Hypoperfusion plays a central role in shock states, and has been proposed as a coagulopathy trigger. The study of the rotational thromboelastometry (ROTEM) profile during cardiac arrest could offer new insights to the role of hypoperfusion in coagulation during shock states.
OutcomeTo describe the ROTEM profile in a cohort of asystole donors and elucidate the incidence of hyperfibrinolysis.
DesignA prospective observational study was carried out in 18 patients consecutively admitted to the ICU after out-of-hospital non-recovered cardiac arrest (CA). Initial rhythm and time between CA and admission were recorded. Conventional coagulation and ROTEM (EXTEM, APTEM, FIBTEM) tests were performed within 30min after blood sample collection.
ScopeAn asystole donor reference hospital.
ParticipantsPatients admitted to the ICU after out-of-hospital non-recovered CA.
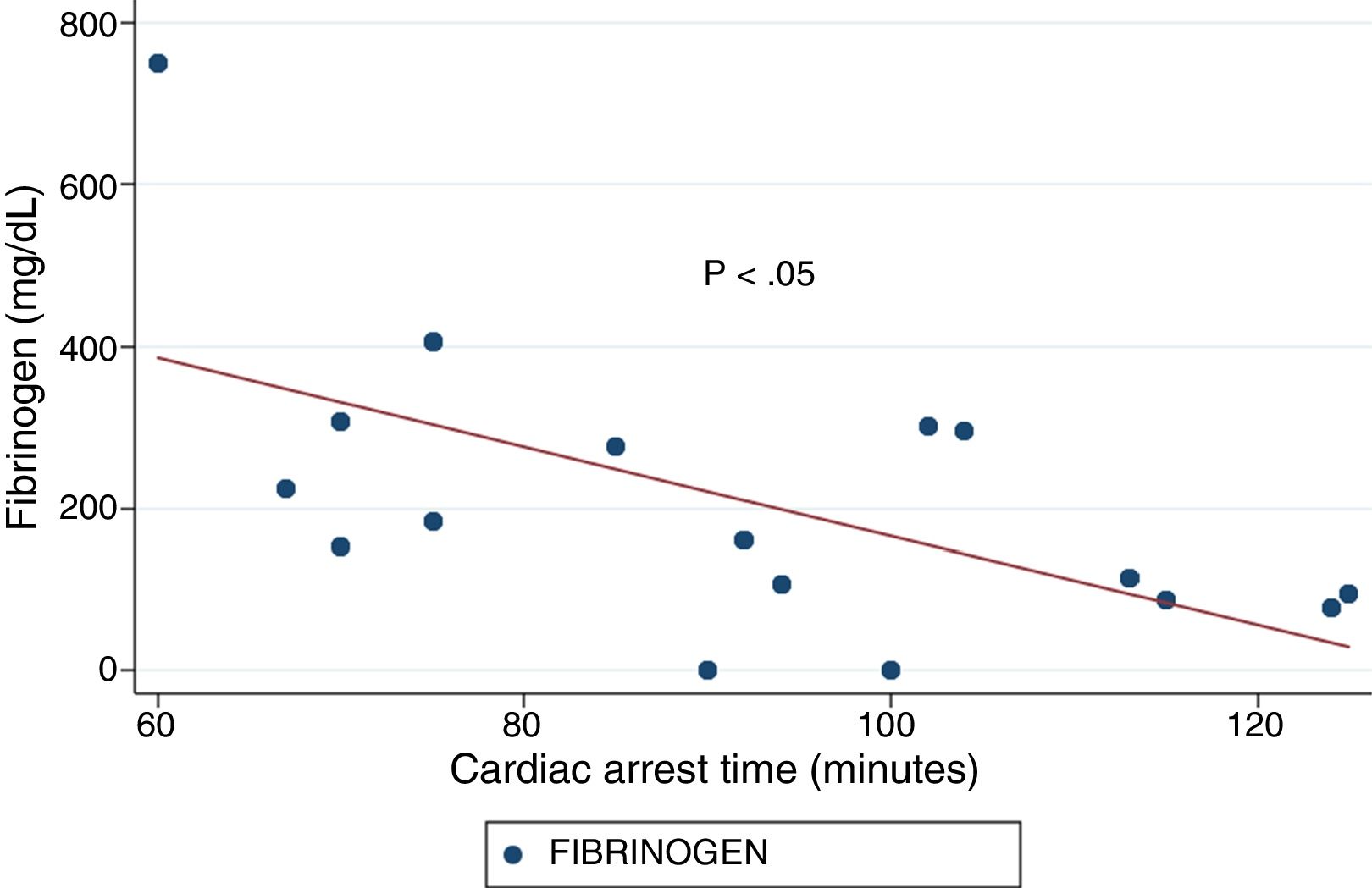
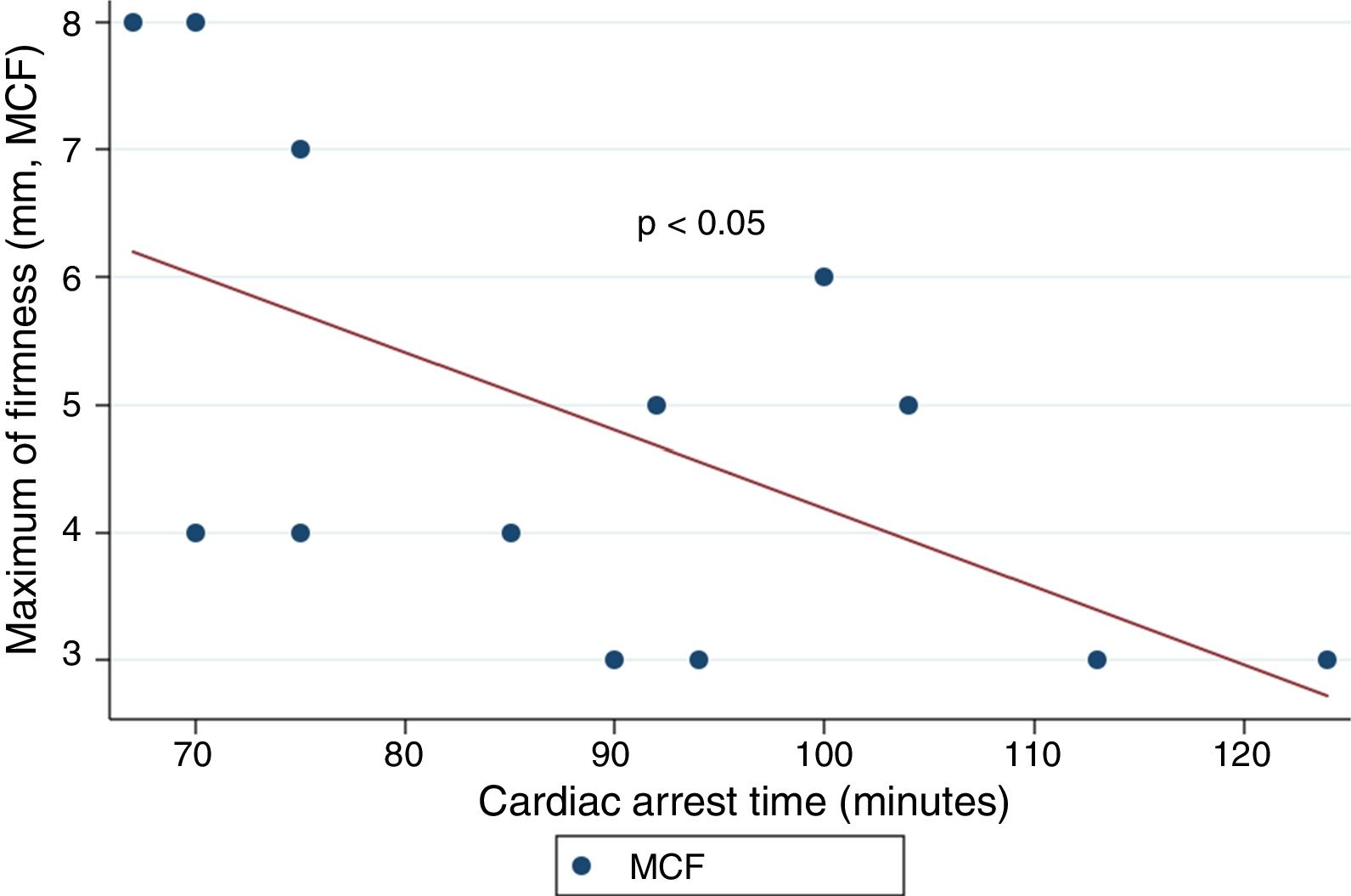
ResultsThe median age was 50years, and 14 of the patients were men (77.8%). The time from CA to hospital admission expressed as the median (interquartile range) was 91min (75–104). The results of the routine tests were: INR 1.25 (1.19–1.34), aPTT 55s (45–73) and fibrinogen 161mg/dL (95–295). For the ROTEM APTEM assay the results were: CT 126s (104–191), CFT 247s (203–694). Hyperfibrinolysis criteria were recorded in 15 patients (83.3%). In addition, MCF improved in APTEM versus EXTEM. Prolonged CA times were associated to lower fibrinogen levels and lower values for MCF FIBTEM (P<.05).
ConclusionsThe ROTEM assays revealed severe alterations of the clot formation parameters and a high incidence of hyperfibrinolysis.
La hipoperfusión juega un papel central en el shock, y es un desencadenante de la coagulopatía. El estudio del perfil ROTEM durante la parada cardíaca prolongada podría ofrecer nuevos conocimientos sobre la fisiopatología de la coagulopatía por shock.
ObjetivoDescribir el perfil de tromboelastometría rotacional en una cohorte de donantes en asistolia y determinar la incidencia de hiperfibrinólisis.
DiseñoCohortes prospectivo. Incluimos 18 pacientes ingresados tras parada cardíaca extrahospitalaria no recuperada. Se recopiló el primer ritmo cardíaco registrado, los tiempos de parada y los de asistencia. Al ingreso se realizaron test de coagulación convencional y ROTEM (EXTEM, APTEM, FIBTEM) en los 30min tras la obtención de la muestra.
ÁmbitoEl estudio se llevó a cabo en un hospital de tercer nivel incluido en un programa de donación en asistolia.
ParticipantesPacientes en parada cardíaca extrahospitalaria no recuperada.
ResultadosLa mediana de edad fue de 50años y 14 de los participantes eran hombres (77.8%). La mediana de tiempo (rango intercuartílico) desde la parada hasta la obtención de muestras fue de 91min (75-104). Los resultados de la coagulación fueron: INR 1,25 (1,19-1,34), TTPA 55s (45-73) y fibrinógeno 161mg/dl (95-295). Los resultados del ROTEM (APTEM): CT 126s (104-191), CFT 247s (203-694). En 15 (83,3%) se cumplió el criterio de hiperfibrinólisis. También se observó mejoría del MCF en APTEM frente a EXTEM. Tiempos más prolongados se asociaron con niveles inferiores de fibrinógeno y un MCF FIBTEM inferior (p<0,05).
ConclusionesEl análisis ROTEM mostró una profunda alteración en la formación del coágulo junto con alta incidencia de hiperfibrinólisis.
Shock from any cause is a common entity that is present in one third of the patients admitted to intensive care units (ICU).1 Although the definition of shock may be a complex one, hypoperfusion and tissue dysfunction are key elements here.2 In sepsis, traumatic disease or cardiogenic shock, hypoperfusion plays an essential role in the onset and persistence of clinical manifestations. Clotting disorders and hypoperfusion-related hyperfibrinolysis have great prognostic significance.3,4 However, other phenomena such as tissue lesions or exposure to toxins are also capable of inducing clotting disorders.5,6 And although it is extremely important, the knowledge we have on the mechanisms that associate hypoperfusion with coagulopathy is limited for a number of reasons. In the first place, it is difficult to isolate the role that hypoperfusion plays with respect to other clotting-capable pathways. The most common thing we see here is the occurrence of several factors such as tissue lesion (trauma), infection (sepsis) or systemic inflammation (pancreatitis). Secondly, it is common that early clotting alterations generate thrombosis in the distal beds, thus contributing and expanding early hypoperfusion (reverse causality).5 Cardiorespiratory arrest (CRA) is the clinical situation that best represents global ischemia–reperfusion.7 Several studies have recently described the incidence of hyperfibrinolysis in these patients by using viscoelastic testing.8,9 The implementation of viscoelastic testing (Tem Innovations GmbH, Munich, Germany) in the clinical practice has allowed us to go deeper in what we know about clotting disorders. We believe that the use of viscoelastic testing can improve our understanding of the underlying mechanisms that correlate clotting and hypoperfusion. Therefore, the goal of this study is to describe clotting alterations using traditional and viscoelastic laboratory modalities (ROTEM) in a cohort of potential donors in uncontrolled asystole (Maastricht type IIa).
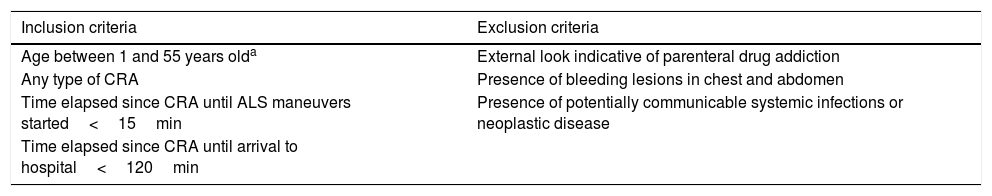
Design and methodsProspective, observational study conducted at Hospital Universitario 12 de Octubre from January 2015 to January 2016. Our hospital ethics committee approved the study (12/160). All the patients from our center considered eligible to enter the program of uncontrolled asystole donation (Maastricht type IIa) were included in this study. The procedure of this donation program is shown in Table 1. The initial out-of-hospital care was provided by our team of specialists in special care. This out-of-hospital care team examined those patients who may be potential donors based on the aforementioned criteria. After confirming the criteria, the hospital service was activated as asystole code and the team proceeded with the transfer. The hospital acceptance of the potential donor was conducted by the Intensive Medicine unit. Upon the patient's arrival, the team confirmed his death, the selection criteria, and the decision to move ahead or terminate the entire process of donation. After making the decision, blood samples were collected for this study. The blood samples were collected after the insertion of one IV cannula and after discarding 2mL of blood. No drugs were administered during hospital care before collecting the blood samples. For conventional clotting study and viscoelastic modality purposes, tubes with citrate (3.2% sodium citrate) were used.
Inclusion and exclusion procedure in the program of uncontrolled asystole donation (UAD).
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age between 1 and 55 years olda | External look indicative of parenteral drug addiction |
| Any type of CRA | Presence of bleeding lesions in chest and abdomen |
| Time elapsed since CRA until ALS maneuvers started<15min | Presence of potentially communicable systemic infections or neoplastic disease |
| Time elapsed since CRA until arrival to hospital<120min |
ALS, advanced life support; CRA, cardiorespiratory arrest; NTO, National Transplant Organization.
The out-of-hospital service report included demographic variables (age and sex), clinical variables (initial heart rhythm) and CRA-related time variables: (a) time elapsed since the cardiac arrest until starting life support; (b) time elapsed since the cardiac arrest until starting advanced life support, and (c) time elapsed since the cardiac arrest until proceeding to collect the blood samples (cardiac arrest time).
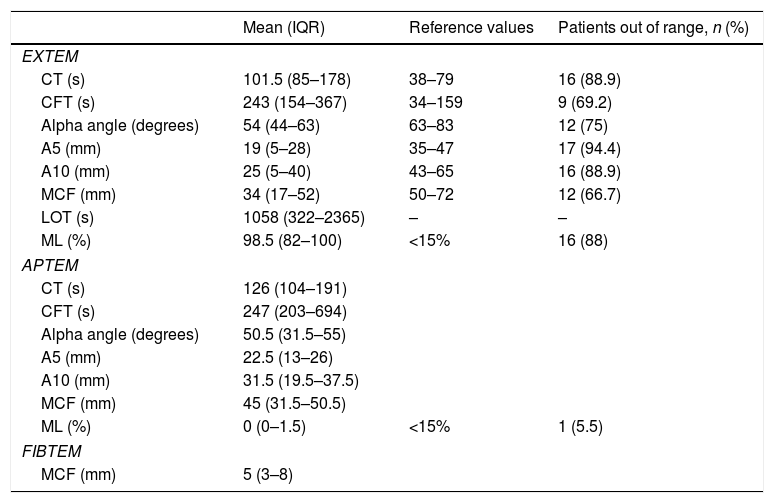
In order to determine the aforementioned parameters, the ROTEM Delta® system was used (Tem Innovations GmbH, Munich, Germany). The reference values already published in the medical literature were used.10 The ROTEM analysis was conducted within the 30min that followed the collection of every blood sample. The EXTEM (ex-tem® recombinant reactive tissue factor activation), APTEM (same activation as EXTEM, fibrinolytic activation with aprotinin) and FIBTEM (same activation as EXTEM, platelet activation with cytochalasin D) tests were run on all the blood samples collected. The following variables were recorded: clotting time (CT, seconds); time elapsed since the initial measurement to the formation of a 2mm-wide blood blot; clot formation time (CFT, seconds), time elapsed since the end of CT until achieving a 20mm relatively firm clot; alpha angle (in degrees), angle between the central line and a tangent to the curve after reaching a 2mm-wide blood blot; maximum clot firmness (MCF, in mm); clot final resistance resulting from the interaction of fibrin, activated platelets, and factor XIII.
The fibrinolytic parameters included are the Lysis Index (LI), the Maximum Lysis (ML) and the Lysis Onset Time (LOT). The ROTEM Delta® software provides such fibrinolytic parameters automatically. Maximum lysis (ML; %) describes the MCF relative reduction due to the lysis of the clot. LOT (seconds) is the time elapsed since the beginning of the clotting reaction until reaching ≥15% lysis of the blood clot. According to the medical literature, hyperfibrinolysis was defined as APTEM maximum lysis (ML)>15%.11,12
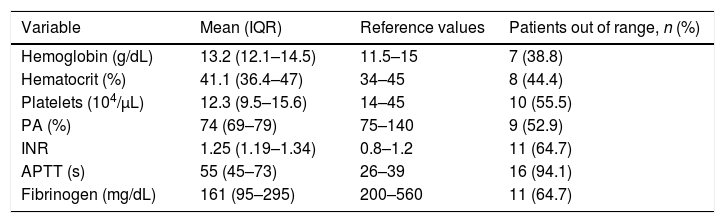
Also, the following traditional clotting tests were run: international normalized ratio (INR); activated partial thromboplastin time (APTT), expressed in seconds, and fibrinogen concentrate, expressed in mg/dL. The reference values provided by the center lab were used.
When it comes to the statistical analysis, the continuous variables were expressed as mean and interquartile range (IQR) unless specified otherwise. The categorical variables were expressed as absolute and relative frequencies. The study of the correlation among the different quantitative variables was conducted using the univariate linear regression analysis. P values=.05 were considered statistically significant. The statistical analysis was conducted using the STATA 12 statistical software package (StataCorp. 2011). The results of this study were disclosed according to the recommendations established for STROBE observational studies.13
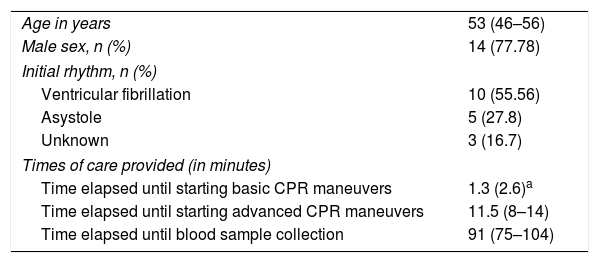
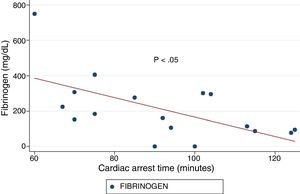
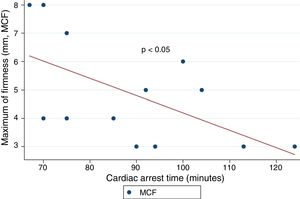
ResultsA total of 18 patients who met the uncontrolled asystole donation criteria of the hospital protocol were included in the study. No patient was excluded during the study period. Fourteen (14) patients were males (77.8%) and the mean age of our sample was 50±8.6years. The first heart rhythm recorded in the out-of-hospital care was ventricular fibrillation in 10cases (55.6%), asystole in 5cases (27.8%), and unknown in 3cases. During the hospital assessment for eligibility in the donation program, all patients showed EKC tracings that were compatible with asystole and met the remaining criteria established. The mean time elapsed from the CRA until the blood sample was collected (cardiac arrest time) was 91min (75–104). The remaining times associated with care are shown in Table 2. In over half the patients, alterations in traditional clotting parameters were seen. APPT alterations were present in almost the entire sample (94.1%) as well as hypofibrinogenemia (Table 3). The detailed results from the ROTEM analysis for the parameters on clot formation and lysis are shown on Table 4. There was an increase in the times of blood clot initiation and formation (APTEM): CT, 126s (104–191); CFT, 247s (203–694). Fifteen (15) patients (83.3%) showed hyperfibrinolysis according to the criteria established. In order to confirm this finding, the results obtained from the EXTEM and APTEM tests were compared to one another. MCF improved in the APTEM test (34mm vs 42mm; mean difference 7.4mm; P<.001). The LOT mean was 1058s (322–2365). The univariate regression analysis studied the correlation between cardiac arrest time and clotting tests. Longer cardiac arrest times were associated with lower levels of fibrinogen at admission (P<.05). There was also an inverse correlation between the duration of the cardiac arrest and MCF in the FIBTEM test (P<.05) (Figs. 1 and 2). The remaining clotting parameters and viscoelastic testing were not statistically significant when it comes to the cardiac arrest times reported.
General characteristics of the sample.
| Age in years | 53 (46–56) |
| Male sex, n (%) | 14 (77.78) |
| Initial rhythm, n (%) | |
| Ventricular fibrillation | 10 (55.56) |
| Asystole | 5 (27.8) |
| Unknown | 3 (16.7) |
| Times of care provided (in minutes) | |
| Time elapsed until starting basic CPR maneuvers | 1.3 (2.6)a |
| Time elapsed until starting advanced CPR maneuvers | 11.5 (8–14) |
| Time elapsed until blood sample collection | 91 (75–104) |
CPR, cardiopulmonary resuscitation.
The quantitative variables are expressed as mean (IQR) unless specified otherwise.
Results from traditional clotting tests.
| Variable | Mean (IQR) | Reference values | Patients out of range, n (%) |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.2 (12.1–14.5) | 11.5–15 | 7 (38.8) |
| Hematocrit (%) | 41.1 (36.4–47) | 34–45 | 8 (44.4) |
| Platelets (104/μL) | 12.3 (9.5–15.6) | 14–45 | 10 (55.5) |
| PA (%) | 74 (69–79) | 75–140 | 9 (52.9) |
| INR | 1.25 (1.19–1.34) | 0.8–1.2 | 11 (64.7) |
| APTT (s) | 55 (45–73) | 26–39 | 16 (94.1) |
| Fibrinogen (mg/dL) | 161 (95–295) | 200–560 | 11 (64.7) |
APTT, activated partial thromboplastin time; INR, international normalized ratio; IQR, interquartile range; PA, prothrombin activity.
Results from the ROTEM analysis.
| Mean (IQR) | Reference values | Patients out of range, n (%) | |
|---|---|---|---|
| EXTEM | |||
| CT (s) | 101.5 (85–178) | 38–79 | 16 (88.9) |
| CFT (s) | 243 (154–367) | 34–159 | 9 (69.2) |
| Alpha angle (degrees) | 54 (44–63) | 63–83 | 12 (75) |
| A5 (mm) | 19 (5–28) | 35–47 | 17 (94.4) |
| A10 (mm) | 25 (5–40) | 43–65 | 16 (88.9) |
| MCF (mm) | 34 (17–52) | 50–72 | 12 (66.7) |
| LOT (s) | 1058 (322–2365) | – | – |
| ML (%) | 98.5 (82–100) | <15% | 16 (88) |
| APTEM | |||
| CT (s) | 126 (104–191) | ||
| CFT (s) | 247 (203–694) | ||
| Alpha angle (degrees) | 50.5 (31.5–55) | ||
| A5 (mm) | 22.5 (13–26) | ||
| A10 (mm) | 31.5 (19.5–37.5) | ||
| MCF (mm) | 45 (31.5–50.5) | ||
| ML (%) | 0 (0–1.5) | <15% | 1 (5.5) |
| FIBTEM | |||
| MCF (mm) | 5 (3–8) | ||
CFT, clot formation time; CT, clotting time; IQR, interquartile range; LOT, lysis onset time; MCF, maximum clot firmness; ML, maximum lysis.
This study describes the ROTEM profile of a group of patients in uncontrolled asystole. As far as the authors know, the ROTEM profile has never been determined in a cohort of patients with these characteristics and so long cardiac arrest times. The patients included in the study revealed severe clotting data in the traditional clotting tests performed and in the ROTEM system. We should mention here that the incidence of hyperfibrinolysis (APTEM maximum lysis>15%) was far more superior than previously thought.
It has been over 40 years since CRA-related coagulopathy was first described and still few studies include viscoelastic testing.14,15 In a series of 30pacientes with out-of-hospital cardiac arrest, the cardiac arrest mean times were 27min and 53% of the patients had hyperfibrinolysis.8 In a new study, both the APTT and CT (EXTEM) alterations were associated with poor prognoses. In this study, 35.8% of the patients had hyperfibrinolysis and cardiac arrest times of 21min.9 The patients included in our study showed severe coagulopathy patterns both in the traditional tests performed and in the viscoelastic testing run. Traditional clotting levels were out of the range of normalcy in over half of the patients together with decreased fibrinogen levels. Also, the incidence of hyperfibrinolysis was higher to the one described by the medical literature (83%) and cardiac arrest times were longer (91min). We find some relevant differences in our study compared to former studies. The goal of these former studies was to validate the no-reflow hypothesis and the effect of fibrinolytic drugs in patients who suffer cardiac arrests.16 Newer studies have confirmed the existence of predictive factors of spontaneous circulation recovery through the analysis of viscoelastic testing.8,9 This study describes the viscoelastic pattern of a given subgroup of patients with unrecovered cardiac arrest (donors in asystole). Thus, the times of cardiac arrest and ischemia are much longer compared to the ones already published by the medical literature. Secondly, but also having to do with what we just talked about, the incidence of hyperfibrinolysis was higher compared to other series (83% vs 53 and 35%). Except for three (3) patients, all of them showed hyperfibrinolysis. The improved MCF and lysis correction in the APTEM test confirm these results. Also, we saw statistically significant associations among the cardiac arrest times, the levels of fibrinogen, and MCF in the FIBTEM test. We attribute the higher incidence of hyperfibrinolysis to the longer cardiac arrest times of our sample. This may actually reinforce the hypoperfusion hypothesis after seeing a dose–response effect between the degree of hypoperfusion (cardiac arrest time) and the incidence of coagulopathy and hyperfibrinolysis.
Hypoperfusion is one of the leading mechanisms involved in the development of coagulopathy and hyperfibrinolysis.3 The increased activated protein C (APC) levels in this setting can lead to the deterioration of certain clotting factors (Va, VIIa) and induce fibrinolysis.4,11,17 Increased levels of t-PA (tissue plasminogen activator) and reduced levels of PAI-1 (plasminogen activator inhibitor-1) during the first phases of a CRA have been reported here.18 Sustaining this situation in time may explain the incidence of hyperfibrinolysis reported.
Recently, the use of the ROTEM pattern has been proposed as a tool for prognostic assessment during a CRA. Apart from the obvious clinical interest, here we should ask ourselves whether the alterations observed in the viscoelastic testing are but a reflection of sustained hypoperfusion or they are capable of perpetuating CRA in some cases.19 Nevertheless, we should mention here that in studies like this it is difficult to isolate the effect of hypoperfusion from other factors such as exposure to catecholamines, tissue lesion or endothelial response to a combination of both.20,21
Our study has some limitations. In this study, cardiac arrest is proposed as a hypoperfusion model although this should be explained in detailed. In the first place, the cardiac massage during the cardiac arrest can cause chest trauma, meaning that our results may not be attributable to hypoperfusion only.22 Secondly, we never knew what the D-dimer levels were in the patients included in the study. The assessment of these levels may have completed the findings reported and their association with hyperfibrinolysis. Finally, this was a single-center study with a limited number of asystole patients. Coagulopathy is one dynamic process where several phenomena coexist such as ischemia–reperfusion, hyperfibrinolysis, and fibrinolysis-related cardiac arrest (fibrinolytic shutdown). Our study only included patients with sustained hypoperfusion (asystole), so we cannot not provide any information on these other phenomena.
This study tried to look into the underlying mechanisms of hypoperfusion and coagulopathy. With the data collected in this study we cannot draw final conclusions for their implementation in the routine clinical practice, but they can be hypothesis-generating in this area of knowledge.
FundingNone whatsoever.
Author's contributionOriginal idea: M. Chico-Fernández, L.J. Terceros-Almanza, C. García-Fuentes.
Data mining: M. Valiente-Fernández, A. Rodríguez-Biendicho, I.J. Prieto del Protillo, C. Mudarra-Reche, S. Bermejo-Aznárez.
Data analysis: L.J. Terceros-Almanza, J.A. Barea-Mendoza, C. García-Fuentes.
Interpretation of results: J.A. Barea-Mendoza, L.J. Terceros-Almanza, C. García-Fuentes, M. Chico-Fernández.
Writing of the manuscript: J.A. Barea-Mendoza, L.J. Terceros-Almanza, M. Chico-Fernández.
Review of final manuscript: All authors.
Conflicts of interestThe authors declare no conflicts of interest whatsoever.
Please cite this article as: Barea-Mendoza JA, Terceros-Almanza LJ, García-Fuentes C, Bermejo-Aznárez S, Prieto del Portillo IJ, Mudarra-Reche C, et al. Perfil de tromboelastometría rotacional (ROTEM) en una cohorte de asistolia no controlada. Med Intensiva. 2019;43:410–415.