To identify pretransplant predictors of early mortality (90 days after transplantation) and evaluate their discriminating capacity in adult liver transplant recipients (LTRs).
DesignAn observational, retrospective, nested cases–controls study from a consecutive cohort of LTRs was carried out.
SettingUniversity hospital.
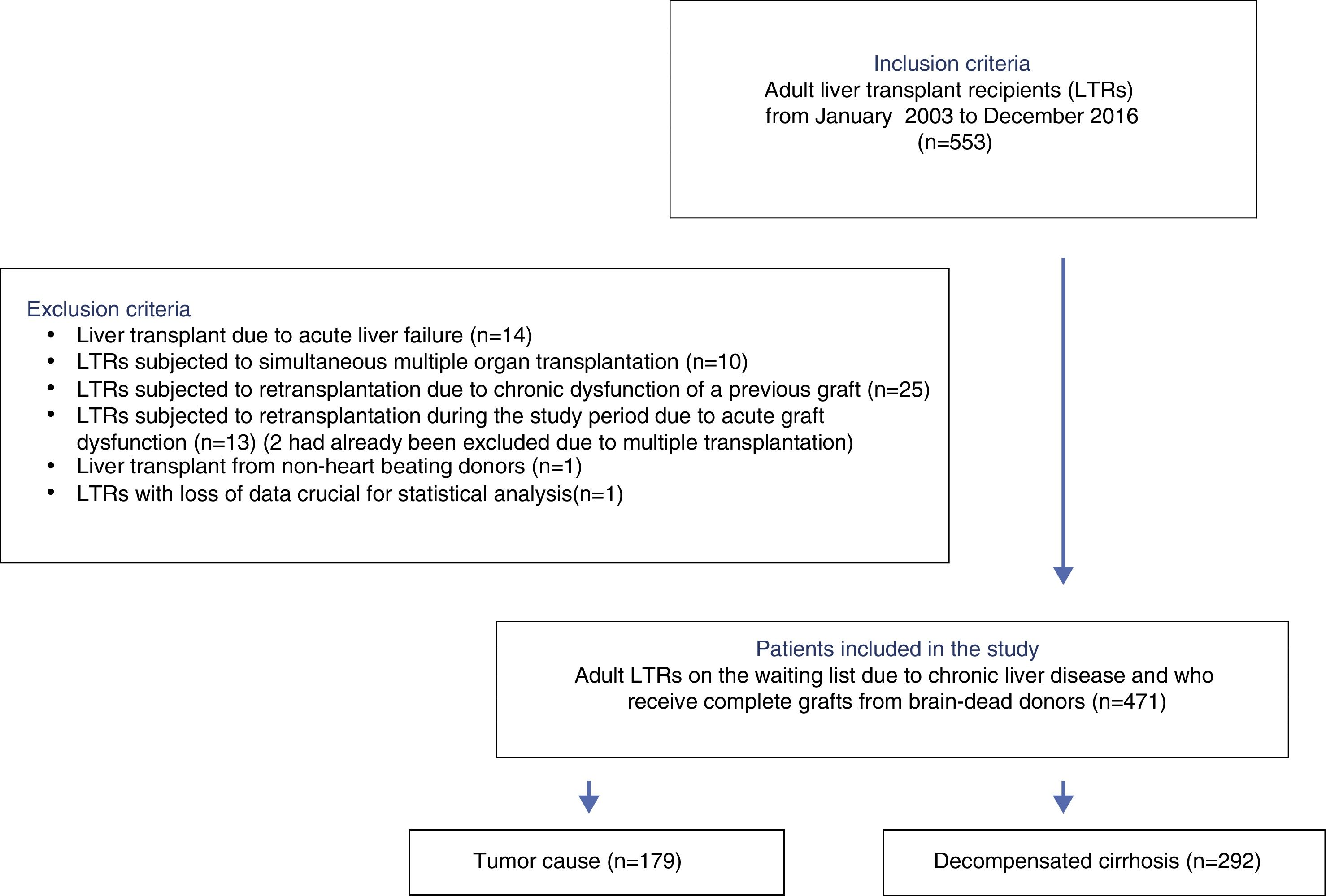
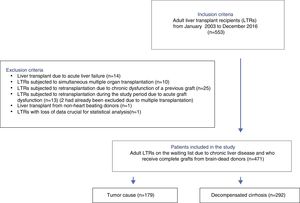
PatientsAll consecutive LTR between January 2003 and December 2016 were eligible for inclusion. Patients with acute liver failure, previous graft dysfunction, simultaneous multiple organ transplantation, non-heart beating donors, and those needing urgent retransplantation during the study period were excluded. The analysis comprised 471 patients.
Main variables of interestPretransplant characteristics were the main variables of interest. The LTR were grouped according to the dependent variable (early mortality). Multivariate logistic regression analysis was conducted to identify predictors of early mortality. The discriminating capacity of the models obtained was evaluated by comparing ROC curves (models versus MELD-Na).
ResultsThe MELD-Na score (OR=1.069, 95% CI=1.014–1.127), age >60 years (OR=2.479, 95% CI=1.226–5.015), and LTR height <163cm (OR=4.092, 95% CI=2.115–7.917) were identified as independent predictors of early mortality. The cause of transplantation (hepatocellular carcinoma or decompensated cirrhosis) was identified as a confounding factor.
ConclusionsIn LTR due to decompensated cirrhosis, the MELD-Na score, age >60 years, and height <163cm are independent predictors of early mortality. These factors provide a better classification model than the MELD-Na score for early post-transplant mortality.
Identificar predictores pretrasplante de mortalidad precoz (90 días postrasplante) y evaluar su capacidad discriminante en receptores adultos de trasplante hepático (RTH).
DiseñoEstudio observacional, retrospectivo, de casos y controles anidados sobre una cohorte consecutiva de RTH.
ÁmbitoHospital Universitario.
PacientesDesde enero de 2003 a diciembre de 2016, todos los receptores adultos de trasplante hepático fueron elegibles para inclusión. Fueron excluidos los RTH por fallo hepático agudo, disfunción de un injerto previo, trasplante simultáneo de órganos, de donantes en asistolia, y aquellos que precisaron retrasplante durante el periodo de estudio. Para el análisis se incluyeron 471 pacientes.
Principales variables de interésLas características pretrasplante fueron las variables de interés. Los RTH fueron agrupados de acuerdo con la variable dependiente (mortalidad precoz). Los predictores se obtuvieron mediante análisis multivariante de regresión logística. La capacidad discriminante de los modelos obtenidos se evaluó mediante comparación de curvas ROC.
ResultadosSe identificaron como predictores independientes de mortalidad precoz: la puntuación MELD-Na (OR=1,069; IC95%=1,014-1,127), la edad mayor de 60 años (OR=2,479; IC95%=1,226-5,015), y la estatura del RTH inferior a 163cm (OR=4,092; IC95%=2,115-7,917), considerándose el motivo del trasplante (carcinoma hepatocelular o cirrosis descompensada) como variable de confusión.
ConclusionesEn los RTH por cirrosis descompensada, la puntuación MELD-Na, la edad mayor de 60 años y la estatura del receptor inferior a 163cm son predictores independientes de mortalidad precoz. Esos predictores producen un modelo que clasifica a los pacientes significativamente mejor que el MELD-Na en relación con la mortalidad precoz.
Liver transplantation is the only curative treatment option for patients with end-stage liver disease. However, transplantation involves significant risks which liver transplant recipients (LTRs) must be able to overcome. Although consensus is lacking, a mortality rate of under 10% in the first 90 days after transplantation (early mortality) may be regarded as representative of good clinical practice in patients subjected to elective transplantation.1,2
The disproportion between donors and potential transplant candidates made it necessary to establish an order of priority on the waiting lists. In the past, the Child-Turcotte-Pugh scale or the United Network for Organ Sharing (UNOS) score were regarded as the standard references in this respect,3 though organ distribution systems have evolved since then, seeking to apply principles of individual fairness and maximum yield in donation.4
The introduction of the Model for End-stage Liver Disease (MELD)5 score afforded objectiveness and transparency in prioritization,6 and the MELD is now used throughout the world for the distribution of organs, thanks to the balance it affords between usefulness and distributive fairness,7 except in those scenarios where the patient prognosis is not directly related to liver function (i.e., exceptions to the MELD) – hepatocellular carcinoma (HCC) being the paradigm in this respect.8
The MELD has been well established as a predictor of mortality before transplantation, and is used as organ distribution criterion. Nevertheless, there has been much research and debate seeking to improve the tool,9–11 and it has recently been revised.12 In this regard, since January 2016, the UNOS includes serum sodium for calculation of the MELD score (MELD-Na).
Studies have also been made on the role of the MELD as a predictor of mortality after transplantation – though there is controversy on this issue.13,14 As a result, other risk factors have been investigated, such as prior admission to the Intensive Care Unit (ICU),15 the need for extrarenal replacement therapy,16 a history of abdominal surgery,17 or other comorbidities which when added to the MELD could result in the development of specific models capable of improving the prediction of early postoperative mortality.18
Specialists in Intensive Care Medicine should participate in the committees for the selection of liver transplant candidates, and must know the risk factors capable of incrementing postoperative mortality.
The primary objective of the present study was to identify other clinical characteristics of transplant candidates on the waiting list (i.e., in the pretransplant setting) which in combination with the MELD-Na could act as independent predictors of early mortality (in the first 90 days) after liver transplantation in elective adult recipients. As a secondary objective, the study explored the predictive and discriminatory capacity of the variables included in the model obtained.
Patients and methodsA retrospective, observational nested case–controls study of a consecutive cohort of adult LTRs was carried out in a single institution in the university setting.
All adult LTRs between January 2003 and December 2016 were considered eligible for inclusion. The following exclusion criteria were applied: patients with acute liver failure, patients subjected to simultaneous multiple organ transplants, living donor or non-heart beating donor LTRs, recipients elective for second transplantation due to chronic dysfunction of a previous graft, and recipients requiring urgent retransplantation during the study period.
Cases were defined as patients meeting the criterion of mortality within 90 days after transplantation, while the rest of the transplant recipients were regarded as controls.
We recorded anthropometric parameters of the recipients (age, gender, weight, height and body mass index [BMI]), the etiology of liver disease, and the pretransplantation scores related to the prognosis of liver disease (Child-Pugh, MELD). The MELD-Na score was calculated (http://www.rccc.eu/calculadoras/MELD.html) from the laboratory test data obtained at the time of hospital admission for transplantation, and plasma urea and creatinine were also registered at this time. The case history in turn provided information referred to other pretransplantation comorbidities capable of influencing postoperative mortality, such as diabetes mellitus, the implantation of a transjugular intrahepatic portosystemic shunt (TIPS) or supramesocolic surgery, as well as data referred to antecedents during the month before transplantation of hepatorenal syndrome, treatment with terlipressin, the need for renal support (dialysis in any of its modalities) and hospital admissions (both ward and ICU).
The study complied with all the ethical requirements defined by the World Health Organization (WHO) (Declaration of Helsinki), and was approved by the local Clinical Research Ethics Committee, which considered that the obtainment of informed consent was not required.
Statistical analysisThe patients were grouped according to the dependent variable (mortality 90 days after transplantation). The normal distribution of the quantitative study variables of the cohort was analyzed using the Kolmogorov–Smirnov test, with calculation of the median and percentiles 25–75 where pertinent. In the case–control study, all factors except the MELD-Na score were categorized for improved understanding of the results from the clinical perspective, with individualization into dummy variables where indicated. The cut-off point for the categorization of quantitative factors was selected arbitrarily according to a clinical criterion based on the greatest risk quartile. In the case of the variable decompensated cirrhosis, the hepatocellular, biliary and miscellaneous etiologies were pooled.
The bivariate analysis was carried out using simple binary logistic regression analysis. We calculated the odds ratio (OR) and corresponding 95% confidence interval (95% CI) as measure of association. Statistical significance was considered for p<0.05.
The multivariate analysis was carried out using binary logistic regression analysis, including all those factors with p≤0.10 in the initial model. The possible confounding factors and interactions (height and gender) were controlled by stratified analysis and logistic regression. An intermediate model was obtained, regarding those factors with p≤0.15 as significant. Adjustment was made by backward stepwise binary logistic regression analysis with manual removal of predictors (likelihood ratio) and maximum parsimony, considering those predictors with p≤0.05 as significant for obtaining the final model. We generated the receiver operating characteristic (ROC) curves of the models and contrasted their discriminating capacity according to the indications of transplantation using the DeLong test. The statistical analysis was carried out using the PASW version 18 package (SPSS Inc. Chicago, IL, USA) and MedCalc statistical software version 18 (Ostend, Belgium).
ResultsA total of 533 liver transplants were performed among eligible LTRs during the study period. Of these, 62 were excluded due to the presence of one or more exclusion criteria. A total of 471 patients were thus included for analysis (Fig. 1).
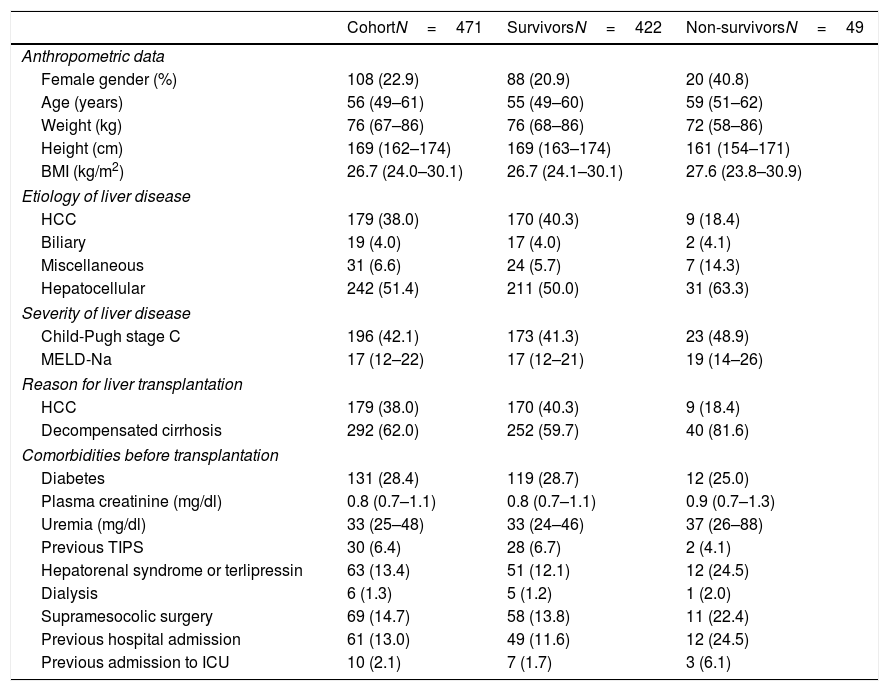
The pretransplant characteristics of the study groups are reported in Table 1. The median age of the LTRs was 55 years (P25–P75=49–60 years), with a predominance of males (77.1%). The median BMI was 26.7kg/m2 (P25–P75=24.0–30.1). The indications for transplantation were decompensated cirrhosis (62%) and liver tumors (38%).
Description of the pretransplantation characteristics of the cohort of liver transplant recipients and cases–controls (N=471).
| CohortN=471 | SurvivorsN=422 | Non-survivorsN=49 | |
|---|---|---|---|
| Anthropometric data | |||
| Female gender (%) | 108 (22.9) | 88 (20.9) | 20 (40.8) |
| Age (years) | 56 (49–61) | 55 (49–60) | 59 (51–62) |
| Weight (kg) | 76 (67–86) | 76 (68–86) | 72 (58–86) |
| Height (cm) | 169 (162–174) | 169 (163–174) | 161 (154–171) |
| BMI (kg/m2) | 26.7 (24.0–30.1) | 26.7 (24.1–30.1) | 27.6 (23.8–30.9) |
| Etiology of liver disease | |||
| HCC | 179 (38.0) | 170 (40.3) | 9 (18.4) |
| Biliary | 19 (4.0) | 17 (4.0) | 2 (4.1) |
| Miscellaneous | 31 (6.6) | 24 (5.7) | 7 (14.3) |
| Hepatocellular | 242 (51.4) | 211 (50.0) | 31 (63.3) |
| Severity of liver disease | |||
| Child-Pugh stage C | 196 (42.1) | 173 (41.3) | 23 (48.9) |
| MELD-Na | 17 (12–22) | 17 (12–21) | 19 (14–26) |
| Reason for liver transplantation | |||
| HCC | 179 (38.0) | 170 (40.3) | 9 (18.4) |
| Decompensated cirrhosis | 292 (62.0) | 252 (59.7) | 40 (81.6) |
| Comorbidities before transplantation | |||
| Diabetes | 131 (28.4) | 119 (28.7) | 12 (25.0) |
| Plasma creatinine (mg/dl) | 0.8 (0.7–1.1) | 0.8 (0.7–1.1) | 0.9 (0.7–1.3) |
| Uremia (mg/dl) | 33 (25–48) | 33 (24–46) | 37 (26–88) |
| Previous TIPS | 30 (6.4) | 28 (6.7) | 2 (4.1) |
| Hepatorenal syndrome or terlipressin | 63 (13.4) | 51 (12.1) | 12 (24.5) |
| Dialysis | 6 (1.3) | 5 (1.2) | 1 (2.0) |
| Supramesocolic surgery | 69 (14.7) | 58 (13.8) | 11 (22.4) |
| Previous hospital admission | 61 (13.0) | 49 (11.6) | 12 (24.5) |
| Previous admission to ICU | 10 (2.1) | 7 (1.7) | 3 (6.1) |
Quantitative variables are reported as the median and percentiles 25–75 (P25–P75). Qualitative variables are reported as frequency and percentage of column. Hepatorenal syndrome or terlipressin, previous hospital or ICU admission are referred to comorbidities during the month before transplant.
HCC: hepatocellular carcinoma; BMI: body mass index; TIPS: transjugular intrahepatic portosystemic shunt.
A large proportion of the LTRs had comorbidities: diabetes mellitus (28.4%), criteria of hepatorenal syndrome or the need for terlipressin therapy in the last month (13.4%), renal support in any of its modalities at least on one occasion (1.3%), previous supramesocolic surgery (14.7%), admission to hospital in the last month or admission in the moment when organ donation for transplantation was accepted (n=61, 13.0%). Ten patients were in the ICU at the time of transplantation (2.1% of the total).
A total of 49 patients (10.4%) met the criterion of the primary endpoint (mortality in under 90 days after transplant).
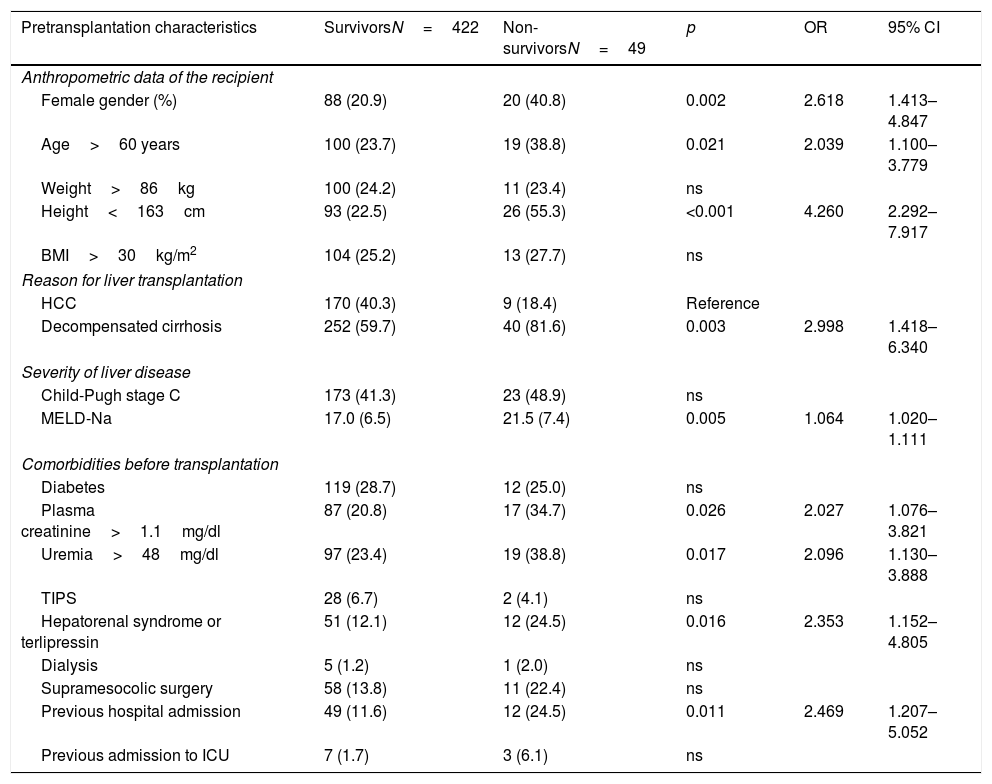
The analysis of the potential mortality risk factors is shown in Table 2. Mortality was greater among women (OR=2.618, 95% CI=1.413–4.847), in LTRs over 60 years of age (OR=2.039, 95% CI=1.100–3.779) and in recipients less than 163cm in height (OR=4.260, 95% CI=2.292–7.917). The interaction between height and gender was not significant (OR=0.908, 95% CI=0.820–1.005). Among the LTRs in which decompensated cirrhosis was the indication for transplantation, early post-transplantation mortality was greater than in the patients that received a liver due to neoplastic disease (HCC) (OR=2.998, 95% CI=1.418–6.340). Mortality was also related to the severity of disease (MELD-Na, OR=1.064 for each point increment, with 95% CI=1.020–1.111), though not only due to alteration of liver function (Child-Pugh grade C, p=ns). The LTRs that died presented higher blood creatinine (OR=2.027, 95% CI=1.076–3.821) and uremia levels (OR=2.096, 95% CI=1.130–3.888)], and a greater frequency of hepatorenal syndrome (OR=2.353, 95% CI=1.152–4.805) and pretransplant dialysis (p=0.615). They also had a greater need for hospital admission in the month before transplantation (OR=2.469, 95% CI=1.207–5.052) or were in the ICU at the time of transplantation (OR=3.866, 95% CI=0.0967–15.467). Diabetes was not identified as an early mortality risk factor (p=ns).
Evaluation of the pretransplantation predictors of early mortality after liver transplant. Bivariate analysis (N=471).
| Pretransplantation characteristics | SurvivorsN=422 | Non-survivorsN=49 | p | OR | 95% CI |
|---|---|---|---|---|---|
| Anthropometric data of the recipient | |||||
| Female gender (%) | 88 (20.9) | 20 (40.8) | 0.002 | 2.618 | 1.413–4.847 |
| Age>60 years | 100 (23.7) | 19 (38.8) | 0.021 | 2.039 | 1.100–3.779 |
| Weight>86kg | 100 (24.2) | 11 (23.4) | ns | ||
| Height<163cm | 93 (22.5) | 26 (55.3) | <0.001 | 4.260 | 2.292–7.917 |
| BMI>30kg/m2 | 104 (25.2) | 13 (27.7) | ns | ||
| Reason for liver transplantation | |||||
| HCC | 170 (40.3) | 9 (18.4) | Reference | ||
| Decompensated cirrhosis | 252 (59.7) | 40 (81.6) | 0.003 | 2.998 | 1.418–6.340 |
| Severity of liver disease | |||||
| Child-Pugh stage C | 173 (41.3) | 23 (48.9) | ns | ||
| MELD-Na | 17.0 (6.5) | 21.5 (7.4) | 0.005 | 1.064 | 1.020–1.111 |
| Comorbidities before transplantation | |||||
| Diabetes | 119 (28.7) | 12 (25.0) | ns | ||
| Plasma creatinine>1.1mg/dl | 87 (20.8) | 17 (34.7) | 0.026 | 2.027 | 1.076–3.821 |
| Uremia>48mg/dl | 97 (23.4) | 19 (38.8) | 0.017 | 2.096 | 1.130–3.888 |
| TIPS | 28 (6.7) | 2 (4.1) | ns | ||
| Hepatorenal syndrome or terlipressin | 51 (12.1) | 12 (24.5) | 0.016 | 2.353 | 1.152–4.805 |
| Dialysis | 5 (1.2) | 1 (2.0) | ns | ||
| Supramesocolic surgery | 58 (13.8) | 11 (22.4) | ns | ||
| Previous hospital admission | 49 (11.6) | 12 (24.5) | 0.011 | 2.469 | 1.207–5.052 |
| Previous admission to ICU | 7 (1.7) | 3 (6.1) | ns | ||
Hepatorenal syndrome or terlipressin, previous hospital or ICU admission are referred to the month before transplant.
With the exception of the MELD-Na score – a quantitative variable expressed as the mean (standard deviation [SD]) – all the variables were categorized according to the cut-off point of the greatest risk quartile, and are described as frequencies and percentages. Hypothesis contrasting was made by simple binary logistic regression analysis, with expression of significance (p-value), odds ratio (OR) and 95% confidence interval (95% CI).
HCC: hepatocellular carcinoma; BMI: body mass index; ns: nonsignificant; TIPS: transjugular intrahepatic portosystemic shunt.
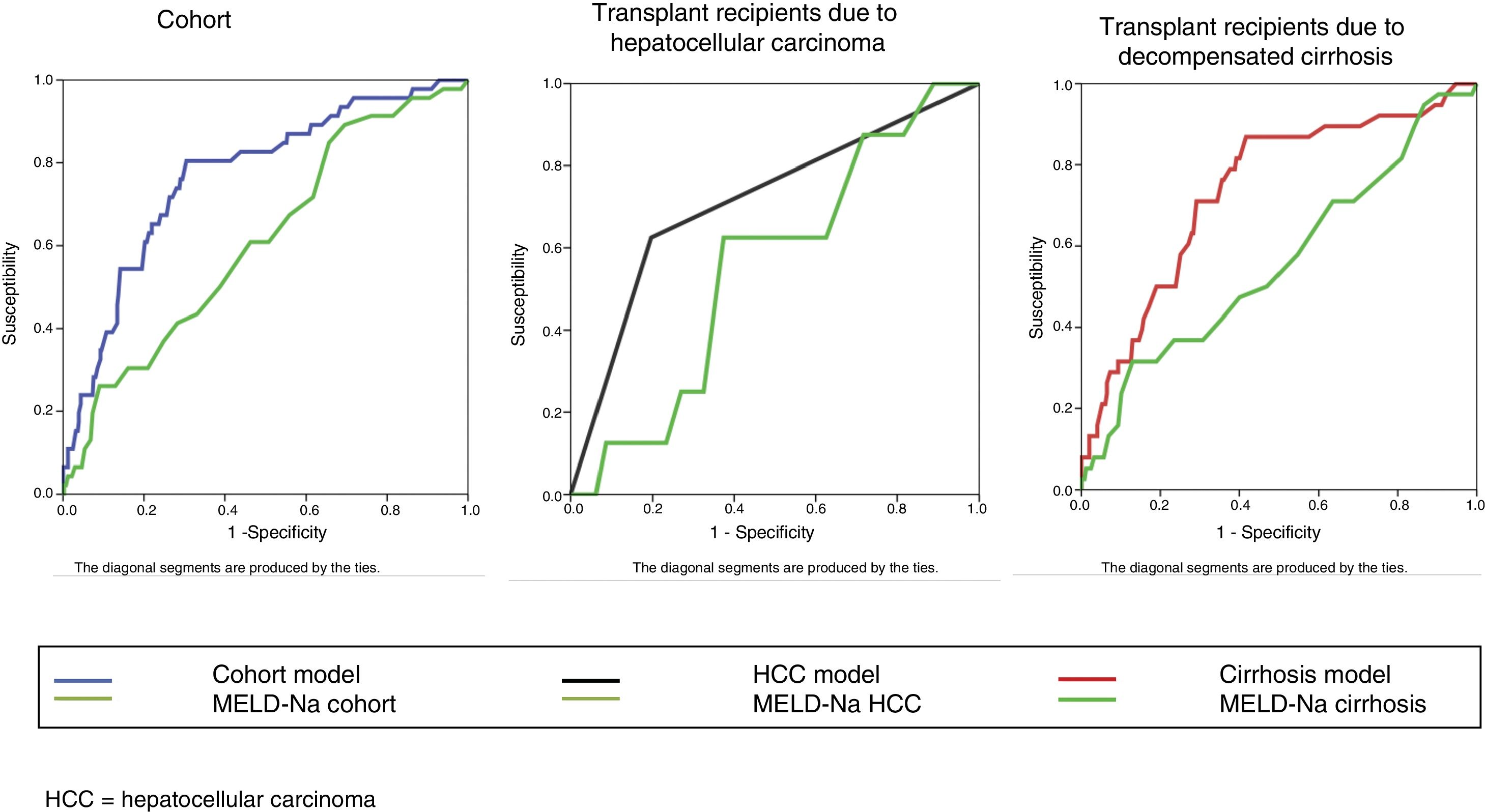
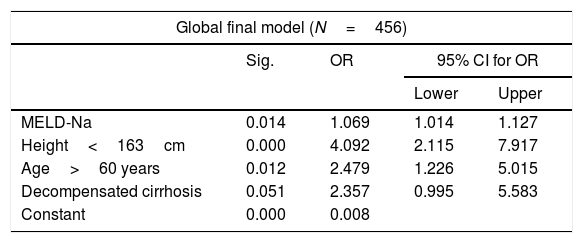
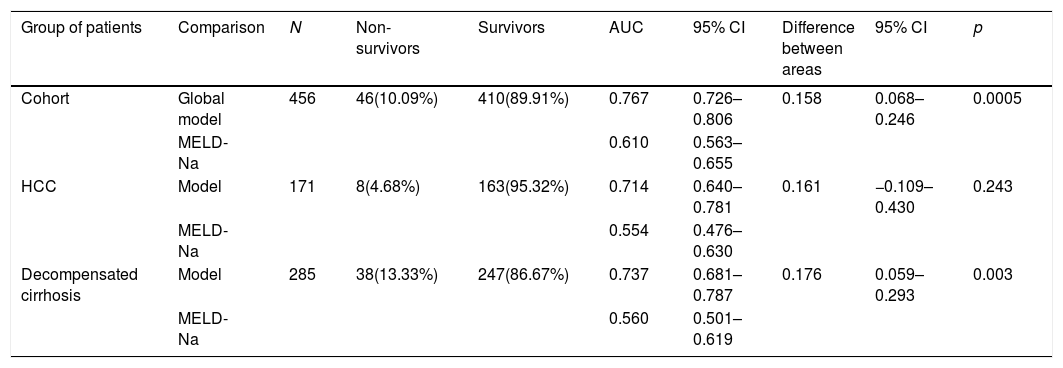
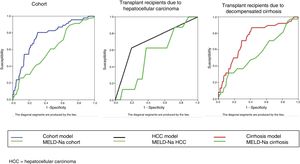
The bivariate analysis identified 10 predictors of early mortality (p≤0.10) that were entered in the multivariate analysis. Creatinine was excluded due to colinearity, with the inclusion of previous surgery due to clinical criterion. This initial model explained between 10.0 and 20.7% of the total variance, with a global percentage correctness of 90.3%. Specificity was very high (99.7%), but sensitivity was very low (6.5%). Backward stepwise logistic regression analysis yielded the final model, which identified three independent predictors of early mortality and a confounding factor (reason for transplant) that resulted in a 27% increase in the regression coefficient β of the MELD-Na score. The factor was therefore included in the final equation (Table 3). This table shows the independent predictors both globally and according to the two reasons for transplant (decompensated cirrhosis and liver tumor disease). The ROC curves of both were obtained and compared with the ROC curve of the MELD-Na (Fig. 2), and the predictive capacity of the final models was compared versus MELD-Na by means of the DeLong test (Table 4) – confirming that the model obtained in the LTRs with decompensated cirrhosis was significantly better in predicting survival than MELD-Na (p=0.003), while the model in LTRs with HCC failed to improve upon the predictive capacity of MELD-Na.
Independent pretransplantation predictors of early mortality after liver transplant. Multivariate logistic regression analysis. Final models.
| Global final model (N=456) | ||||
|---|---|---|---|---|
| Sig. | OR | 95% CI for OR | ||
| Lower | Upper | |||
| MELD-Na | 0.014 | 1.069 | 1.014 | 1.127 |
| Height<163cm | 0.000 | 4.092 | 2.115 | 7.917 |
| Age>60 years | 0.012 | 2.479 | 1.226 | 5.015 |
| Decompensated cirrhosis | 0.051 | 2.357 | 0.995 | 5.583 |
| Constant | 0.000 | 0.008 | ||
| Final model for LTRs due to HCC (N=171) | ||||
|---|---|---|---|---|
| Sig. | OR | 95% CI for OR | ||
| Lower | Upper | |||
| Height<163cm | 0.005 | 7.765 | 1.847 | 32.650 |
| Constant | 0.000 | 0.023 | ||
| Final model for LTRs due to decompensated cirrhosis (N=285) | ||||
|---|---|---|---|---|
| Sig. | OR | 95% CI for OR | ||
| Lower | Upper | |||
| MELD-Na | 0.012 | 1.082 | 1.018 | 1.150 |
| Height<163cm | 0.001 | 3.581 | 1.711 | 7.497 |
| Age>60 years | 0.011 | 2.845 | 1.269 | 6.376 |
| Constant | 0.000 | 0.015 | ||
HCC: hepatocellular carcinoma; 95% CI: 95% confidence interval; MELD-Na: Mayo End-Liver Disease score; OR: odds ratio; LTRs: liver transplant recipients.
Comparison of ROC using the DeLong test.
| Group of patients | Comparison | N | Non-survivors | Survivors | AUC | 95% CI | Difference between areas | 95% CI | p |
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Global model | 456 | 46(10.09%) | 410(89.91%) | 0.767 | 0.726–0.806 | 0.158 | 0.068–0.246 | 0.0005 |
| MELD-Na | 0.610 | 0.563–0.655 | |||||||
| HCC | Model | 171 | 8(4.68%) | 163(95.32%) | 0.714 | 0.640–0.781 | 0.161 | −0.109–0.430 | 0.243 |
| MELD-Na | 0.554 | 0.476–0.630 | |||||||
| Decompensated cirrhosis | Model | 285 | 38(13.33%) | 247(86.67%) | 0.737 | 0.681–0.787 | 0.176 | 0.059–0.293 | 0.003 |
| MELD-Na | 0.560 | 0.501–0.619 |
AUC: area under the curve; HCC: hepatocellular carcinoma; 95% CI: 95% confidence interval; N: sample size; p: probability; %: percentage.
It is known that complications of surgery are the factors with the strongest impact upon early mortality among LTRs,17,19,20 though such complications generally cannot be predicted or controlled before transplantation. We therefore need to establish pretransplant predictors that can help us to assess risk among the transplant candidates on the waiting list and predict mortality after transplantation. Although many studies have been made in this field, the results have been inconclusive.21–25
The results of our study demonstrate the different associations of mortality risk factors and indications of transplantation (HCC versus decompensated cirrhosis), and include the score MELD-Na as an independent predictor of early mortality in LTRs with decompensated cirrhosis.
Our findings also confirm the data from other studies26–28 that identify a patient age of over 60 years as an independent mortality risk factor. However, this predictor is not useful in LTRs with tumor disease, and should only be considered in LTRs subjected to transplantation due to decompensated cirrhosis – this possibly being related to greater comorbidity in these patients.
To the best of our knowledge, this study is the first to identify LTR body height as an independent mortality risk factor. The reason underlying this relationship cannot be established from the data of our study, though it would seem reasonable to associate it to both a greater incidence of surgical complications in patients offering a smaller surgical field and to a greater probability of discrepancies between donor size and liver recipient size in small patients.29 In our study this association was also evidenced by the greater proportion of male donors (60.5%) and higher mortality among women (OR=2.618, 95% CI=1.413–4.847); gender therefore may act as a confounding factor. However, in our series height remained significant after controlling for gender. These results do not coincide with those of other authors30 and should be validated.
Lastly, our study shows that even on considering numerous pretransplantation factors (18 variables), we are unable to significantly differentiate those patients that die after liver grafting, since our models have low sensitivity. This has very important implications for the pretransplant evaluation of possible candidates.
From the clinical perspective, it is reasonable to assume that patients with greater liver dysfunction, greater portal hypertension and greater renal dysfunction (higher MELD-Na score) will have greater perioperative risk. In our patients, each 10-point increment in the MELD-Na score increased mortality by about 10%. However, even accepting the pretransplantation MELD-Na score as an independent predictor of early post-transplantation mortality, its predictive capacity is poor.20,30,31 This is consistent with our own results, since the MELD-Na only explained between 1.7 and 3.5% of the variance in early mortality (R2 of Cox-Snell and Nagelkerke). Consequently, studies made in patients with a MELD score of over 40 points are unable to demonstrate the futility of transplantation in such individuals based only on this predictor.24,32 We therefore could not discard the option of transplantation in patients with a high MELD-Na score based on this parameter alone.
It must be taken into account that in our case the MELD-Na score was obtained from the laboratory test data at the time of patent admission for transplantation, and that – most importantly – it refers to post-transplantation mortality predictive capacity, not to patients on the waiting list without transplantation. For this reason the predictive capacity of the MELD-Na was lower than that published by these authors.33,34 Furthermore, the post-transplantation mortality predictive capacity also decreased on disaggregating the global model (Table 4).
A weakness of our data, evidenced by the results of the study, is that a predictor (body height of the graft recipient) that proved to be so significant from the statistical perspective is usually not measured directly but is reported by the patient. This may represent a source of bias,35 though the problem can be reduced by categorizing recipient body height as a dichotomic variable. In any case, the clinical issue is whether a difference in median height of 8cm between the two groups is clinically relevant or only statistically significant. In order to answer this question, we would have to corroborate our results in other different patient populations or study a validation cohort. This latter option was not possible due to the low frequency of mortality and the consequent small sample size – thus representing a limitation of our study.
Our models are simple, homogeneous in their criteria, and are centered on the patients on the waiting list in the absence of evident contraindications to transplantation (severe heart disease, active sepsis). The offer very high specificity and negative predictive values, but they have very poor sensitivity in differentiating those patients that do not survive transplantation. Other studies that include information on the donor and the surgical process likewise do not show better prediction of mortality.36,37 We therefore feel that the models could be considered by transplant candidate evaluating committees as tools for assessing risk in individualized patients, though always taking into account that mortality predicting capacity based on pretransplantation factors is very poor in individual cases.
In conclusion, the models obtained with the studied pretransplantation factors have very low positive predictive capacity and low discriminating potential (area under the ROC curve [AUROC]<0.8), and are therefore unable to exclude the possibility of transplantation in any of the candidates, since they are not able to reliably differentiate those patients that will die in the early post-transplantation period. However, these predictors may help transplant candidate evaluating committees to assess the mortality risk of the candidates on the basis of the indication of transplantation (HCC or non-HCC), and thus attempt to minimize other risk factors. Patients subjected to liver transplantation due to neoplastic disease (HCC) present greater early mortality risk if they measure under 163cm in height. In turn, the early mortality risk in patients subjected to liver transplantation due to decompensated cirrhosis increases with increasing MELD-Na scores, an age of over 60 years, and a body height of under 163cm – and this model is significantly better in predicting survival than MELD-Na on an isolated basis.
Authorship/collaboratorsJCPL and MRP contributed to the study design and definition of the inclusion and exclusion criteria.
MCMV, JCPL and MRP contributed to the statistical study.
JCPL, FRE, JMT and IDG contributed to data registry.
JCPL was responsible for the manuscript.
All the authors were responsible for final review of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pozo-Laderas JC, Rodríguez-Perálvarez M, Muñoz-Villanueva MC, Rivera-Espinar F, Durban-García I, Muñoz-Trujillo J, et al. Predictores pretrasplante de mortalidad precoz en receptores adultos de trasplante hepático en la era MELD-Na. Med Intensiva. 2019;43:261–269.