Lung transplantation is a therapeutic option for pulmonary diseases in which the other treatment options have failed or in cases of rapid disease progression. However, transplantation is not free from complications, and primary graft dysfunction is one of them. Primary graft dysfunction is a form of acute lung injury. It characteristically develops during the immediate postoperative period, being associated to high morbidity and mortality, and increased risk of bronchiolitis obliterans. Different terms have been used in reference to primary graft dysfunction, leading to a consensus document to clarify the definition in 2005. This consensus document regards primary graft dysfunction as non-cardiogenic pulmonary edema developing within 72h of reperfusion and intrinsically attributable to alteration of the lung parenchyma. A number of studies have attempted to identify risk factors and to establish the underlying physiopathology, with a view to developing potential therapeutic options. Such options include nitric oxide and pulmonary surfactant together with supportive measures such as mechanical ventilation or oxygenation bypass.
El trasplante pulmonar representa una opción terapéutica para procesos pulmonares en los que los tratamientos han fallado o que presenten una evolución rápidamente progresiva. Sin embargo, no está libre de complicaciones, siendo la disfunción primaria del injerto una de ellas. Se trata de una forma de lesión pulmonar aguda, y caracterizada por desarrollarse durante el postoperatorio inmediato, estar asociada a una alta morbi-mortalidad y aumentar el riesgo de bronquiolitis obliterante. Ha presentado diferentes acepciones terminológicas conduciendo a un documento de consenso que precisara su definición en el año 2005. En ese consenso se acordó considerar la disfunción primaria del injerto como edema pulmonar no cardiogénico en las primeras 72 horas de la reperfusión y debido a una alteración del propio parénquima pulmonar. Se han llevado a cabo estudios que tratan de identificar factores de riesgo y de conocer la fisiopatología subyacente para secundariamente desarrollar posibles opciones terapéuticas. Entre las opciones de tratamiento se encuentran el óxido nítrico o el surfactante pulmonar junto con las medidas de soporte como ventilación mecánica o la oxigenación extracórporea.
Lung transplantation is a treatment option in those pulmonary disease processes in which the other therapies have failed, or when the disease progresses rapidly. Nevertheless, transplantation is not without complications, and primary graft dysfunction (PGD) is one of them. PGD is characterized by acute lung damage developing during the immediate postoperative period, and is associated to important patient morbidity–mortality and an increased risk of bronchiolitis obliterans.1–4 PGD is the end result of a series of circumstances involving brain death, graft preservation, graft implantation, and posterior reperfusion. The condition has received a variety of names, reflecting the difficulty of establishing a definition or categorization based on unified and standardized clinical criteria. In this sense, use has been made of terms such as “post-reperfusion edema”, “ischemia-reperfusion syndrome”, “reimplantation response”, “implantation edema” or “early graft dysfunction”.
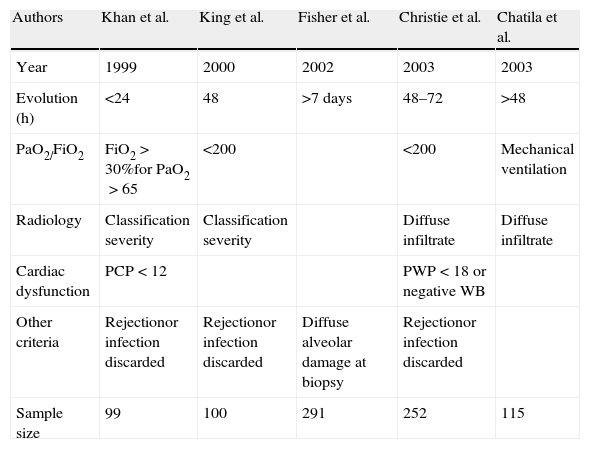
Terminological approachThe literature offers references from quite diverse angles. Khan et al.5 described “pulmonary reimplantation response” as hypoxemia in the context of alveolar condensations after discarding left heart failure, rejection or infection. Christie et al.6–8 in turn referred to radiologically manifest infiltrates and hypoxemia (PaO2/FiO2<200) in the first 24h, in the absence of other possible etiological explanations. Chatila et al.9 analyzed transplant patients subjected to mechanical ventilation for over 48h or who required reintubation, and established a series of homogeneous criteria referred to what they called reperfusion-induced ischemic damage. King et al.10 evaluated transplant recipients with reperfusion damage and established a correlation to pulmonary hypertension but not to ischemia times. Thabut et al.11 in turn established similar criteria of hypoxemia (PaO2/FiO2<200) and radiological infiltrates, after excluding other potential causes (Table 1).
Criteria used to define primary lung graft dysfunction in different studies prior to the consensus conference.
| Authors | Khan et al. | King et al. | Fisher et al. | Christie et al. | Chatila et al. |
| Year | 1999 | 2000 | 2002 | 2003 | 2003 |
| Evolution (h) | <24 | 48 | >7 days | 48–72 | >48 |
| PaO2/FiO2 | FiO2>30%for PaO2>65 | <200 | <200 | Mechanical ventilation | |
| Radiology | Classification severity | Classification severity | Diffuse infiltrate | Diffuse infiltrate | |
| Cardiac dysfunction | PCP<12 | PWP<18 or negative WB | |||
| Other criteria | Rejectionor infection discarded | Rejectionor infection discarded | Diffuse alveolar damage at biopsy | Rejectionor infection discarded | |
| Sample size | 99 | 100 | 291 | 252 | 115 |
WB: water balance; PWP: pulmonary wedge pressure (mmHg)
In view of this terminological diversity, the International Society of Heart and Lung Transplantation (ISHLT) identified the need to unify criteria with a view to standardizing and comparing results. In this context, in the year 2005 the ISHLT drafted a consensus document on the diagnostic criteria of PGD. The mentioned document defined PGD as non-cardiogenic lung edema developing in the first 72h of reperfusion and attributable to alteration of lung parenchyma proper.12 A number of timepoints for evaluation were proposed (6h after the start of graft perfusion, and again after 24, 48 and 72h), with the purpose of defining lung involvement (Table 2). Other possible causes offering an alternative diagnosis must be ruled out in such cases.
Primary graft dysfunction grading recommendations proposed by Christie et al.
| Grade | PaO2/FiO2 | Chest X-rays: lung infiltrates compatible with lung edema |
| 0 | >300 | Absent |
| 1 | >300 | Present |
| 2 | 200–300 | Present |
| 3 | <200 | Present |
PGD is one of the main causes of patient morbidity–mortality in the early stages following lung transplantation. It has been estimated to affect between 10 and 25% of all transplant recipients, and according to the ISHLT is related to 30% of all deaths occurring in the immediate postoperative period. The presence of PGD has been associated to mortality one month after surgery, with figures even exceeding 50% in some series.1,6,8,10,12–15
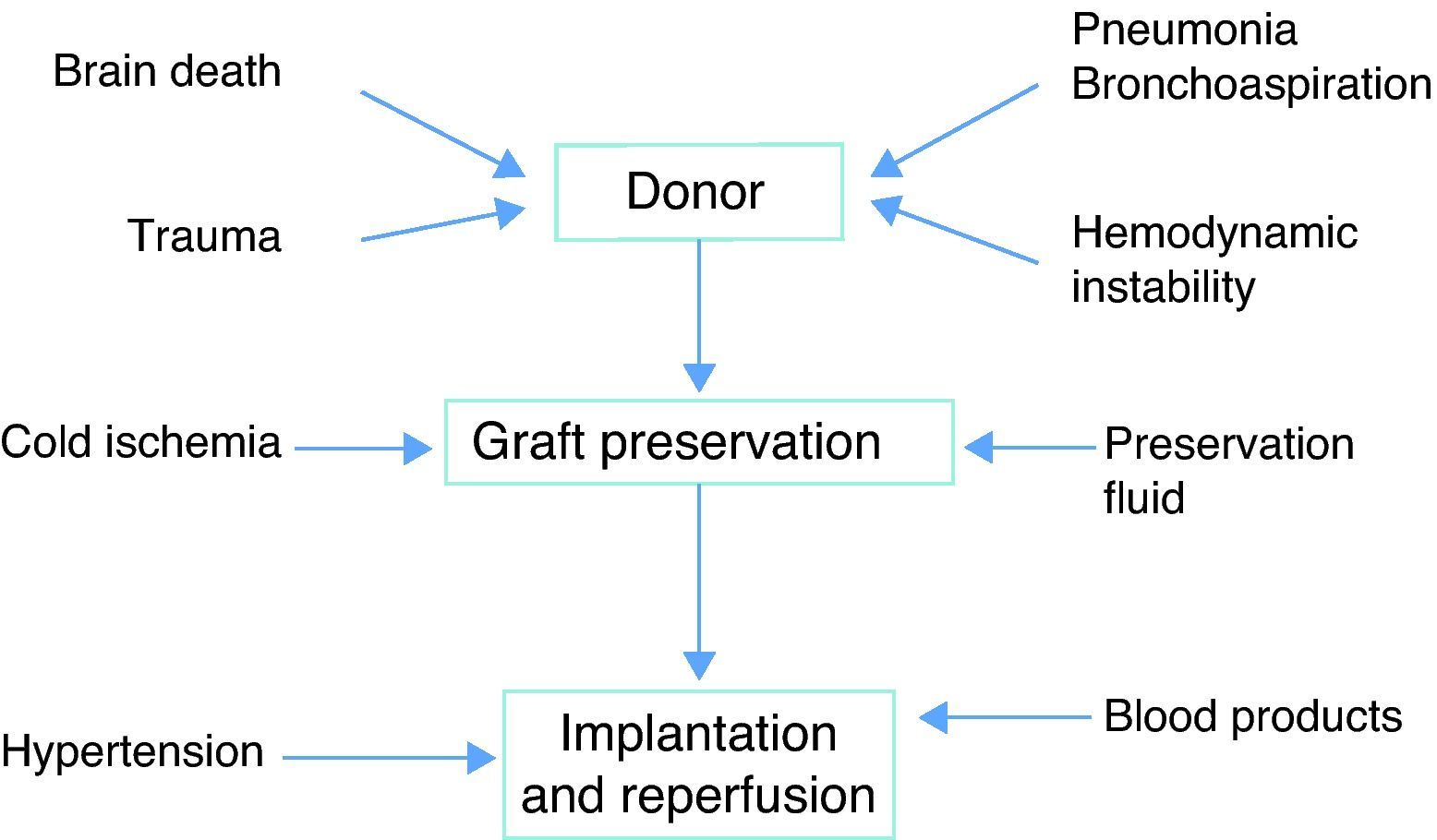
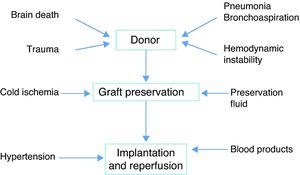
PhysiopathologyThe pathogenesis PGD is multifactorial, though ischemia-reperfusion is the main cause. The triggering event is continuous ischemia-reperfusion with the generation of reactive oxygen species (ROS). The target “organ” in this case is the endothelium, and its alteration gives rise to edema. Underlying ischemia-reperfusion is the activation of an inflammatory response that can be induced by different factors related to both the donor and to the preservation process (Fig. 1).
From an academic perspective, three aspects can be distinguished in offering a physiopathological approach to PGD.
Brain deathAlterations in cell homeostasis take place at the time of brain death, with changes in endocrine function and an intense inflammatory reaction. These alterations in turn reduce graft tolerance of ischemia.
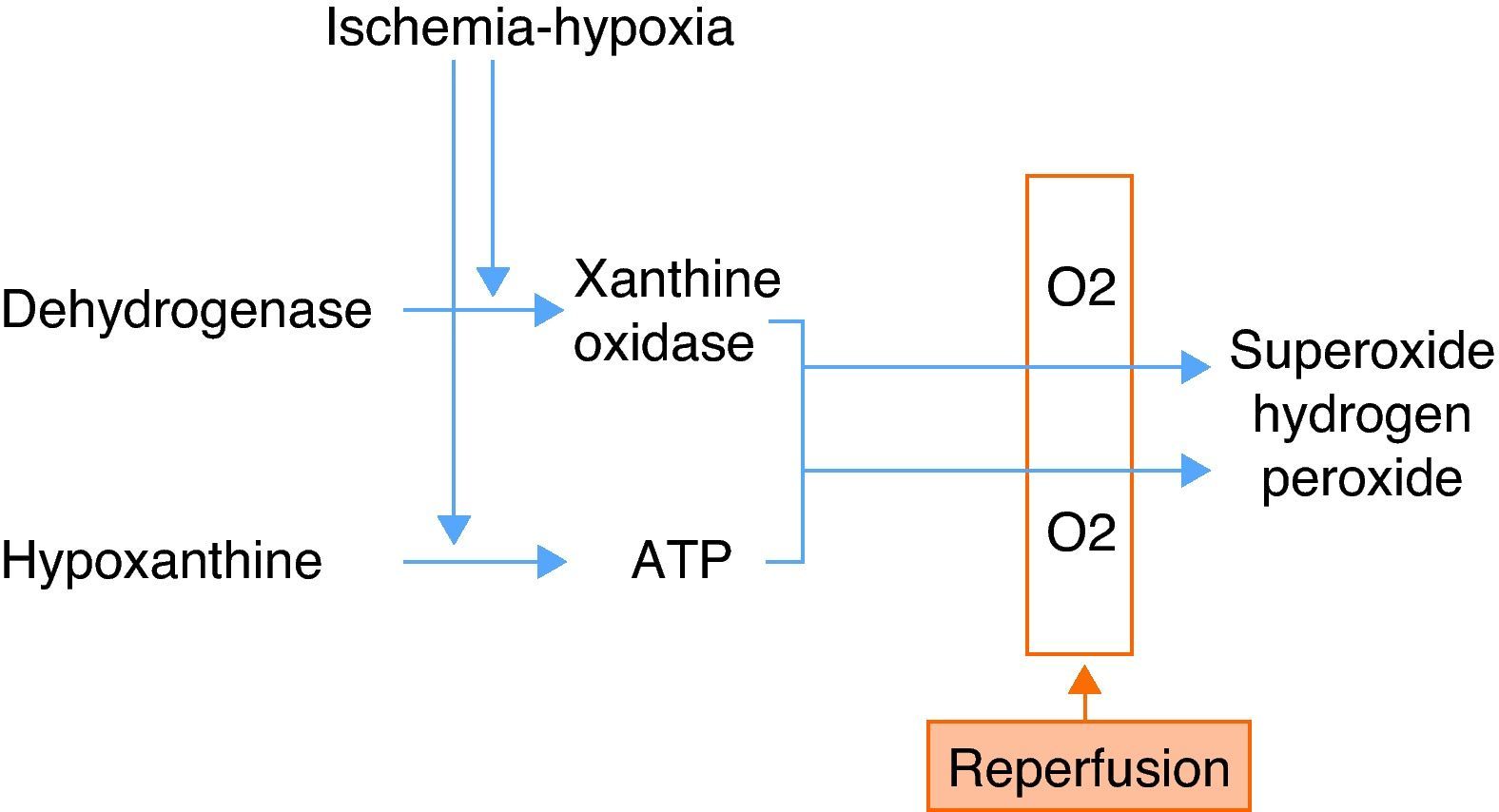
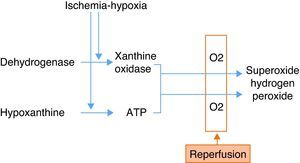
Ischemia-hypoxiaDuring ischemia, the xanthine levels increase as a result of the degradation of ATP molecules. Ischemia in turn activates the macrophages, which release proinflammatory cytokines (IL-8, IL-12, IL-18, TNF-alpha and IFN-gamma). These cytokines activate the T lymphocytes and neutrophils during reperfusion. The activation and accumulation of neutrophils, the release of reactive oxygen species (ROS), and the activation of proteolytic enzymes in turn damage the endothelial cells, producing alterations in their permeability, and edema. The lung surfactant is likewise altered by ischemia and reperfusion, giving rise to alveolar collapse, alteration of the ventilation/perfusion ratio, and decreased oxygenation.
On the other hand, hypoxia depletes the ATP levels, with an increase in hypoxanthine degradation products—this in turn generating superoxide radicals when oxygen is introduced during ventilation–perfusion (Fig. 2).
ReperfusionReperfusion gives rise to neutrophil release, capillary obstruction and the triggering and spread of the inflammatory cascade. The introduction of oxygen generates ROS, as commented above, which results in increased tissue damage. Complement activation likewise increases tissue damage through different pathways.
Recently, some authors have examined the role of autoimmunity in the development of PGD, specifically as regards the existence of humoral immunity in the recipient.16,17 Yoshida et al.18 have found that specific cell immune reactions to type V collagen can have an impact in the development of PGD.
The increase in capillary permeability with alveolo-interstitial edema, the rise in pulmonary vascular resistance, and the loss of lung compliance inevitably lead to the deterioration of ventilation, hypoxia, hypercapnia, and eventually to right heart failure.
Risk factorsAttempts have been made to identify the risk factors associated to the development of primary graft failure.19–22 This field is of great clinical interest, since the identification of those patients most susceptible to graft failure could allow the early adoption of measures designed to minimize its deleterious effects. However, the studies carried out in this sense suffer a series of limitations, since they are not multicentric, involve small sample sizes, and are not homogeneous since they have been carried out in different periods characterized by different lung transplant management protocols.20
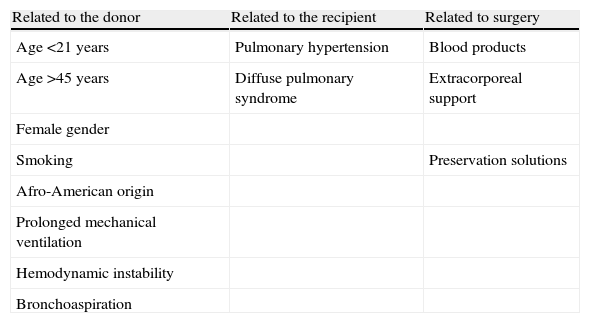
In relation to the donor, there appears to be a greater incidence of PGD in females, Afro-Americans, and in extreme age intervals. Prolonged mechanical ventilation, hemodynamic instability and aspiration pneumonia have also been associated to the development of PGD.21
The risk factors associated to the recipient in turn include pulmonary hypertension and those circumstances which imply increased surgical trauma. The presence of pulmonary hypertension prior to transplant or during the surgical process has been related to the development of PGD.8,20,21
Regarding the surgical technique, different multicenter studies have reported an independent association between the administration of blood products and an increased risk of PGD, though the precise relationship between them is not clear20,23 (Table 3).
Risk factors of primary graft dysfunction.
| Related to the donor | Related to the recipient | Related to surgery |
| Age <21 years | Pulmonary hypertension | Blood products |
| Age >45 years | Diffuse pulmonary syndrome | Extracorporeal support |
| Female gender | ||
| Smoking | Preservation solutions | |
| Afro-American origin | ||
| Prolonged mechanical ventilation | ||
| Hemodynamic instability | ||
| Bronchoaspiration |
The preventive measures are targeted to potentially modifiable factors and focus on optimizing lung preservation, minimizing the ischemia times, and avoiding barotrauma during lung donor maintenance. In this context, the intensivist plays a key role in the management of the lung donor, and must actively seek optimum lung graft preservation. Once brain death has been diagnosed, the administration of corticosteroids (methylprednisolone 15mg/kg) has been suggested, with the purpose of protecting the lung from dysregulation and the surrounding proinflammatory tendencies. Some authors postulate the use of thyroid hormone, as well as the administration of vasoactive drugs on an early basis, to achieve hemodynamic stability.24–26 Fluid management is another important element – the recommendation being to maintain these patients with central venous pressures of <10mmHg and/or an extrapulmonary lung water index (ELWI) of <10ml/kg, in order to protect against fluid loss secondary to pulmonary capillary alteration.27
Following explantation, hypothermia is needed for graft preservation, since it slows the enzymatic and proteolytic activity, but also exerts a deleterious effect upon the tissues. A temperature of 4°C is considered advisable, though experimentally 10°C seems more appropriate, but would require the continuous supply of nutrients due to the correspondingly more active metabolic rate.
Preservation using solutions such as Perfadex has been beneficial, due to the combination of low concentrations of potassium and the presence of dextran. These low potassium levels make it possible to maintain the structural and functional integrity of the endothelial cells, since lesser amounts of pulmonary vasoconstrictors are released and fewer oxidants are produced. Dextran is a macromolecule that avoids platelet aggregation, improves red cell deformability and exerts an antithrombotic effect. These properties improve the pulmonary microcirculation and preserve the endothelial barrier, reducing the extravasation of proteins and water during reperfusion. The use of Perfadex vs other preservation fluids has been associated with a decrease in the incidence of graft dysfunction.28
Lung vasodilators such as the prostaglandins (prostaglandin E1) have been shown to be beneficial, thanks to their immune modulating effects, vasodilatory and antiinflammatory properties, and their effects upon platelet aggregation. They have been used in graft preservation fluid and in the lung graft recipient in both the reperfusion stage and in the immediate postoperative period.29 Randomized studies are needed to establish whether the routine use of such vasodilators during the early postoperative period is able to reduce the deleterious impact of PGD and offer a globally positive effect without systemic repercussions.21
TreatmentOnce graft dysfunction damage has been established, the management strategies are similar to those used in patients with adult respiratory distress syndrome. The aims are to improve oxygenation and hemodynamic support, affording low tidal volumes and optimum PEEP levels, and using FiO2 values as scantly toxic as possible, in order to preserve the parenchyma from ventilation induced damage.
Inhalatory nitric oxide (NO) (10–20ppm) may be beneficial in the treatment of PGD.30–32 Its effects are attributed to improved ventilation, lessened mean pulmonary pressure, and a shortening of mechanical ventilation.33 However, the contradictory results found in the literature, together with the lack of randomized studies, has prevented its use from becoming widespread. Nitric oxide would be indicated in selected cases of severe hypoxemia, accompanied by pulmonary arterial hypertension.34,35
Lung surfactant is presently little used, due to the lack of evidence supporting its efficacy. In this sense, further randomized studies are needed to establish its usefulness.
In very serious cases, involving refractory hypoxia, extracorporeal membrane oxygenation (ECMO) can be used, allowing associated cardiovascular support in the case of hemodynamic instability and cardiac impairment.33,36,37 The venous–venous modality would be preferable, due to the fewer complications involved. However, some studies have found no differences in terms of survival according to the support mode used (venous–venous vs venous-arterial).37 The Toronto group presented a review of 151 lung transplants in which PGD had developed, and which were managed with ECMO.36 The hospital survival rate was 42%. Survival in turn was found to be lower in those patients requiring extracorporeal support for over a week. In our experience, ECMO is useful when the rest of the measures prove insufficient.
Other therapies such as platelet stimulating factor antagonists, captopril, antithrombin III or metalloproteinase inhibitors, the development of modulation therapies with the administration of antioxidants, protease inhibitors, and the depletion of neutrophils are all in the experimental stages.
Re-transplantation may be an option in highly selected cases, in the absence of other types of organ failure, and in very high risk patients accompanied by a poor life expectancy.38
PrognosisPrimary graft dysfunction is responsible for important morbidity–mortality in lung transplant patients.39 Christie et al.,7 in a retrospective series of 255 lung grafts, found the incidence of PGD to be 11.8%. In this subpopulation, the mortality rate after one month reached 63% (vs only 9% in patients who do not develop PGD), both hospital stay and mechanical ventilation were longer, and posterior lung function was diminished. In this context, the classification of the ISHLT has been evaluated to determine whether it may be useful as a predictive factor. In a retrospective study of 402 patients, the T-score after 48h was found to be correlated to increased mortality over the short and long term, and to longer hospital stay, among patients with grade 3 PGD.40 Whitson et al.41 observed a correlation between the severity of PGD and an increased development of bronchiolitis obliterans, in a retrospective study of 374 patients. This finding suggests an association between the development of chronic rejection and increased immune activity favored by the inflammatory response generated by ischemia-reperfusion.42 However, the precise mechanism whereby PGD leads to bronchiolitis obliterans is not known.
The identification and development of markers capable of predicting PGD is one of the future lines of research. Understanding the pathogenesis of PGD is essential for the development of clinical, genetic or biochemical markers.
The study of the inflammatory cascade and of autoimmunity is a pending issue, since it would contribute information on the pathogenesis and would help develop possible therapeutic targets and markers. Given the diverse nature of the pathogenesis of PGD, the use of marker panels is gaining interest.
New concepts in donationGiven the scarcity of lung donors, efforts are being made to optimize the possible donors, extending the criteria for donation, with good results.43 Likewise, non-heart beating donors are becoming increasingly important due to the decrease in optimum brain death donors. This poses new challenges, including the need to overcome technical difficulties referred to preservation and conservation. Concepts such as warm ischemia require definition of the optimum time of such ischemia. The warm ischemia time is the time elapsed from cardiorespiratory arrest to the start of the thoracic infusion of cold fluids. It has been postulated that the tolerable ischemia time for the lung graft is approximately 120min. To date, some studies have attempted to relate PGD to the type of donation involved. In this sense, De Vleeschauwer et al.44 compared 5 lung grafts obtained from non-heart beating donors vs 10 grafts obtained from brain death donors. The former group tended to be associated with shorter mechanical ventilation, a lesser infection rate, and improved lung function tests after 30 days.
In an attempt to limit the damage derived from hypothermic preservation, normothermal ex vivo perfusion techniques are being developed, supplying oxygen and nutrients, with a view to optimizing the management and preservation of the lung graft.45 The growing experience gained and the technical efforts made to improve the preservation techniques will allow us to define and minimize the consequences of warm ischemia.
Primary graft dysfunction is an important cause of morbidity–mortality in the lung transplant patient, with a complex and multifactorial etiopathogenesis, and increases the risk of bronchiolitis obliterans (chronic rejection). Improved understanding is required of the underlying mechanisms, with a view to developing markers and therapeutic targets. The new forms of organ donation and the use of marginal donors make ongoing and further studies of PGD necessary. Likewise, the specification of diagnostic and grading criteria will allow us to conduct quality research capable of establishing the true impact of PGD.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Suárez López VJ, et al. Disfunción primaria del injerto tras el trasplante pulmonar. Med Intensiva. 2012;36:506–12.