fundamentally ethical and methodological barriers.1 Accordingly, most therapeutic agents have not been studied in children, and the benefit–risk balance of their use is therefore often supported by only limited evidence. All this in many cases has caused quality information on pediatric recommendations to be scarce or inexistent.
The Summary of Product Characteristics (SPC) approved by the Spanish Medicines Agency (Agencia Española del Medicamento y Productos Sanitarios [AEMPS]) is the official document reflecting the authorized conditions of use of the medication. Based on the findings of the clinical trials made, the SPC summarizes the essential scientific information addressed to health professionals. When the information on the use of a drug in children is limited or inexistent, its prescription is made outside the corresponding licensed or authorized terms–a situation internationally known as off-label (OL) and unlicensed (UL) drug use. Unlicensed prescription is generally understood as the prescription of drugs that have not been approved for the pediatric population, while off-label prescription involves the use of drugs that have been approved for children but which are used under conditions different from those authorized in the corresponding SPC, i.e., involving different indications, doses, age ranges or administration routes. The proportion of drugs prescribed outside the authorized terms has been studied in recent years, though the results have been highly varied.2 Most studies have been conducted on an in-hospital basis, and those carried out in neonatal Intensive Care Units (ICUs) are particularly relevant in this respect. Among newborn infants, the number of patients that receive at least one OL or UL prescription is much higher than in any other medical or surgical pediatric ward–with percentages reaching 70–97% of the patients.3
The use of these OL or UL prescriptions is not necessarily incorrect: it simply means that the evidence and evaluations required by the health authorities (AEMPS and the European Medicines Agency [EMA]) are not available or are insufficient, and therefore cannot be reflected in the SPC. Nevertheless, it has been demonstrated that such drug use can be associated to certain safety problems.4 In fact, a number of studies have already shown that these prescriptions are a risk factor for the development of adverse drug reactions (ADRs), medication error and possible treatment failure.5 It has been estimated that OL or UL prescriptions are implicated in between 23% and 60% of all ADRs in children.6
An added problem is the lack of specific pediatric formulations, which favors the use of extemporaneous or magistral formulations aimed at adapting certain treatments to the oral route. These preparations are also related to possible medication error and involve uncertainty regarding the stability of the products used.7
Considering that intensive care is characterized by great and very diverse exposure to drugs, we were interested in knowing the frequency of prescriptions of this kind in this concrete area of practice. We were also particularly interested in exploring commonly used drugs such as dopamine, midazolam, fentanyl or furosemide–many of which have not been specifically investigated in the pediatric population, despite extensive experience with their use. On the other hand, most of the studies published to date have been carried out in the United Kingdom and in the Netherlands,6 with very few data referred to the Spanish population. Indeed, the existing studies are limited to two areas unrelated to intensive care, namely gastroenterology8 and out-hospital prescription practice.9 According to current European Union regulations referred to pediatric medications,10 one of the necessary important tasks is to compile information on drug use in children in all the member states, with a view to evaluating the current situation and detecting those therapeutic areas where the need for additional pediatric studies is more pressing.
The aim of the present study therefore was to determine the prescription profile and OL (outside SPC specifications) and UL (medicines not specifically authorized in children) uses in pediatric (PICUs) and neonatal Intensive Care Units (NICUs).
Patients and methodsA cross-sectional observational study was conducted of all the prescriptions made in a cohort of children admitted to the ICU of a third-level public hospital in Granada (Andalusia, Spain). This ICU distributed the patients into neonatal (newborn infants up until one month of age) and pediatric patients (from the first month to 14 years of age).
Based on consecutive recruiting over a 5-month period (July–November), we prospectively recorded the data on those patients in which at least one drug treatment was indicated. The information source was the case history and medical instructions. Information was collected referred to patient age, weight and reason for admission, as well as to drug treatment: indication, dose, frequency, dosing form and administration route. The drugs studied were grouped according to the Anatomical Therapeutic Chemical (ATC) classification index.
All prescriptions were classified as OL or UL according to the information referred to pediatric use contained in the corresponding SPC. The criteria used were those published by Turner,11 who classified OL as prescriptions involving doses, age ranges, administration routes or indications different from those reflected in the official drug authorization documentation. In turn, the definition of UL included drug substances still not authorized, drugs specifically contraindicated in children, the modification of formulations, and drugs for which no information at all is available referred to pediatric use.
A descriptive analysis was made using the SPSS version 19.0 statistical package (Chicago, IL, USA). The comparison of means of quantitative variables between two groups of patients was based on the Student's t-test, while the chi-squared test was used for the comparison of proportions in the case of qualitative variables. Statistical significance was considered for p<0.05 in all cases. All personal information was anonymized, and the study was approved by the local Research Ethics Committee.
ResultsThe study included a total of 81 patients, of which 59.3% (N=48) corresponded to the neonatal ICU (NICU) and 40.7% (N=33) to the pediatric ICU (PICU). The male gender was seen to predominate (59%), with no significant differences between the two Units. In the PICU the mean age was 4.4±4.7 years (range: 2 months–14 years), and among the newborn infants the mean gestational age was 34.5±4.2 weeks (range: 25–41 weeks), with a mean weight of 2335±949g (range: 780–4750g). One-third of the newborn infants weighed less than 2500g, though only 15% were very low weight.
The most frequent reason for admission in the combined Units was prematurity-low weight (22.2%), followed by infections (21%), respiratory disease (17.2%) and neurological disorders (15%).
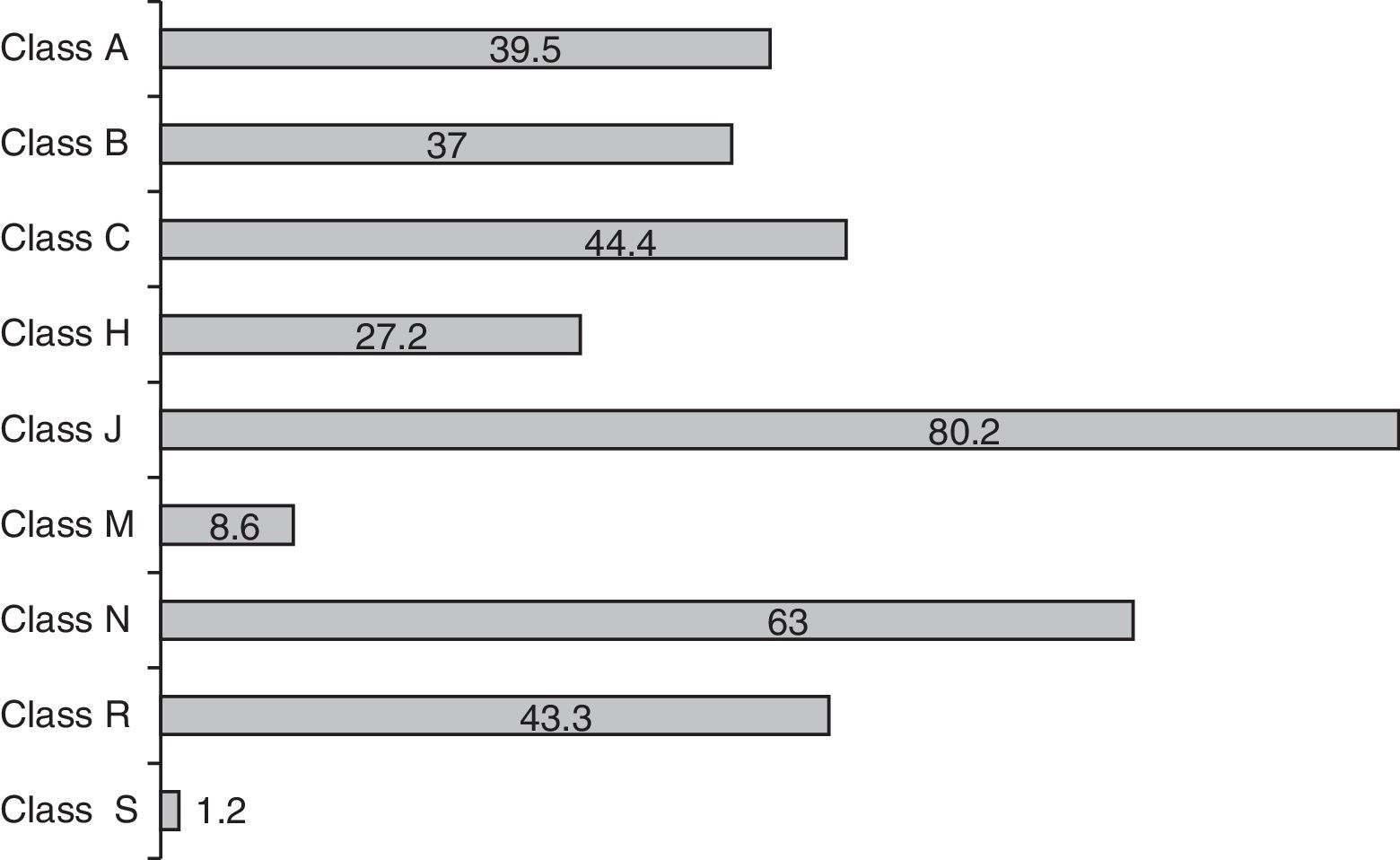
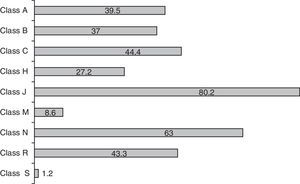
The mean number of drugs prescribed per patient was 7.4±6 (range: 1–32; median 6). The neonates received more drugs than the pediatric patients, though the difference failed to reach statistical significance (8.3 vs 6.1 drugs; p=0.09). Overall, 601 prescriptions were made, involving 91 different drug substances. Fig. 1 shows the prescription profile according to therapeutic groups based on the ATC classification. Eighty percent of the children received at least one antibiotic–class J drugs (systemic antiinfectious agents) being the most frequently used, followed by classes N (nervous system) and C (cardiovascular). It was precisely in these three classes where differences were detected between the neonatal and pediatric patients–with significantly greater use among the neonates of antibiotics (92 vs 64%; p<0.001), nervous system drugs (58 vs 49%; p<0.05) and class C agents (62.5 vs 18.2%; p<0.001). The rest of the drug classes showed no differences in distribution. The 10 most frequently administered drug substances in the ICU were ampicillin (53% of the patients), gentamycin (52%), midazolam (44%), furosemide (34.5%), dopamine (33%), cefotaxime (31%), metamizol (29%), fentanyl (28%), vancomycin (25%) and methylprednisolone (22%).
Distribution of medication use by ATC drug classes (values expressed as % of children receiving some drug within the class). Anatomical, therapeutic, chemical (ATC) classification system. A: digestive system and metabolism; B: blood and hematopoietic organs; C: cardiovascular system; H: systemic hormonal formulations; J: systemic antiinfectious agents; M: musculoskeletal system; N: nervous system; R: respiratory system; S: sensory organs.
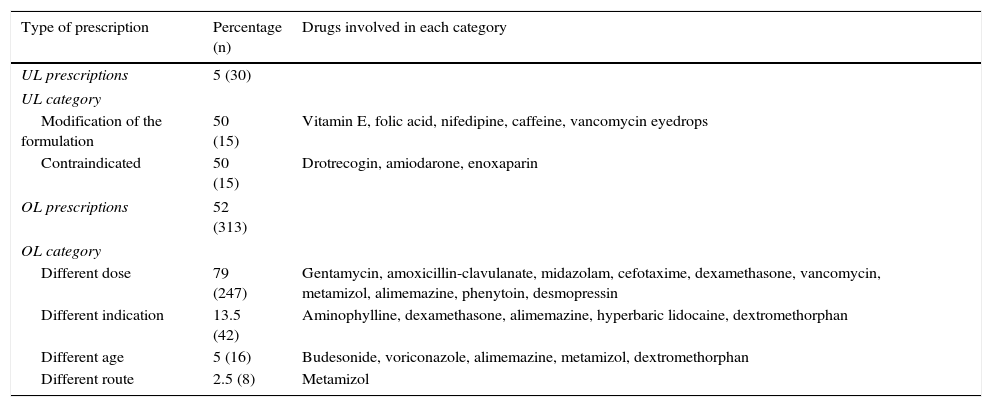
On analyzing the 601 prescriptions made, 52% were found to correspond to OL, while 5% were UL (Table 1). The number of OL and UL prescriptions per patient ranged from 0 to 8 (mean 2.39±1.7) and from 0 to 4 (mean 0.32±0.6), respectively. There was a slight tendency toward more OL prescriptions per patient among the neonates versus the pediatric patients (2.5 vs 1.8; p=0.06). A total of 22.3% of the children in the sample received at least one UL prescription, while 89% received at least one OL prescription. The few differences between neonatal and pediatric patients failed to reach statistical significance.
Prescription classification according to the conditions of authorization for children in the Summary of Product Characteristics (N=601 prescriptions).
| Type of prescription | Percentage (n) | Drugs involved in each category |
|---|---|---|
| UL prescriptions | 5 (30) | |
| UL category | ||
| Modification of the formulation | 50 (15) | Vitamin E, folic acid, nifedipine, caffeine, vancomycin eyedrops |
| Contraindicated | 50 (15) | Drotrecogin, amiodarone, enoxaparin |
| OL prescriptions | 52 (313) | |
| OL category | ||
| Different dose | 79 (247) | Gentamycin, amoxicillin-clavulanate, midazolam, cefotaxime, dexamethasone, vancomycin, metamizol, alimemazine, phenytoin, desmopressin |
| Different indication | 13.5 (42) | Aminophylline, dexamethasone, alimemazine, hyperbaric lidocaine, dextromethorphan |
| Different age | 5 (16) | Budesonide, voriconazole, alimemazine, metamizol, dextromethorphan |
| Different route | 2.5 (8) | Metamizol |
OL: off-label; UL: unlicensed.
The causes of UL prescriptions were equally distributed between modification of the formulations and contraindications in children. As examples of the former we have extemporaneous (magistral) formulations of nifedipine, folic acid, vitamin E, caffeine and vancomycin eyedrops. Contraindications and warnings due to non-established efficacy and safety in children in turn affected the use of drotrecogin, amiodarone and enoxaparin. On the other hand, in relation to the OL prescriptions, the main cause was the administration of doses different from those recommended in the SPC (79% of the OL cases), followed by different indications (13.5%), different patient age ranges (5%) and different administration routes (2.5%) (Table 1). The indications not contemplated by the SPC were bronchopulmonary dysplasia in the case of dexamethasone, arginosuccinic aciduria in the case of dextromethorphan, irritability episodes in the case of alimemazine, hyperbaric lidocaine use as an antiarrhythmic agent, and aminophylline as respiratory center stimulant in apnea. Off-label use due to administration in patients with ages different from those contemplated in the SPC were mainly referred to the use of drugs in newborn infants and in general in patients under two years of age, for which the safety and efficacy profiles have not been established to date (e.g., budesonide and voriconazole in patients under two years of age). Some drugs were found to be prescribed under OL conditions due to more than one cause simultaneously, e.g., metamizol (dose, age and route different from the authorized specifications), alimemazine (dose, age and indication different from the authorized specifications), and dextromethorphan (indication and age different from the authorized specifications).
DiscussionThe single-center nature of our study could limit its external validity. However, even accepting the variability between prescribers and centers, we consider that the results obtained may adequately reflect this often protocolized type of drug prescription practice in the intensive care setting. Furthermore, and from the perspective of a Spanish ICU, the data obtained may contribute to the assessment of current prescription practices requested by the European Medicines Agency.10
The publications on prescriptions of this kind offer very heterogeneous results, due to differences in the methodology, classification criteria and information sources used–a fact that complicates comparisons among the different studies. In the present study we chose the most widely employed classification of OL and UL prescription,12–17 originally described by Turner.11 In the same way as in most of the more recent studies, our main information source was the Summary of Product Characteristics (SPC) of the EMA. The SPC is regarded as the official document, since its data have been subjected to regulatory review, and it serves as a consulting tool for clinicians, offering guidance on the authorized conditions of prescription and administration of drugs. However, the need to update the SPCs should be considered, since in many cases they are outdated with respect to the generation of new uses based on quality studies. In effect, research and knowledge in certain areas modify clinical practice, though such changes are not reflected in the SPCs–particularly in those referred to old drugs or substances of little commercial interest for the drug industry.
Drug exposure among the children of our ICU was highly variable and somewhat greater (mean of 7.4 and median of 6 drugs per patient) than in other studies (median of 3 drugs in the study corresponding to Turkey, 4 in Estonia, and 4.2 in Finland).3,18,19 Nevertheless, taking into account that our data are referred to the critical care setting, we feel that the figures are reasonable and similar to other published data in this same clinical setting.20 The prescription profile is coherent with the reasons for admission and with the predominance of neonates in the ICU. In this regard, antibiotics are typically the most commonly used drugs in such Units.3,12,18,21 On the other hand, the fact that 63% of the children received some drug belonging to class N is explained by the needs for sedation and analgesia–with midazolam and fentanyl ranking among the 10 most frequently prescribed drugs. In our sample there was a significantly greater proportion of antibiotic, nervous system and cardiovascular drug prescription in neonates than in pediatric patients–this being consistent with the most frequent clinical problems found in newborn infants. We also detected slight differences in profile: while the most commonly used antibiotics in neonates were ampicillin and gentamycin, the prescription of amoxicillin-clavulanate and cefotaxime predominated in the pediatric patients. This is logical, considering the microbiological spectrum to be covered in each case. Likewise, in relation to class N drugs, sedation was more frequent among newborn infants, while in the older children the use of this drug class more often involved analgesia and antifever prescription (e.g., paracetamol and metamizol).
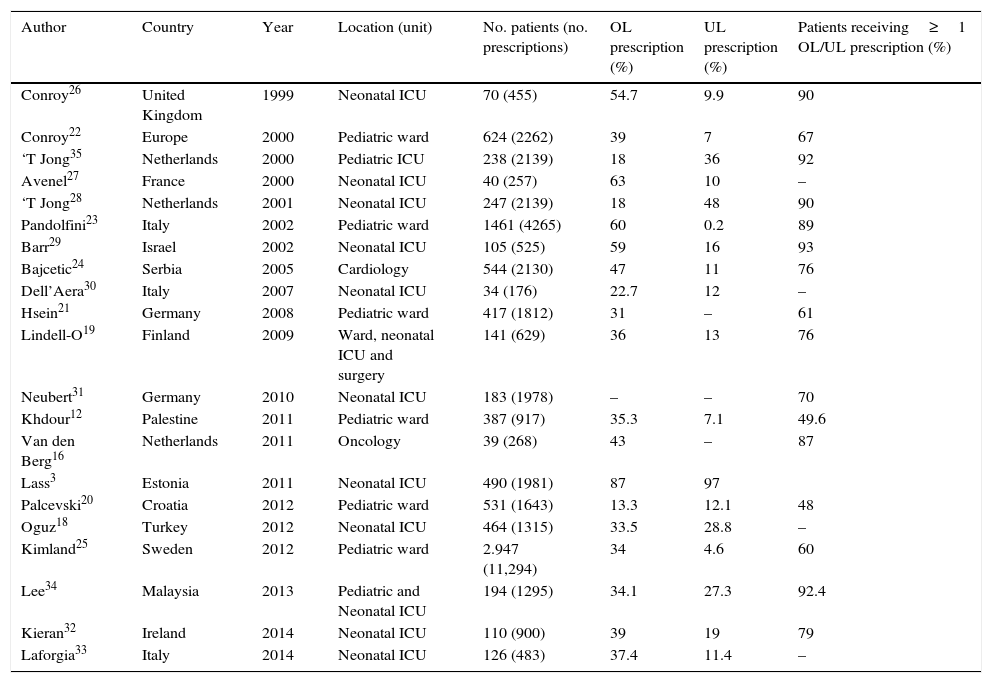
Table 2 reviews the main in-hospital studies found in the literature. In our case, a little over one-half of the prescriptions were OL–this figure being somewhat higher than those recorded in hospital pediatric wards8,12,16,19–25 (between 35% and 45%), closer to those recorded in NICUs,3,18,26–33 and perhaps a little higher than in other PICUs.34,35 This is logical, considering the important proportion of newborn infants in the present study. In this respect, it is not surprising that 89% of the admitted children received some prescription of this type. As is clearly seen in Table 2, the OL prescription rates differ greatly between countries and studies. Thus, in the case of NICUs, the proportions range from 22.7% in Italy30 to 87% in Estonia.3 These very distinct results again reflect differences not only in the methodology and classifications used, but also in clinical practice as such. According to Palcevski, another possible source of variability is the type of hospital involved. In this regard, higher OL prescription rates would be expected in centers offering more sophisticated care, or in which the patients present more complicated disease processes.20
Summary of the studies on OL/UL prescriptions in different hospital units.
| Author | Country | Year | Location (unit) | No. patients (no. prescriptions) | OL prescription (%) | UL prescription (%) | Patients receiving≥1 OL/UL prescription (%) |
|---|---|---|---|---|---|---|---|
| Conroy26 | United Kingdom | 1999 | Neonatal ICU | 70 (455) | 54.7 | 9.9 | 90 |
| Conroy22 | Europe | 2000 | Pediatric ward | 624 (2262) | 39 | 7 | 67 |
| ‘T Jong35 | Netherlands | 2000 | Pediatric ICU | 238 (2139) | 18 | 36 | 92 |
| Avenel27 | France | 2000 | Neonatal ICU | 40 (257) | 63 | 10 | – |
| ‘T Jong28 | Netherlands | 2001 | Neonatal ICU | 247 (2139) | 18 | 48 | 90 |
| Pandolfini23 | Italy | 2002 | Pediatric ward | 1461 (4265) | 60 | 0.2 | 89 |
| Barr29 | Israel | 2002 | Neonatal ICU | 105 (525) | 59 | 16 | 93 |
| Bajcetic24 | Serbia | 2005 | Cardiology | 544 (2130) | 47 | 11 | 76 |
| Dell’Aera30 | Italy | 2007 | Neonatal ICU | 34 (176) | 22.7 | 12 | – |
| Hsein21 | Germany | 2008 | Pediatric ward | 417 (1812) | 31 | – | 61 |
| Lindell-O19 | Finland | 2009 | Ward, neonatal ICU and surgery | 141 (629) | 36 | 13 | 76 |
| Neubert31 | Germany | 2010 | Neonatal ICU | 183 (1978) | – | – | 70 |
| Khdour12 | Palestine | 2011 | Pediatric ward | 387 (917) | 35.3 | 7.1 | 49.6 |
| Van den Berg16 | Netherlands | 2011 | Oncology | 39 (268) | 43 | – | 87 |
| Lass3 | Estonia | 2011 | Neonatal ICU | 490 (1981) | 87 | 97 | |
| Palcevski20 | Croatia | 2012 | Pediatric ward | 531 (1643) | 13.3 | 12.1 | 48 |
| Oguz18 | Turkey | 2012 | Neonatal ICU | 464 (1315) | 33.5 | 28.8 | – |
| Kimland25 | Sweden | 2012 | Pediatric ward | 2.947 (11,294) | 34 | 4.6 | 60 |
| Lee34 | Malaysia | 2013 | Pediatric and Neonatal ICU | 194 (1295) | 34.1 | 27.3 | 92.4 |
| Kieran32 | Ireland | 2014 | Neonatal ICU | 110 (900) | 39 | 19 | 79 |
| Laforgia33 | Italy | 2014 | Neonatal ICU | 126 (483) | 37.4 | 11.4 | – |
–: not indicated; OL: off-label; ICU: Intensive Care Unit; UL: unlicensed.
The main type of OL prescription in our series was referred to the use of drug doses or administration frequencies different from those reflected in the SPC. This coincides with the observations of many other studies.12,14,15,20,21,23 Of note is the fact that the main drugs involving OL use due to doses different from those specified in the SPC are also among the most widely prescribed drug substances–particularly gentamycin, cefotaxime, vancomycin and midazolam. Regarding the non-authorized indications, it may be more interesting at this point to reflect upon the level of evidence supporting such use. Potentially controversial examples are the off-label use of alimemazine (authorized as an H1 antihistamine for the treatment of allergy) as a sedative, or the administration of aminophylline as an anti-apnea drug in premature infants. These clinical situations raise relevant research questions and should stimulate the conduction of independent studies to clarify the existing uncertainties.
In our opinion, a notable observation in the present study was the scarce use of UL prescriptions, with an incidence of only 5% versus the usually reported range of 10–15% of all prescriptions in both the NICU setting26,27,29,30 and in pediatric wards.16,19,20 We consider that the extemporaneous formulation of drugs by the hospital pharmacy, or the modification of authorized products, may also be influenced by the pharmaceutical practices found in each individual country. In fact, in the Netherlands, for example, magistral formulation is a traditional practice–representing up to 48% of all prescriptions.28 Such formulations are also often prepared in Spanish hospital pharmacies, though there were few of them in our series. This might be explained by the critical condition of the patients admitted to our Unit, causing parenteral dosing to be the administration route of choice. After being moved to intermediate care or general wards, where the children are in a more stable condition, the need to modify or adapt oral solutions may be comparatively greater.
It should be remembered that prescription outside the specifications of the SPC need not be inappropriate, and that such practice often represents the most rational and even the best option based on the current evidence and clinical practice guides.2 This is not always the case, however, and UL and OL prescription may be a risk factor for the appearance of adverse drug reactions (ADRs). It therefore must be guaranteed that children receive the safest and most effective drugs for each given disease condition. For this reason, European Directive 1901/2006 establishes as objective the increased development of pediatric medications, ensuring quality research and improving the available information on the use of drugs in children.10 In this regard, the EMA has launched initiatives such as the creation of a Pediatric Committee; Pediatric Investigation Plans (PIPs); the publication of a list of medications for which development for pediatric use is recommended; or the European Network of Pediatric Research. Independently of the implementation of these measures, we consider it reasonable to promote studies on drug utilization in children, divulgating the implications of OL and UL use and–in those cases where such prescription outside the specifications of the SPC or lacking authorization are not supported by clinical practice guides or quality studies–to propose prospective studies to collect information on efficacy and safety with a view to affording greater evidence and better elements of judgment in selecting drug products. We feel that studies of this kind are particularly interesting in critical care setting, due to the pharmacological profile involved in this setting.
Lastly, a comment should be made on the legal aspects of drug use not contemplated in the SPC, for as has already been mentioned, the latter is an official document with a legally binding character. Drug use outside the specifications of the SPC has been contemplated since the year 2009 in the Spanish Royal Decree (RD) on the Availability of Medications in Special Situations,36 which administratively facilitates such use when supported by the clinical practice guides or scientific evidence, and offers clarifications on the responsibilities in this respect. The mentioned Decree establishes that physicians can use medicines in a way different from the specifications contained in the SPC provided such use is justified, prescription is reflected in the patient case history, and at least verbal consent is obtained from the parents. This procedure has not yet been implemented in clinical practice, however, probably due to a lack of knowledge or because more time is needed. In this regard, the results of a recent national survey found 71.5% of the participating pediatricians to be aware of the meaning of the term “OL”; 61% claimed to prescribe drugs with indications not contemplated by the SPC; and 47% were aware that the indications should be reflected in the mentioned SPC. In contrast, a little under one-half of those interviewed claimed to inform the parents, and only 22% recorded such prescription practice in the patient case history.37
Study sitePediatric ICU of San Cecilio University Hospital (Granada, Spain).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Blanco-Reina E, Medina-Claros AF, Vega-Jiménez MA, Ocaña-Riola R, Márquez-Romero EI, Ruiz-Extremera Á. Utilización de fármacos en niños en cuidados intensivos: estudio de las prescripciones off-label. Med Intensiva. 2016;40:1–8.