To determine the clinical characteristics and prognostic factors of patients with severe sepsis/septic shock admitted to the Intensive Care Unit of Donostia University Hospital (Guipuzcoa, Spain).
DesignA prospective, observational study was carried out during a consecutive 6-year period (1st February 2008–31st December 2013).
SettingThe Intensive Care Unit of Donostia University Hospital, the only third level hospital in the province of Guipuzcoa, with a recruitment population of 700,000 inhabitants.
ResultsNumber of patients with severe sepsis/septic shock has progressively increased over the last years to reach 1136 patients, yet significant changes in age, sex, Acute Physiology and Chronic Health Evaluation II, procalcitonin and lactate values could not be observed. In the last years, admission rate from Emergency Department has increased in comparison to admissions from hospitalization ward, with a higher incidence of urological sepsis. Hemodynamic and renal dysfunctions have been the most prevalent disorders, respiratory involvement and thrombocytopenia have gone down while coagulopathy has increased significantly. Mortality has decreased significantly. We have performed a multivariate analysis of the early prognostic factors. Type, origin, sepsis etiology, lactate and the presence of organ dysfunction–except for hyperbilirubinemia and hypotension–were the most important mortality factors.
ConclusionsSevere sepsis and septic shock result in growing ICU admissions. Although clinical features have barely changed over the last years, we have observed a decrease in mortality. We find important knowing these early prognostic factors to improve the management of these patients.
Examinar las características clínicas y los factores pronósticos de los pacientes ingresados por sepsis grave/shock séptico en la Unidad de Cuidados Intensivos del Hospital Universitario Donostia.
DiseñoEstudio observacional prospectivo durante un período consecutivo de 6 años (1-2-2008 a 31-12-2013).
ÁmbitoServicio de Medicina Intensiva del Hospital Universitario Donostia, único hospital terciario de Guipúzcoa.
ResultadosEl número de pacientes con sepsis grave/shock séptico ha aumentado progresivamente hasta un total de 1.136, sin observarse cambios significativos en la edad, el sexo, la puntuación del Acute Physiology and Chronic Health Evaluation II, los valores de procalcitonina ni en los de lactato sérico. En los últimos años ha habido un aumento significativo de los ingresos desde Urgencias respecto a los procedentes de planta, con una mayor incidencia de la sepsis urológica. La afectación hemodinámica y renal han sido las disfunciones más prevalentes, descendiendo la afectación respiratoria y la trombocitopenia y aumentando la coagulopatía. La mortalidad ha descendido significativamente. Mediante un análisis multivariante analizamos factores pronósticos precoces: el tipo de paciente, su procedencia, la etiología de la sepsis, la cifra de lactato y la presencia de disfunciones orgánicas, exceptuando la hiperbilirrubinemia y la hipotensión, fueron las variables más influyentes en la mortalidad.
ConclusionesLa sepsis grave/shock séptico genera un creciente número de ingresos. A pesar de que las características clínicas han variado poco en los últimos años, hemos observado un descenso de la mortalidad. Consideramos importante el conocimiento de los factores pronósticos precoces para mejorar el abordaje de estos pacientes.
Sepsis results in important morbidity and mortality, particularly when associated to organ dysfunction and/or shock. The Surviving Sepsis Campaign was launched in the year 2002 with the aim of reducing the mortality of sepsis through the development and introduction of clinical practice guides, published for the first time in 20041 and last revised in 2012.2 Different studies have shown that adherence to these guides,3,4 and particularly early antibiotic administration, improves patient survival.5 In Spain, the Edusepsis study6,7 made it possible to know the clinical characteristics and epidemiological data of these patients in our country, and demonstrated that the application of an educational program is able to increase compliance with the treatment “bundles” and lessen mortality in severe sepsis/septic shock (SeS/SSh). The ABISS-Edusepsis study was subsequently carried out (with results pending publication) with the purpose of evaluating a multiple intervention for improving early empirical antibiotic treatment in sepsis, with the aim of reducing patient mortality.
In our case, participation in both of the mentioned studies allowed us to develop a registry and conduct educational campaigns in our center and in the different district hospitals that refer patients to our hospital. Through data collection over these 6 years, we aimed to determine the existence of possible variations in the characteristics and evolution of these patients, and to identify early prognostic factors capable of helping us to improve their clinical management.
Material and methodsA prospective observational study was made over a 6-year period (from 1 January 2008 to 31 December 2013) in the Intensive Care Unit (ICU) of Donostia University Hospital (San Sebastián, Spain). Our Unit belongs to a third-level hospital and has 48 beds, with a total recruitment population of 700,000 inhabitants.
We included all patients who upon admission to or during their stay in the ICU presented SeS/SSh according to the definitions of the International Sepsis Conference of 2001.8 In all cases follow-up was carried out until hospital discharge.
Study variables:
- -
Demographic data: patient age, gender and origin.
- -
Patient type and origin of sepsis.
- -
Data on the severity of the condition: Acute Physiology and Chronic Health Evaluation II (APACHE II score) (in all patients, except those who died in the first 24h), organ dysfunction secondary to sepsis, presence or absence of hypoglycemia, procalcitonin peak (100ng/ml being the maximum value offered by the laboratory), and lactate in the first 6h of sepsis onset or of admission to the ICU.
- -
Patient management data: lactate measurement request, extraction for blood cultures, and administration of antibiotics (previous and upon admission to the ICU).
- -
In-ICU and global (ICU+hospital) mortality.
With regard to determination of the prognostic factors, we analyzed all the variables corresponding to the first 12h of sepsis onset, excluding the patients admitted before June 2008, since the procalcitonin values were not available for these individuals.
Statistical analysisQuantitative variables were expressed as the mean and standard deviation, while qualitative variables were reported as absolute numbers and percentages. The comparison of means was made using the Student t-test or analysis of variance (ANOVA), while the comparison of proportions was carried out using the chi-squared test. With regard to evolution over the 6-year period, when the comparison of means using ANOVA yielded statistically significant differences, we applied an increasing or decreasing linear trend test with a one-way procedure through polynomial sub-instruction=2. When the comparison of proportions with the chi-squared test proved significant, we checked whether the proportions showed an increasing or decreasing trend by means of the Mantel–Haenszel linear trend test. Statistical significance was considered for p<0.05. The SPSS® version 20 statistical package was used throughout.
The multivariate analysis was carried out with construction of the logistic regression model adopting the stepwise-backwards strategy, and including those variables which in the univariate analysis yielded p<0.30, as well as the relevant control variables with good theoretical justification. Calibration of the model was made using the Hosmer–Lemeshow goodness of fit test. The validity of the logistic regression model was estimated by calculating the area under the curve with a 95% confidence interval (95%CI).
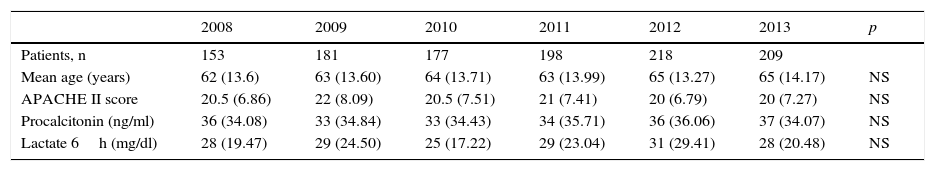
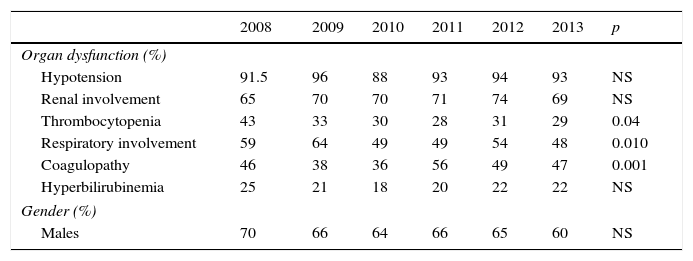
ResultsIn the last 6 years we have observed a gradual increase in the number of patients with sepsis admitted to our Unit (Table 1). In total, 1136 patients presented SeS/SSh upon admission or during the course of stay in the ICU. Some of the clinical characteristics were seen to remain stable, with no significant differences in mean age (between 62 and 65 years), gender (with a clear male predominance), the Acute Physiology and Chronic Health Evaluation II scores (between 20 and 22), procalcitonin concentration (between 33 and 37ng/ml) or arterial lactate levels in the first 6h (between 25 and 31mg/dl). Regarding organ dysfunction (Table 2), hemodynamic and renal alterations were the most prevalent problems during this period. A significant decreasing trend was observed in relation to respiratory problems and thrombocytopenia, with an increasing trend in the case of coagulopathy. The percentage of patients with hyperbilirubinemia remained without significant variations.
Clinical characteristics of the patients admitted to the ICU due to severe sepsis/septic shock.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | p | |
|---|---|---|---|---|---|---|---|
| Patients, n | 153 | 181 | 177 | 198 | 218 | 209 | |
| Mean age (years) | 62 (13.6) | 63 (13.60) | 64 (13.71) | 63 (13.99) | 65 (13.27) | 65 (14.17) | NS |
| APACHE II score | 20.5 (6.86) | 22 (8.09) | 20.5 (7.51) | 21 (7.41) | 20 (6.79) | 20 (7.27) | NS |
| Procalcitonin (ng/ml) | 36 (34.08) | 33 (34.84) | 33 (34.43) | 34 (35.71) | 36 (36.06) | 37 (34.07) | NS |
| Lactate 6h (mg/dl) | 28 (19.47) | 29 (24.50) | 25 (17.22) | 29 (23.04) | 31 (29.41) | 28 (20.48) | NS |
APACHE II: Acute Physiology Score and Chronic Health Evaluation II. The results are reported as the mean (standard deviation).
Organ dysfunction and gender of the patients admitted to the ICU due to severe sepsis/septic shock.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | p | |
|---|---|---|---|---|---|---|---|
| Organ dysfunction (%) | |||||||
| Hypotension | 91.5 | 96 | 88 | 93 | 94 | 93 | NS |
| Renal involvement | 65 | 70 | 70 | 71 | 74 | 69 | NS |
| Thrombocytopenia | 43 | 33 | 30 | 28 | 31 | 29 | 0.04 |
| Respiratory involvement | 59 | 64 | 49 | 49 | 54 | 48 | 0.010 |
| Coagulopathy | 46 | 38 | 36 | 56 | 49 | 47 | 0.001 |
| Hyperbilirubinemia | 25 | 21 | 18 | 20 | 22 | 22 | NS |
| Gender (%) | |||||||
| Males | 70 | 66 | 64 | 66 | 65 | 60 | NS |
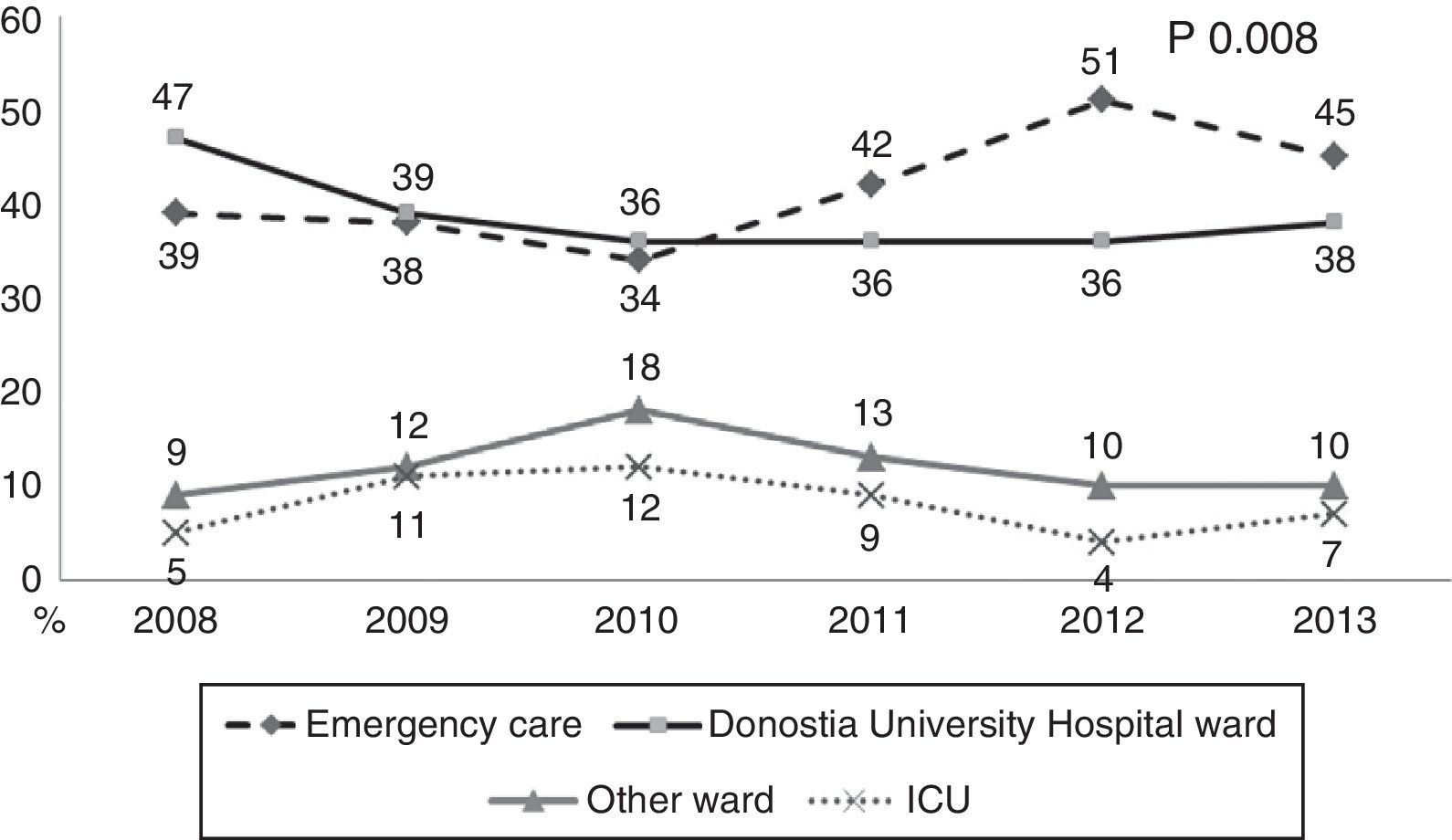
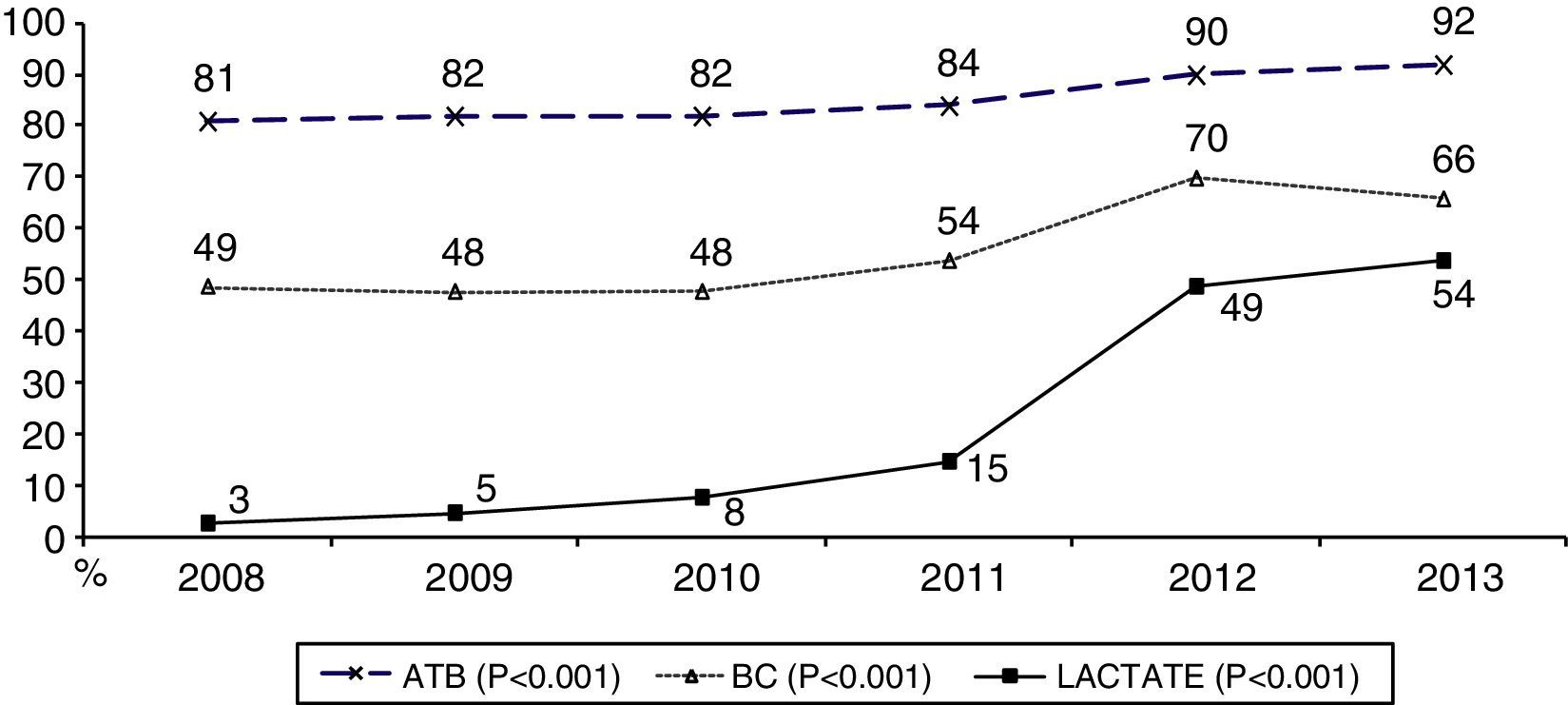
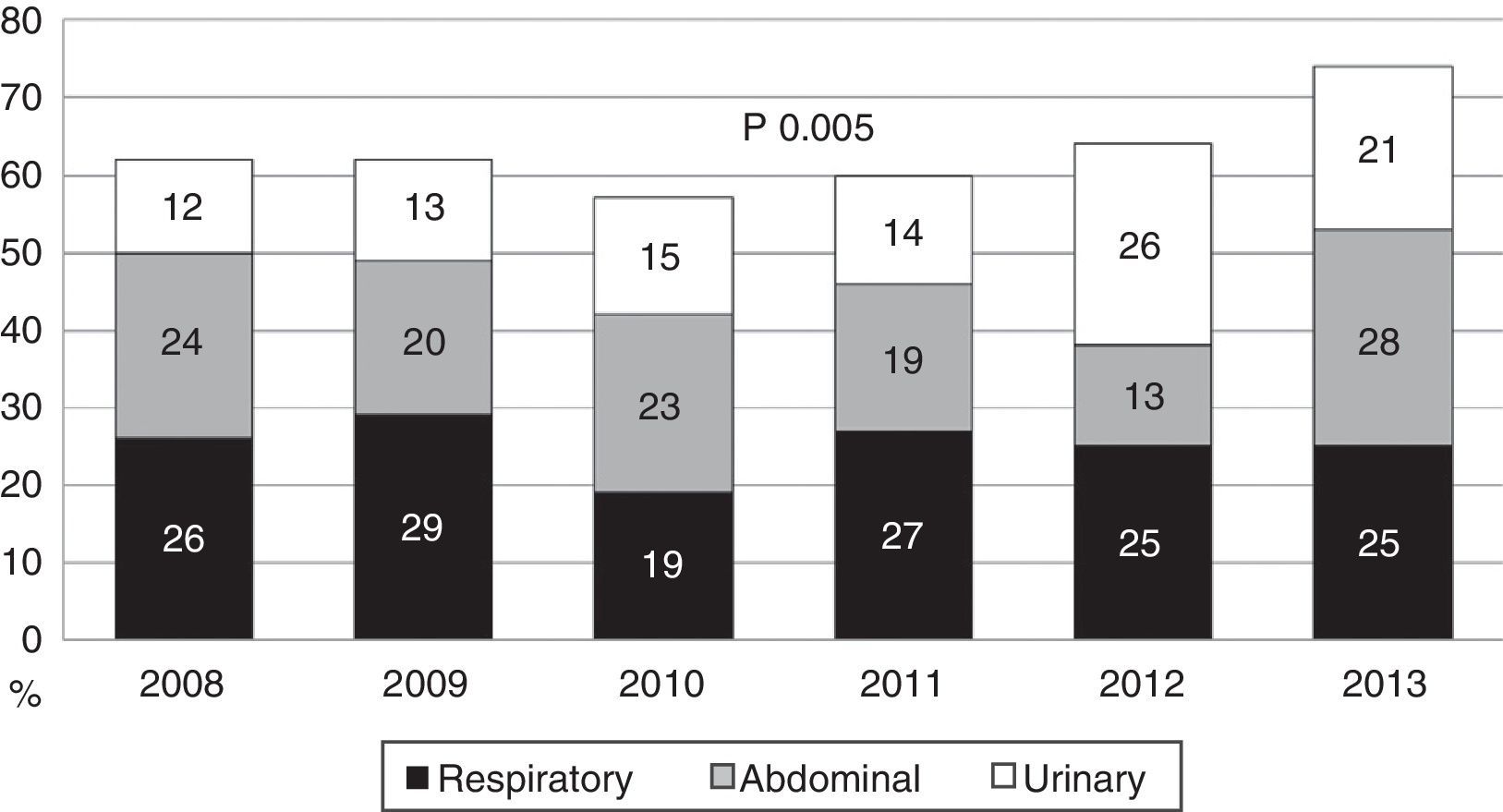
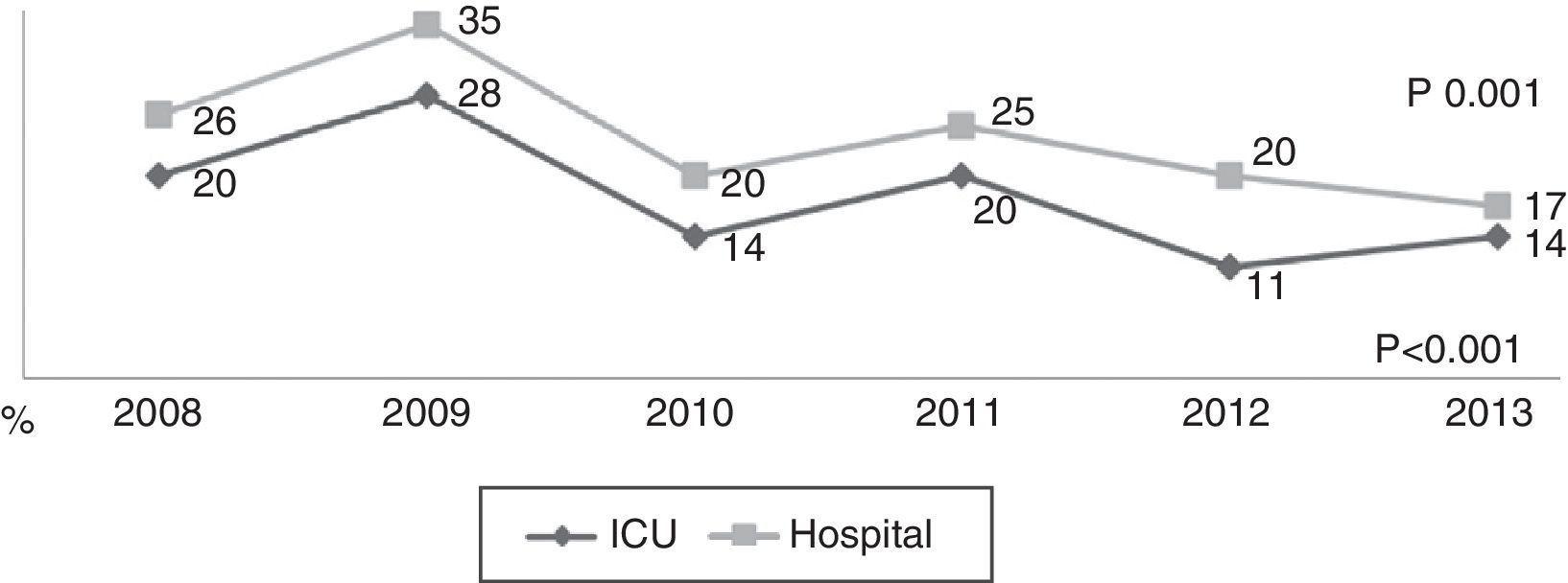
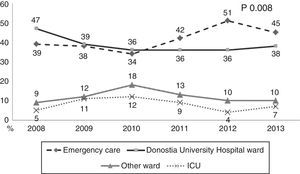
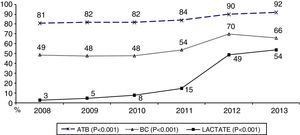
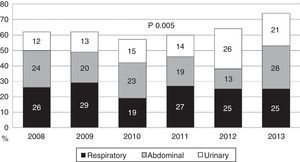
During the first three years, the patients were admitted mainly from hospital wards, though in the last years there has been a significant increase in admissions from emergency care (Fig. 1). Likewise, there have been significant increases in lactate measurement requests, extractions for blood cultures, and the administration of antibiotics prior to admission to the ICU (Fig. 2). Regarding the origin of the disease (Fig. 3), sepsis of respiratory and abdominal origin predominated in the course of the 6-year study period, followed by urinary tract infections, which have experienced an increase to the point of becoming the first cause of SeS/SSh in 2012. Mortality both in the ICU and globally in hospital has experienced a significant decrease (Fig. 4).
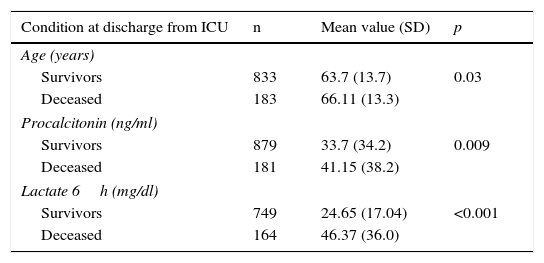
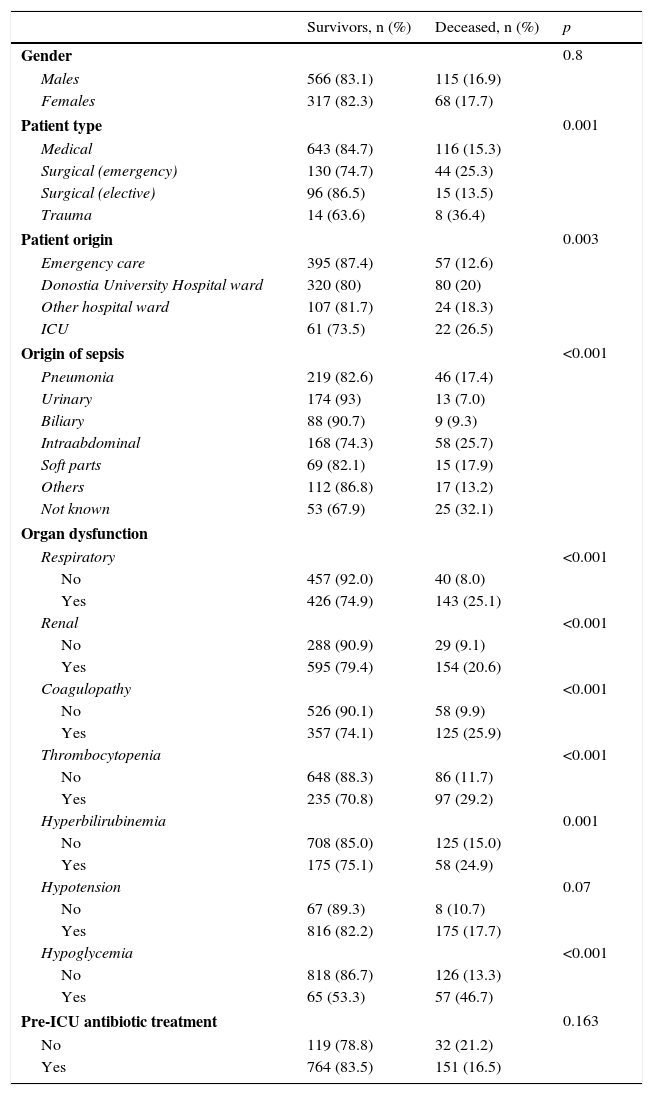
As early predictors of mortality in the ICU, we only analyzed the variables present in the first 12h of sepsis onset. The univariate analysis (Tables 3 and 4) show the deceased patients to be significantly older and with significantly higher procalcitonin and lactate levels, as well as a larger percentage of organ disorders–with the exception of hypotension. Patient gender and the administration of antibiotics before admission to the ICU had no significant impact. The mortality rate among the patients admitted from hospital wards or who developed the condition in the ICU was greater than among those admitted directly from emergency care. Trauma patients showed greater mortality, followed by emergency surgery patients and medical patients. Elective surgery patients showed the best survival figures. With respect to the underlying etiology, sepsis of unknown origin involved the highest mortality, followed by intraabdominal infections, while sepsis of biliary or urinary origin showed better survival.
Comparative analysis of the quantitative variables in relation to mortality.
| Condition at discharge from ICU | n | Mean value (SD) | p |
|---|---|---|---|
| Age (years) | |||
| Survivors | 833 | 63.7 (13.7) | 0.03 |
| Deceased | 183 | 66.11 (13.3) | |
| Procalcitonin (ng/ml) | |||
| Survivors | 879 | 33.7 (34.2) | 0.009 |
| Deceased | 181 | 41.15 (38.2) | |
| Lactate 6h (mg/dl) | |||
| Survivors | 749 | 24.65 (17.04) | <0.001 |
| Deceased | 164 | 46.37 (36.0) | |
SD: standard deviation; ICU: Intensive Care Unit.
Comparative analysis of the qualitative variables in relation to mortality.
| Survivors, n (%) | Deceased, n (%) | p | |
|---|---|---|---|
| Gender | 0.8 | ||
| Males | 566 (83.1) | 115 (16.9) | |
| Females | 317 (82.3) | 68 (17.7) | |
| Patient type | 0.001 | ||
| Medical | 643 (84.7) | 116 (15.3) | |
| Surgical (emergency) | 130 (74.7) | 44 (25.3) | |
| Surgical (elective) | 96 (86.5) | 15 (13.5) | |
| Trauma | 14 (63.6) | 8 (36.4) | |
| Patient origin | 0.003 | ||
| Emergency care | 395 (87.4) | 57 (12.6) | |
| Donostia University Hospital ward | 320 (80) | 80 (20) | |
| Other hospital ward | 107 (81.7) | 24 (18.3) | |
| ICU | 61 (73.5) | 22 (26.5) | |
| Origin of sepsis | <0.001 | ||
| Pneumonia | 219 (82.6) | 46 (17.4) | |
| Urinary | 174 (93) | 13 (7.0) | |
| Biliary | 88 (90.7) | 9 (9.3) | |
| Intraabdominal | 168 (74.3) | 58 (25.7) | |
| Soft parts | 69 (82.1) | 15 (17.9) | |
| Others | 112 (86.8) | 17 (13.2) | |
| Not known | 53 (67.9) | 25 (32.1) | |
| Organ dysfunction | |||
| Respiratory | <0.001 | ||
| No | 457 (92.0) | 40 (8.0) | |
| Yes | 426 (74.9) | 143 (25.1) | |
| Renal | <0.001 | ||
| No | 288 (90.9) | 29 (9.1) | |
| Yes | 595 (79.4) | 154 (20.6) | |
| Coagulopathy | <0.001 | ||
| No | 526 (90.1) | 58 (9.9) | |
| Yes | 357 (74.1) | 125 (25.9) | |
| Thrombocytopenia | <0.001 | ||
| No | 648 (88.3) | 86 (11.7) | |
| Yes | 235 (70.8) | 97 (29.2) | |
| Hyperbilirubinemia | 0.001 | ||
| No | 708 (85.0) | 125 (15.0) | |
| Yes | 175 (75.1) | 58 (24.9) | |
| Hypotension | 0.07 | ||
| No | 67 (89.3) | 8 (10.7) | |
| Yes | 816 (82.2) | 175 (17.7) | |
| Hypoglycemia | <0.001 | ||
| No | 818 (86.7) | 126 (13.3) | |
| Yes | 65 (53.3) | 57 (46.7) | |
| Pre-ICU antibiotic treatment | 0.163 | ||
| No | 119 (78.8) | 32 (21.2) | |
| Yes | 764 (83.5) | 151 (16.5) | |
ICU: Intensive Care Unit.
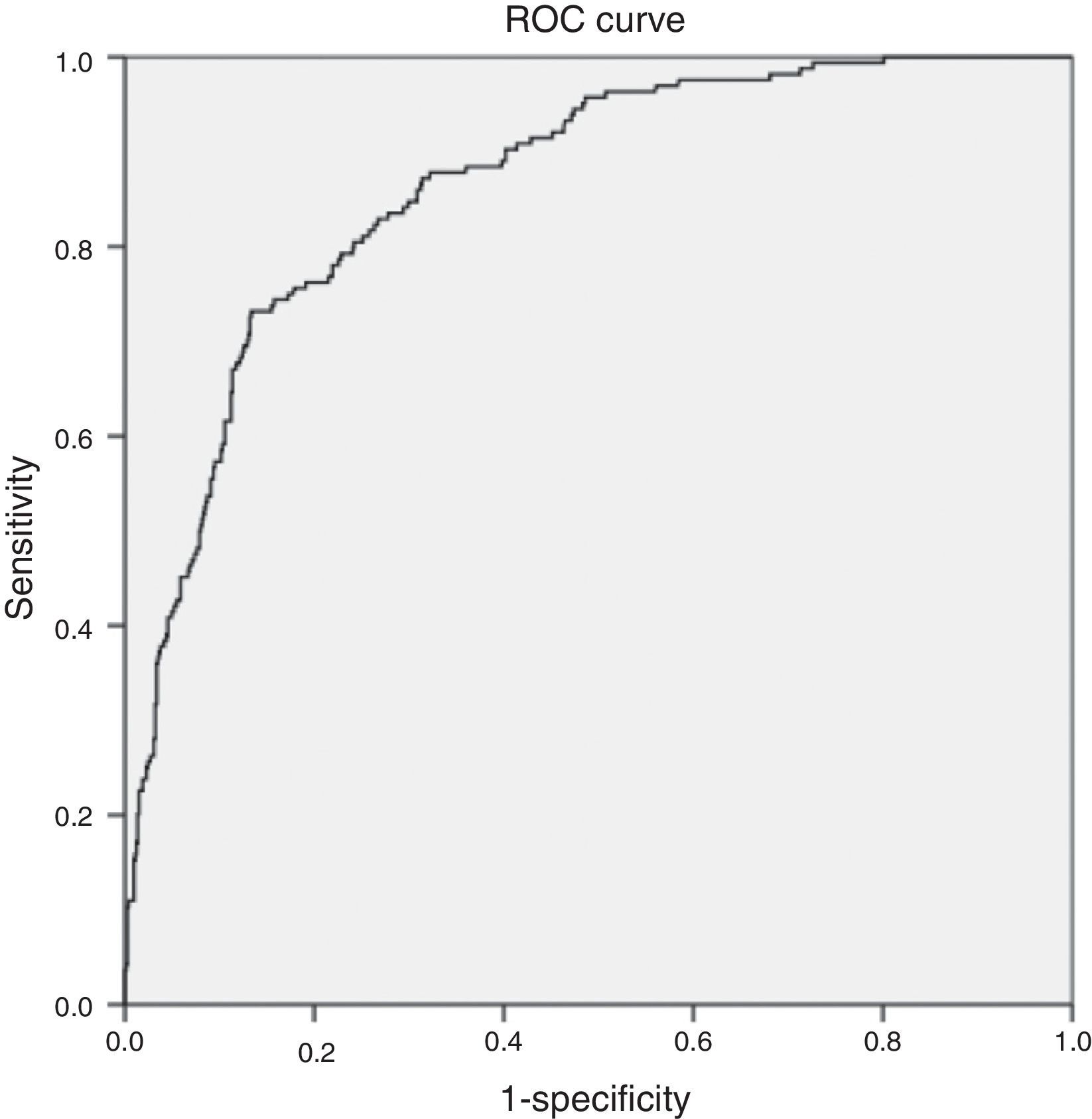
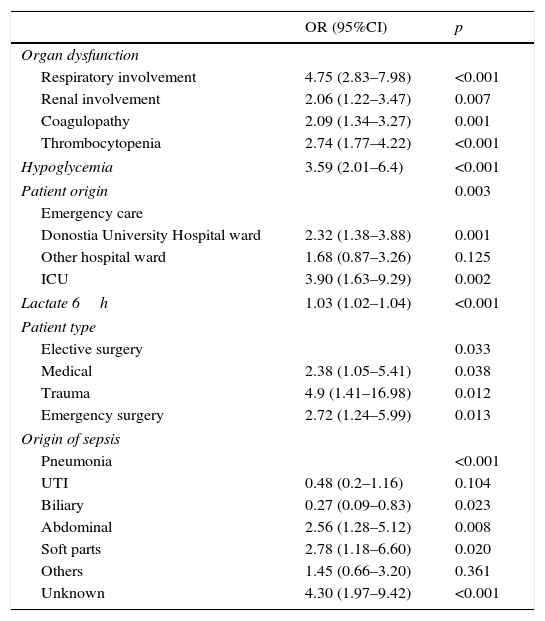
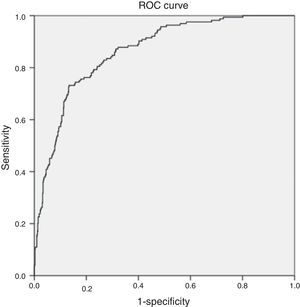
The multivariate analysis (Table 5) found the variables significantly correlated to mortality to be the lactate levels, the presence of respiratory alterations, hypoglycemia, thrombocytopenia, coagulopathy and renal involvement, patient type and origin, and the origin of sepsis. Mortality was not conditioned by the presence of hypotension, hyperbilirubinemia, procalcitonin concentration, or patient age. The Hosmer–Lemeshow test revealed an excellent fit of the model (0.084, with an area under the curve [AUC] of 0.863 [95%CI 0.834–0.892; p<0.0001]) (Fig. 5).
Multivariate analysis of the variables associated to mortality.
| OR (95%CI) | p | |
|---|---|---|
| Organ dysfunction | ||
| Respiratory involvement | 4.75 (2.83–7.98) | <0.001 |
| Renal involvement | 2.06 (1.22–3.47) | 0.007 |
| Coagulopathy | 2.09 (1.34–3.27) | 0.001 |
| Thrombocytopenia | 2.74 (1.77–4.22) | <0.001 |
| Hypoglycemia | 3.59 (2.01–6.4) | <0.001 |
| Patient origin | 0.003 | |
| Emergency care | ||
| Donostia University Hospital ward | 2.32 (1.38–3.88) | 0.001 |
| Other hospital ward | 1.68 (0.87–3.26) | 0.125 |
| ICU | 3.90 (1.63–9.29) | 0.002 |
| Lactate 6h | 1.03 (1.02–1.04) | <0.001 |
| Patient type | ||
| Elective surgery | 0.033 | |
| Medical | 2.38 (1.05–5.41) | 0.038 |
| Trauma | 4.9 (1.41–16.98) | 0.012 |
| Emergency surgery | 2.72 (1.24–5.99) | 0.013 |
| Origin of sepsis | ||
| Pneumonia | <0.001 | |
| UTI | 0.48 (0.2–1.16) | 0.104 |
| Biliary | 0.27 (0.09–0.83) | 0.023 |
| Abdominal | 2.56 (1.28–5.12) | 0.008 |
| Soft parts | 2.78 (1.18–6.60) | 0.020 |
| Others | 1.45 (0.66–3.20) | 0.361 |
| Unknown | 4.30 (1.97–9.42) | <0.001 |
CI: confidence interval; ITU: urinary tract infection; OR: odds ratio. Results are expressed as odds ratio (95% confidence interval).
Our registry shows that SeS/SSh generates an increasing number of admissions to the ICU, though the patient characteristics have changed little over these years. Findings such as the increasing trend in patients admitted directly from the emergency service, and the increase in requests for lactate determinations and blood cultures, as well as the growing tendency to administer antibiotics prior to admission to the ICU, suggest that the education campaigns in our province have contributed to improve the management of sepsis. As a result of these campaigns, we have established consensus on measures such as the use of noradrenalin as vasopressor treatment in the emergency services of all the district hospitals, which until the year 2012 lacked such treatment. In this way we are able to ensure earlier hemodynamic resuscitation, which may have contributed to the observed decrease in certain organ dysfunctions, such as thrombocytopenia and lung damage, and fundamentally to the decrease in patient mortality.
These data encourage us to intensify the educational campaigns and continue to register our clinical activity with a view to identifying opportunities for improvement. To this effect, and through the logistic regression analysis, we have attempted to identify early predictors of mortality due to sepsis. In this regard, mention must be made of the identification of arterial lactate concentration, which coincides with the findings of many other studies,9,10 where the magnitude of lactacidemia is seen to reflect the severity of hypoperfusion and is directly related to patient mortality. The same cannot be said of procalcitonin, for although this marker is clearly useful in diagnosing bacterial infections,11 its prognostic relevance is less clear.12 In our registry, the procalcitonin levels were not found to be related to mortality. However, it must be underscored that in all patients we recorded the peak value, not the clearance value–which according to some investigators13,14 could constitute a better prognostic marker.
Regarding organ dysfunction, its usefulness as a predictor of mortality has been firmly established.15,16 In our case there were two exceptions: hypotension and hyperbilirubinemia. Marchick et al.17 showed that the presence of hypotension–even when not sustained over time–in emergency care in septic patients increases mortality risk during hospital admission. In our registry, however, this was not found to be related to increased mortality. The explanation for this may be that hypotension could have acted as an alarm signal helping to recognize the severity of sepsis earlier, start resuscitation measures earlier, and accelerate admission to the ICU. On the other hand, the group of “hypotensive” patients is very heterogeneous and comprises both subjects requiring low-dose vasopressor treatment and rapid hemodynamic stabilization, and patients with refractory shock. The results might have been different on considering the severity and duration of hypotension. In this context, arterial lactate appears to be a more objective measure of severity.
The presence of hyperbilirubinemia in sepsis is often related to liver disorders, though cholestasis, circulating endotoxin and hemolysis may also cause hyperbilirubinemia. We found few literature references to its potential prognostic utility. In this regard, Zhai et al.18 reported a relationship between bilirubin elevation upon admission to the ICU and the development of adult respiratory distress syndrome and increased mortality. In our registry these patients did not show a poorer prognosis, possibly because an important number of them presented sepsis of biliary origin, which is associated to lesser mortality and could affect the global results.
In reference to organ dysfunction, it should be mentioned that hypoglycemia, while not considered a criterion of severe sepsis, has been shown to be related to mortality in many studies.19 We perform conventional, non-intensive, blood glucose monitoring with the aim of securing levels of ≤150mg/dl. As a result, the presence of hypoglycemia is rarely secondary to insulin therapy. Although the underlying etiology may be multifactorial,20 we consider it important to point out that hypoglycemia was secondary to severe liver dysfunction in a significant proportion of our patients–manifesting before hyperbilirubinemia and involving high mortality.
Other prognostic variables were the patient type, the origin of sepsis and the patient origin. In this regard, patients undergoing elective surgery showed greater survival, while trauma patients showed greater mortality–this coinciding with the observations of large both trauma21,22 and surgical registries.23 In our case, and in coincidence with Reyes et al.,24 sepsis of unknown origin was associated to increased mortality. In contrast, Zahar et al.25 considered that mortality is not influenced by the origin of sepsis but by the early and adequate start of antibiotherapy. Although this is a very relevant prognostic factor in the literature, our registry showed no differences between patients who had or had not received antibiotic treatment prior to admission to the ICU. This observation draws our attention, though a more in-depth analysis is probably required–in all cases examining the timing of administration from the onset of symptoms, and investigating whether the antibiotic used was adequate once the microbiological data became known. Since in many of our patients administration took place outside the hospital, we lack the data needed for such an analysis.
Lastly, those patients admitted to the ICU from the hospital ward and who developed sepsis once in the Unit showed significantly greater mortality. We consider that a number of factors influenced this situation, including the nosocomial nature of some infections,26 and delays in recognizing the disease–particularly among patients coming from hospital wards. We probably should intensify our educational efforts in the ward setting, with the introduction of organizational changes–creating multidisciplinary teams and a sepsis code, with a view to improving the management of these patients.
Our study has a number of limitations. On one hand, it is a single-center study, and although the sample size is quite large, the validity of the findings may have been affected as a result. On the other hand, since in many cases the management measures were started outside our hospital, we lack information on relevant aspects such as the timing of antibiotic administration and other initial measures recommended in the Surviving Sepsis Campaign.
In conclusion, it should be underscored that severe sepsis/septic shock is responsible for an increasing number of admissions to the ICU, with high morbidity-mortality. Through our clinical activity we have attempted to define the epidemiological characteristics of our patients and the prognostic factors involved, with a view to ensuring earlier identification and a better approach to the management of this disease. In this regard, we consider it important to continue the introduction of educational campaigns and to promote the creation of a sepsis code with the intervention of multidisciplinary teams.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Azkárate I, Choperena G, Salas E, Sebastián R, Lara G, Elósegui I, et al. Epidemiología y factores pronósticos de la sepsis grave/shock séptico. Seis años de evolución. Med Intensiva. 2016;40:18–25.