To study the characteristics, evolution and prognosis of patients with infectious endocarditis requiring treatment in the Intensive Care Unit.
DesignA prospective, observational cohort study of patients admitted due to infectious endocarditis.
SettingNuestra Señora de Candelaria University Hospital, a third-level center with a recruitment population of 493,145.
PatientsAll patients consecutively diagnosed with infectious endocarditis in our center according to the Duke criteria, between 1st January 2005 and 31st July 2011.
Study variablesDemographic data, clinical severity scores, microbiological and echocardiographic data, hospital mortality and complications.
ResultsOut of 102 patients diagnosed with endocarditis, 38 (37%) were admitted to Intensive Care. Compared with those patients not admitted to the ICU, these subjects suffered more frequent mitral valve alterations (OR=7.13; 95%CI: 2.12–24; p=0.002) and cerebral embolism (OR=3.89; 95%CI: 1.06–14.3; p=0.041). In turn, mortality was greater (42.1% vs 18.8%, p=0.011), as was the proportion of emergency surgeries (45.8% vs 5.9%, p<0.001). The identified mortality predictors were Staphylococcus aureus infection (OR=3.49; 95%CI 1.02–11.93; p=0.046), heart failure (OR=4.18; 95%CI: 1.17–14.94; p=0.028), cerebral embolism (OR=8.45; 95%CI: 1.89–37.74; p=0.005) and the SAPS II upon admission (OR=1.09; 95%CI: 1.04–1.15; p<0.001).
ConclusionsA large proportion of patients with endocarditis require admission to the Intensive Care Unit, presenting a much poorer prognosis. Staphylococcus aureus infection, heart failure, cerebral embolism and SAPS II scores are independent predictors of hospital mortality.
Conocer las características, evolución y pronóstico de los pacientes con endocarditis infecciosa que requieren tratamiento en la Unidad de Medicina Intensiva.
DiseñoEstudio observacional de cohortes prospectivo en pacientes ingresados por endocarditis infecciosa.
ÁmbitoHospital Universitario Nuestra Señora de Candelaria, centro con 824 camas y población asignada de 493,145 personas.
PacientesTodos los pacientes diagnosticados de endocarditis siguiendo los criterios de Duke entre el 1 de Enero de 2005 y el 31 de Julio de 2011.
Variables de interésVariables demográficas, clínicas, scores de gravedad, hallazgos microbiológicos y ecocardiográficos, mortalidad intrahospitalaria y complicaciones.
ResultadosDe 102 pacientes diagnosticados de endocarditis, 38 (37%) ingresaron en Medicina Intensiva. Comparándolos con los que no lo hicieron, sufrieron con más frecuencia afectación mitral (OR=7.13; IC del 95%, 2.12–24; p=0.002) y embolia cerebral (OR=3.89; IC del 95%, 1.06–14.3; p=0.041). La mortalidad fue mayor (42.1% vs 18.8%, p=0.011), así como la proporción de cirugías urgentes (45.8% vs 5.9%, p<0.001). Resultaron predictores de mortalidad la infección por Estafilococo aureus (OR=3.49; IC 95%: 1.02–11.93; p=0.046), la insuficiencia cardiaca (OR=4.18; IC 95%: 1.17–14.94; p=0.028), el embolismo cerebral (OR=8.45; IC 95%: 1.89–37.74; p=0.005) y la puntuación en el score SAPS II al ingreso (OR=1,09; IC 95% 1.04–1.15; p<0.001).
ConclusionesUna elevada proporción de pacientes con endocarditis requieren ingreso en la Unidad de Medicina Intensiva, presentando un pronóstico mucho más desfavorable. La infección por Estafilococo aureus, la insuficiencia cardiaca, el embolismo cerebral y la puntuación SAPS II resultan predictores de mortalidad intrahospitalaria.
Infectious endocarditis (IE) is an infrequent condition that proves particularly difficult to treat and usually requires close collaboration among intensivists, specialists in infectious diseases, cardiologists and heart surgeons, in an attempt to improve the management outcome.1 There have been important changes in the epidemiological and microbiological characteristics of the disease, particularly in third level hospital centers in developed countries, which typically see a greater proportion of elderly patients with multiple illnesses requiring treatment, with infections produced by aggressive organisms, and involving an acute presentation.2–4 Although there is presently very little doubt regarding the need for multidisciplinary management, scant mention has been made of those patients requiring admission to the Intensive Care Unit (ICU)–despite the high frequency of serious complications that develop in the course of the infection.5,6 In this context, patients can be admitted to the ICU from the emergency area or from other Departments such as Cardiology or Internal Medicine, when the seriousness of the patient condition requires the help of these specialized Units. The in-hospital mortality rate among patients with severe sepsis or septic shock has been estimated to reach 20–50%, though this percentage is probably underestimated and is moreover adversely conditioned by late patient referral to the ICU, despite the existence of clear criteria for admission to Intensive Care in the case of patients in these situations.7 In reference to endocarditis, a large and recent series published in Spain describes an in-hospital mortality rate of 29.5%.8
The present study analyzes the differential characteristics of patients with IE requiring admission to the ICU, as well as their evolution and in-hospital prognosis, based on objective severity scores and criteria–identifying the variables associated with admission to the Unit and with patient mortality.
MethodologyA prospective, observational cohort study was carried out of the patients consecutively admitted due to infectious endocarditis or who developed the disease during their stay in Nuestra Señora de Candelaria University Hospital–a third level center with 824 beds that serves most of the population on the island of Tenerife (493,145 inhabitants), and which is also the reference hospital for the islands of La Gomera and El Hierro.
PatientsThe study patients were diagnosed with IE in our center between 1st January 2005 and 31st July 2011. All met the criteria of definitive or probable endocarditis according to the Duke classification.9 In all cases a history was compiled, including disease antecedents, microbiological study, echocardiographic variables, disease complications, and severity assessment based on the APACHE II (Acute Physiology and Chronic Health Evaluation II),10 SAPS II (Simplified Acute Physiology Score II)11 and SOFA (Sepsis-related Organ Failure Assessment) scores.12
DefinitionDependent variables were taken to be admission to the ICU (regarded as any stay in the Unit, based on the need to treat at least the failure of one organ, and excluding habitual stay after heart surgery) and in-hospital mortality (including deaths before leaving hospital regardless of whether the patient had been subjected to surgery or not).
Acute IE was defined as episodes with an evolutive course of under two weeks from appearance of the first signs and symptoms. The development of acute renal failure was considered based on the RIFLE criteria referred to renal damage or failure. We only considered New York Heart Association (NYHA) functional classes III and IV in reference to the development of heart failure. Cerebral or peripheral embolism had to be evidenced by imaging techniques (CAT, MRI or ultrasound) in order to be diagnosed as such. Emergency surgery in turn referred to all surgery not delayed more than 24h after establishing the indication.
Complementary testsIn all patients at least three blood cultures were performed, with serological testing for Brucella, Legionella, Mycoplasma and Coxiella burnetii in the case of negative blood culture findings and/or under the criterion of the supervising physician.
In the transthoracic and transesophageal echocardiographic study, we registered the location of the vegetation (where present), the existence of new onset valve insufficiency ≥2/4, the appearance of a new prosthetic dehiscence, and the development of periannular complications (abscesses, pseudoaneurysms or fistulas).
Unless precluded by the seriousness of the patient condition, ultrasound or an abdominal scan was performed, together with a cranial imaging study in search of embolic phenomena.
Statistical analysisThe data are presented as the percentage or mean±standard deviation (SD) or median (interquartile range), as applicable. Normality of the distribution of the continuous variables was evaluated using the Kolmogorov–Smirnoff test. Categorical variables in turn are presented as the absolute frequency (percentage), and were compared using the chi-squared test or Fisher exact test, as required. Continuous variables were compared with the Student t-test for non-paired data or the Mann–Whitney U-test as appropriate. The Wilcoxon test was used to assess the evolutive differences in the severity scores. Forward stepwise logistic regression analysis (likelihood ratio test) was carried out in application to those variables showing p<0.05, in order to identify the risk factors for admission to the ICU and for in-hospital mortality. Goodness of fit was assessed using the Hosmer–Lemeshow test. The SPSS (version 16.0) statistical package was used for the analysis of the results.
ResultsDifferential characteristics of the patients admitted to the ICUDuring the study period a total of 102 patients were diagnosed with IE in our center. Admission to the ICU was considered necessary in 38 of these patients. Among these subjects, 25 (65.8%) came directly from the emergency area, 7 (18.4%) from the Department of Cardiology, 3 (7.9%) from Internal Medicine, and the remaining three from other Departments.
The causes indicating intensive care were predominantly severe sepsis or septic shock (14 patients, 36.8%) and severe heart failure or cardiogenic shock (14 cases, 36.8%). In three patients (7.9%) the cause of admission was neurological worsening. In the remaining cases (7 patients, 18.4%) the reasons were diverse but related to the severity of the patient condition, and included resuscitated cardiopulmonary arrest and pneumonia. Nineteen (50%) of the patients required mechanical ventilation.
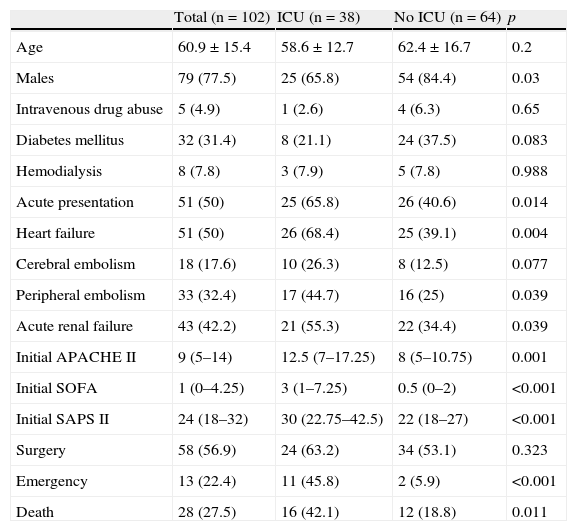
The most relevant demographic and clinical differences between the patients admitted to Intensive Care and those not admitted to the ICU are shown in Table 1. The patients admitted to Intensive Care more frequently presented acute endocarditis and mitral valve disease, and it was more common for the disorder to be complicated by heart failure and embolic phenomena. The mortality predictor scores (SAPS II, APACHE II, SOFA) upon admission to hospital were also higher in the case of admission to the ICU.
Clinical characteristics and complications.
| Total (n=102) | ICU (n=38) | No ICU (n=64) | p | |
| Age | 60.9±15.4 | 58.6±12.7 | 62.4±16.7 | 0.2 |
| Males | 79 (77.5) | 25 (65.8) | 54 (84.4) | 0.03 |
| Intravenous drug abuse | 5 (4.9) | 1 (2.6) | 4 (6.3) | 0.65 |
| Diabetes mellitus | 32 (31.4) | 8 (21.1) | 24 (37.5) | 0.083 |
| Hemodialysis | 8 (7.8) | 3 (7.9) | 5 (7.8) | 0.988 |
| Acute presentation | 51 (50) | 25 (65.8) | 26 (40.6) | 0.014 |
| Heart failure | 51 (50) | 26 (68.4) | 25 (39.1) | 0.004 |
| Cerebral embolism | 18 (17.6) | 10 (26.3) | 8 (12.5) | 0.077 |
| Peripheral embolism | 33 (32.4) | 17 (44.7) | 16 (25) | 0.039 |
| Acute renal failure | 43 (42.2) | 21 (55.3) | 22 (34.4) | 0.039 |
| Initial APACHE II | 9 (5–14) | 12.5 (7–17.25) | 8 (5–10.75) | 0.001 |
| Initial SOFA | 1 (0–4.25) | 3 (1–7.25) | 0.5 (0–2) | <0.001 |
| Initial SAPS II | 24 (18–32) | 30 (22.75–42.5) | 22 (18–27) | <0.001 |
| Surgery | 58 (56.9) | 24 (63.2) | 34 (53.1) | 0.323 |
| Emergency | 13 (22.4) | 11 (45.8) | 2 (5.9) | <0.001 |
| Death | 28 (27.5) | 16 (42.1) | 12 (18.8) | 0.011 |
Data expressed as mean±standard deviation, median (interquartile range) or n (%). No ICU: patients not admitted to ICU; ICU: patients admitted to ICU.
While there were no differences in the global indication of surgery, a total of 11 patients were emergency referred for surgery from the ICU (45.8% of those operated upon), versus only two patients (5.9%) from other medical Departments (p<0.001). The causes leading to surgery were mainly heart failure (36.2%), valve destruction with severe valve insufficiency (27.6%), and persistent fever (15.5%). Other causes included annular alterations and the presence of large vegetations.
Regarding the etiological agent, in the patients admitted to the ICU there was a clear predominance of Staphylococcus aureus, which proved significantly more common than in the patients not admitted to Intensive Care (39.5 vs 20.3%, p=0.036). Other causal organisms were streptococci (18.4%), enterococci (10.5%) and other agents (7.9%). Coagulase-negative staphylococci were less frequent compared with the patients not admitted to the ICU (5.3 vs 20.3%, p=0.038). Lastly, patients with negative blood culture findings represented 18.4% of the cases in the ICU.
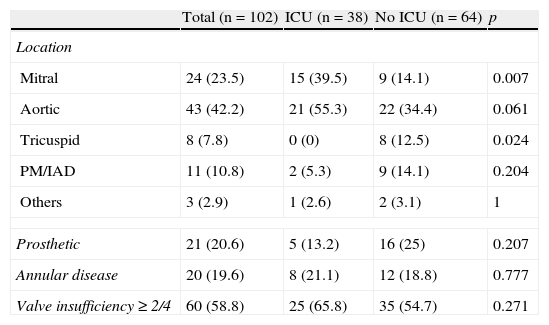
Table 2 reports the echocardiographic characteristics. No significant differences were observed in the proportion of cases of valve prosthesis endocarditis, in the frequency of annular alterations or in the presence of moderate or severe valve insufficiency. However, the percentage of patients with mitral valve disease was greater in the ICU, and no cases of tricuspid valve endocarditis were registered during the period studied.
Differential echocardiographic characteristics.
| Total (n=102) | ICU (n=38) | No ICU (n=64) | p | |
| Location | ||||
| Mitral | 24 (23.5) | 15 (39.5) | 9 (14.1) | 0.007 |
| Aortic | 43 (42.2) | 21 (55.3) | 22 (34.4) | 0.061 |
| Tricuspid | 8 (7.8) | 0 (0) | 8 (12.5) | 0.024 |
| PM/IAD | 11 (10.8) | 2 (5.3) | 9 (14.1) | 0.204 |
| Others | 3 (2.9) | 1 (2.6) | 2 (3.1) | 1 |
| Prosthetic | 21 (20.6) | 5 (13.2) | 16 (25) | 0.207 |
| Annular disease | 20 (19.6) | 8 (21.1) | 12 (18.8) | 0.777 |
| Valve insufficiency≥2/4 | 60 (58.8) | 25 (65.8) | 35 (54.7) | 0.271 |
Data expressed as n (%). PM/IAD: on pacemaker or implantable defibrillator; No ICU: patients not admitted to ICU; ICU: patients admitted to ICU.
As predictors of admission, the multivariate analysis identified cerebral embolism (OR=3.89; 95%CI, 1.06–14.3; p=0.041), the SOFA score upon admission to hospital (OR=1.60; 95%CI, 1.29–2.00; p<0.001), and mitral valve disease (OR=7.13; 95%CI, 2.12–24; p=0.002). The Hosmer–Lemeshow test yielded p=0.87.
In-hospital mortalityThe in-hospital mortality rate reached 42.1% in the ICU versus 18.8% among the patients not admitted to Intensive Care (p=0.011). The predominant cause of death was septic shock (51.8%), followed by severe heart failure or cardiogenic shock (25.9%) and neurological complications (14.8%).
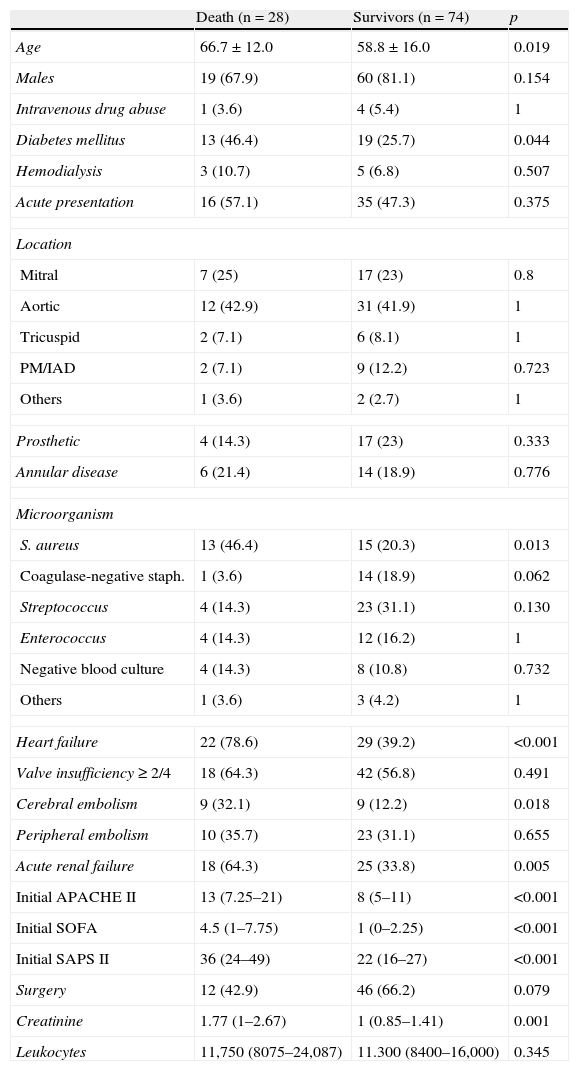
According to the univariate analysis, the patients who died during admission were older, with a greater prevalence of diabetes mellitus, more frequent infection due to S. aureus, and with complications most often in the form of heart failure, cerebral embolism and renal failure. The scores of the severity scales upon admission were also significantly greater. Although the percentage of operated patients was higher among the survivors, statistical significance was not reached. Table 3 shows the main differences between the patients who survived the hospital stage and those who did not.
Influence of different variables upon in-hospital mortality. Univariate analysis.
| Death (n=28) | Survivors (n=74) | p | |
| Age | 66.7±12.0 | 58.8±16.0 | 0.019 |
| Males | 19 (67.9) | 60 (81.1) | 0.154 |
| Intravenous drug abuse | 1 (3.6) | 4 (5.4) | 1 |
| Diabetes mellitus | 13 (46.4) | 19 (25.7) | 0.044 |
| Hemodialysis | 3 (10.7) | 5 (6.8) | 0.507 |
| Acute presentation | 16 (57.1) | 35 (47.3) | 0.375 |
| Location | |||
| Mitral | 7 (25) | 17 (23) | 0.8 |
| Aortic | 12 (42.9) | 31 (41.9) | 1 |
| Tricuspid | 2 (7.1) | 6 (8.1) | 1 |
| PM/IAD | 2 (7.1) | 9 (12.2) | 0.723 |
| Others | 1 (3.6) | 2 (2.7) | 1 |
| Prosthetic | 4 (14.3) | 17 (23) | 0.333 |
| Annular disease | 6 (21.4) | 14 (18.9) | 0.776 |
| Microorganism | |||
| S. aureus | 13 (46.4) | 15 (20.3) | 0.013 |
| Coagulase-negative staph. | 1 (3.6) | 14 (18.9) | 0.062 |
| Streptococcus | 4 (14.3) | 23 (31.1) | 0.130 |
| Enterococcus | 4 (14.3) | 12 (16.2) | 1 |
| Negative blood culture | 4 (14.3) | 8 (10.8) | 0.732 |
| Others | 1 (3.6) | 3 (4.2) | 1 |
| Heart failure | 22 (78.6) | 29 (39.2) | <0.001 |
| Valve insufficiency≥2/4 | 18 (64.3) | 42 (56.8) | 0.491 |
| Cerebral embolism | 9 (32.1) | 9 (12.2) | 0.018 |
| Peripheral embolism | 10 (35.7) | 23 (31.1) | 0.655 |
| Acute renal failure | 18 (64.3) | 25 (33.8) | 0.005 |
| Initial APACHE II | 13 (7.25–21) | 8 (5–11) | <0.001 |
| Initial SOFA | 4.5 (1–7.75) | 1 (0–2.25) | <0.001 |
| Initial SAPS II | 36 (24–49) | 22 (16–27) | <0.001 |
| Surgery | 12 (42.9) | 46 (66.2) | 0.079 |
| Creatinine | 1.77 (1–2.67) | 1 (0.85–1.41) | 0.001 |
| Leukocytes | 11,750 (8075–24,087) | 11.300 (8400–16,000) | 0.345 |
Data expressed as mean±standard deviation, median (interquartile range) or n (%). PM/DAI: on pacemaker or implantable defibrillator.
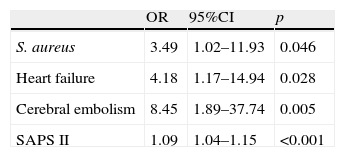
As independent predictors of mortality, the multivariate analysis (Table 4) identified infection due to S. aureus (OR=3.49; 95%CI, 1.02–11.93; p=0.046), the development of heart failure (OR=4.18; 95%CI, 1.17–14.94; p=0.028), cerebral embolism (OR=8.45; 95%CI, 1.89–37.74; p=0.005) and the SAPS II score (OR=1.09; 95%CI, 1.04–1.15; p<0.001). The Hosmer–Lemeshow test yielded p=0.81.
Independent predictors of in-hospital mortality.
| OR | 95%CI | p | |
| S. aureus | 3.49 | 1.02–11.93 | 0.046 |
| Heart failure | 4.18 | 1.17–14.94 | 0.028 |
| Cerebral embolism | 8.45 | 1.89–37.74 | 0.005 |
| SAPS II | 1.09 | 1.04–1.15 | <0.001 |
95%CI: 95% confidence interval; OR: odds ratio; SAPS II: Simplified Acute Physiology Score II; Hosmer–Lemeshow test: p=0.87.
Although it is accepted that hemodynamic impairment and the failure of one or more organs may require intensive care and monitoring, the exact proportion of patients with endocarditis requiring admission to the ICU has not been well established. Furthermore, the number of cases may be underdiagnosed, and in this sense an increased use of transthoracic echocardiography and especially of transesophageal echocardiography in patients with sepsis could prove very useful.13 In our hospital, over one-third of the patients diagnosed with endocarditis during a period of 6 years were admitted to the ICU due to different causes–fundamentally severe sepsis and heart failure complicated or not with shock. The difference in the severity scores between the patients with and without admission to Intensive Care, and the increase in such scores from patient arrival in hospital, objectively reflect the clinical and hemodynamic worsening of the patients, as evidenced by their high morbidity-mortality and the frequent indication of emergency surgery.
Karth et al.14 analyzed 33 patients admitted to the ICU due to a complicated disease course over a period of 4 years (1994–1999), in which the age, percentage of patients subjected to surgery and the microbiological profile were very similar to those of our own series, and where the in-hospital mortality reached 54%. On the other hand, the large retrospective series published by Mourvillier et al.,15 carried out in a similar period (1993–2000), found the mortality rate to be 45%, i.e., slightly higher than in our own study. In any case, there is a clear difference with respect to mortality among the patients not admitted to the ICU, which in our series did not reach 20%. This difference can be explained by the comparatively greater seriousness of the patient condition and the more adverse microbiological profile. On examining the predictors of mortality in endocarditis, important differences can be found among the published series, probably as a result of the heterogeneity of the study samples and the frequently unpredictable course taken by the disease. As predictors of in-hospital mortality, a recent large multinational study identified infection caused by S. aureus, prosthetic or mitral valve disease, peri-valvular complications and lung edema.2 In another Spanish multicenter cohort8 with a study period extending from 1984 to 2006, and with a global mortality rate of 29.5%, the independent predictors were found to be the Charlson morbidity score, neurological manifestations, acute renal failure, septic shock and, once again, prosthetic or mitral valve disease, heart failure and staphylococcal infection. In our own series, infection due to this microorganism, the development of heart failure and neurological complications were likewise identified as predictors of mortality. However, the scores commonly used in the ICU as predictors of in-hospital mortality have been little used in the literature for assessing the prognosis of infectious endocarditis. The SAPS II score upon admission to hospital was found to be an independent mortality predictor in the present study, in the same way as the APACHE II score in the series published by Chu et al.16 It could be postulated here that the evaluation of these scores upon admission of the patient with IE could help us predict the level of care that will be required – though a broader analysis would be needed in this context.
The percentage of survivors was greater in the group of patients subjected to surgery, though the difference failed to reach statistical significance. Given the calculated p-value, we decided to include this parameter in the multivariate analysis, though once again it was not identified as a predictor. Perhaps the lack of uniform benefit among the patients referred to surgery could be obviated by adequate case selection.17,18 Although surgery plays a key role in the treatment of IE, the decision to operate and the timing of surgery are influenced by a range of factors including patient comorbidity, age, previous antibiotic treatment, or the availability of an experienced surgical team.19 The endocarditis guidelines of the European Society of Cardiology indicate surgery in situations of severe heart failure, signs of poor hemodynamic tolerance, persistent fever, signs of uncontrolled local infection such as abscesses, severe mitral or aortic valve regurgitation, and the presence of large vegetations after one or more embolic episodes, despite adequate medical treatment.20 We consider that the indications established in our center are consistent with these recommendations.
ConclusionsIn our setting, the ICU collaborates in the management of patients with infectious endocarditis in a large percentage of cases. These patients suffer a greater number of complications and present an objectively more serious disease condition, in turn reflected by high mortality and an important emergency surgery rate. Independent predictors of in-hospital mortality are infection caused by S. aureus, the development of heart failure, cerebral embolism and the SAPS II score upon admission.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Miranda-Montero S, et al. Endocarditis infecciosa en la Unidad de Medicina Intensiva. Med Intensiva. 2012;36:460–6.