The use of extracorporeal techniques in cardiopulmonary support has spread in the last 20 years. extracorporeal membrane oxygenation (ECMO) devices are the most commonly employed option, and have been used for years in lung transplant programs. Nevertheless, few articles on the results of ECMO involving large numbers of cases have been published to date. The use of ECMO in respiratory failure affords immediate oxygen support in patients with severe hypoxia and/or acidosis, and moreover provides pulmonary protection, since it allows an instantaneous decrease in the ventilator pressure and FiO2 needs. The complications of ECMO have been minimized thanks to the technological improvements found in the latest devices, though renal failure, infections, bleeding, and vascular and mechanical complications are still reported in many studies. At present there is less controversy regarding the use of cardiorespiratory assists with ECMO as an alternative in decompensated patients who are on the waiting list, referred to the intra- and postoperative periods of lung transplantation.
El uso de tecnologías extracorpóreas en el soporte cardiopulmonar se ha extendido en los últimos 20 años. Los dispositivos oxigenador de membrana extracorpóreo (ECMO) son los más utilizados y se emplean desde hace años en los programas de trasplante pulmonar. Sin embargo, hay pocos artículos con series amplias de resultados de ECMO. El empleo del ECMO en el fallo respiratorio, además de otorgar un soporte inmediato de oxígeno en pacientes severamente hipoxémicos y/o acidóticos, proporciona protección pulmonar ya que permite la disminución instantánea en las necesidades de presiones y FiO2 en el ventilador. Las complicaciones del uso de ECMO han ido minimizándose con las mejoras técnicas que presentan los últimos modelos de las asistencias, pero la insuficiencia renal, las infecciones, hemorragias y las complicaciones vasculares y mecánicas siguen siendo referidas en numerosos trabajos. Existen actualmente menos controversias en el empleo de la asistencia cardiorrespiratoria con ECMO como alternativa para pacientes descompensados que están en lista de espera, para el intraoperatorio y postoperatorio del trasplante pulmonar.
Lung transplantation (LT) is a management option in non-malignant end-stage pulmonary disease including alterations of the airway, lung parenchyma and pulmonary circulation. The number of LT procedures is gradually increasing both in Spain and in other countries.1 However, difficulties and complications are found that require the adoption of special measures in order to achieve better perioperative results. In this context, the number of patients on the transplant waiting list is growing due to a lack of organs; as a result, mortality among patients on the waiting list is significant–hence the concern about increasing the number of potential donations by means of different methods.2–4 Based on the data offered by the international LT registry, the mortality rate among patients on the waiting list is 20% during the first year and 40% beyond the second year. Another important aspect to be taken into account is the fact that about 10% of all LTs performed each year are carried out on an emergency basis–some patients requiring pretransplant mechanical ventilation that gives rise to different problems such as barotrauma, volutrauma, a high risk of infection and myopathy, which in turn increase morbidity and mortality.5–7 Furthermore, despite the great advances made in perioperative patient management, primary graft dysfunction and cardiovascular alterations continue to represent the main causes of mortality in the immediate postoperative period.1
Artificial devices offer pulmonary and circulatory support that contributes to lessen morbidity–mortality among selected patients during the different phases of the perioperative period. The present study offers a review of the literature on the utilization of extracorporeal membrane oxygenation (ECMO) devices in the perioperative period of patients subjected to LT.
The use of extracorporeal technologies in cardiopulmonary support has increased over the last 20 years. ECMO devices are the most widely used option and have been used for years in LT programs. However, few large series offering results referred to the use of ECMO in these patients have been published to date. Indeed, even the Extracorporeal Life Support Organization (ELSO) presents a limited series, since it only reports 151 cases with a patient survival rate of 42%.8
ECMO makes use of a pump and an oxygenator to provide prolonged hemodynamic and/or respiratory support–its indications being cardiogenic shock and severe respiratory failure refractory to optimum conventional treatment.
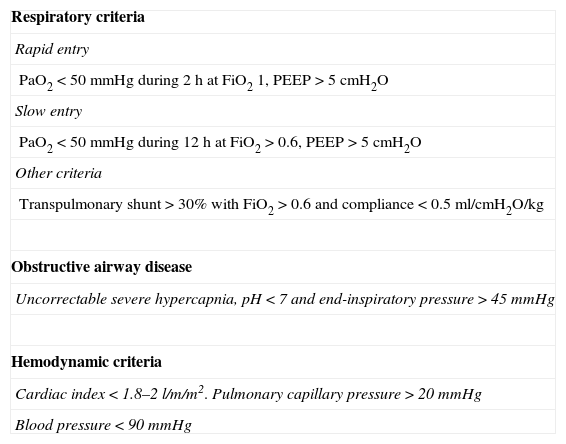
Depending on the intended purpose, we can choose venovenous ECMO (ECMO VV) for respiratory failure and/or venoarterial ECMO (ECMO VA) when moreover there is also hemodynamic impairment. These systems can be used for variable periods of time ranging from days to weeks, though some studies have already reported the results of even longer periods of use.9,10 The criteria employed by our group for using ECMO as respiratory and/or hemodynamic support in patients who fail to respond to usual treatment are specified in Table 1.
Extracorporeal membrane oxygenation entry criteria.
| Respiratory criteria |
| Rapid entry |
| PaO2<50mmHg during 2h at FiO2 1, PEEP>5cmH2O |
| Slow entry |
| PaO2<50mmHg during 12h at FiO2>0.6, PEEP>5cmH2O |
| Other criteria |
| Transpulmonary shunt>30% with FiO2>0.6 and compliance<0.5ml/cmH2O/kg |
| Obstructive airway disease |
| Uncorrectable severe hypercapnia, pH<7 and end-inspiratory pressure>45mmHg |
| Hemodynamic criteria |
| Cardiac index<1.8–2l/m/m2. Pulmonary capillary pressure>20mmHg |
| Blood pressure<90mmHg |
In addition to providing immediate oxygen support in severely hypoxemic and/or acidotic patients, ECMO in respiratory failure affords lung protection, since it allows the use of less damaging mechanical ventilation strategies based on the reduction of airway pressures and the concentration of supplied oxygen. ECMO devices should by applied on an early basis in order to avoid serious worsening of the clinical condition of the patient and to secure greater chances for reverting the lung and multiorgan damage.6 The Minnesota group has published its experience with patients presenting early graft dysfunction, introducing ECMO in the first 24h. Another group of patients with later graft dysfunction required ECMO from the seventh day. The survival rate in the first group was 56%, while none of the patients in the second group were able to survive.11
At present, ECMO use in LT is accepted in three stages of the perioperative period: as a bridge to transplantation, during surgery, and in the postoperative period.
Extracorporeal membrane oxygenation as a bridge to lung transplantationThe use of ECMO in patients on the waiting list is subject to controversy. Some centers consider ECMO to be a contraindication to LT. However, in the light of the recent technological advances, some authors estimate that a relative safety period in this stage of 4–6 weeks of ECMO would be reasonable until a lung donor is found.12
In the year 2012, the Vienna group13 published 38 cases of ECMO used as a bridge to LT. Four patients died before transplantation proved possible, and the rest of the patients could be operated upon. Of these, 8 died in hospital after a mean stay of 24.5 days (range 1–180 days). The survival rate after 1, 3 and 5 years among all the transplanted patients were 60%, 60% and 48%, respectively.
In 2007, Aigner et al. also published two cases of ECMO VA as a bridge to LT in patients with decompensation due to cystic fibrosis.14 One patient died 2 months after surgery due to sepsis, while the other showed prolonged survival.
Although different studies have reported the survival rate of patients subjected to ECMO to be 40% after 12 months, in recent years there have been important advances in the technical aspects of ECMO that have made it possible to obtain better results. The use of polymethylpentene (PMP) oxygenators and of new centrifuge pumps has led to a decrease in failures and in technical problems during handling of the system. Accordingly, the blood transfusion and blood product requirements have decreased considerably, thereby improving gas exchange, reducing the frequency of oxygenator failure, and improving the safety of the device.15–17 In addition, the incorporation of a heat exchanger and the lesser priming volumes of ECMO undoubtedly have contributed to improve the results.
Recent studies have reported high survival rates in the preoperative period. In this context, Hämmäinen et al.18 used both ECMO VV and ECMO VA in 16 patients. Of these subjects, three died with ECMO while waiting for a donor, another patient died 82 days after transplantation, and the survival rate after 1 year in the rest of the transplanted patients was 92%–with a mean duration of ECMO of 16.8±19.2 days.
The technological advances are also leading to experience in the utilization of ECMO in patients on the waiting list and maintaining spontaneous respiration without the need for mechanical ventilation, sedation and immobilization,19–21 thereby even allowing the patients to undergo muscle rehabilitation and to thus face surgery under better clinical conditions. Likewise, the use of ECMO is being incorporated in patients on the LT waiting list who present severe clinical deterioration even in centers located at a considerable distance from the transplanting center–with very encouraging results.22
Patients with idiopathic pulmonary arterial hypertension (PHT) present a high mortality risk (20–30%) while on the waiting list.23–25 Many of these patients present right ventricle failure requiring improvement of the hemodynamic conditions and of the dysfunction of other organs (e.g., liver, kidney). The Ontario group has published its experience from 2006 to 2010 with this group of patients requiring extracorporeal support while on the waiting list. Their results show ECMO to be an excellent choice as a bridge to LT, making it possible to reduce mortality during this period, and with no increase in postoperative mortality or increased risk of graft dysfunction.26 Regarding the middle-term survival of the patients requiring ECMO as a bridge to transplantation, a number of studies have reported survival rates of 74% and 65% after 1 and 3 years, respectively, with no differences in lung function beyond 1 year of follow-up, since the respiratory function test results (FVC and FEV1) were similar to those of patients who had not required ECMO in the pretransplantation period.27
Extracorporeal membrane oxygenation during surgeryIntraoperatively, most groups continue to use extracorporeal circulation in the presence of important respiratory and/or hemodynamic alterations. However, other authors recommend the use of ECMO as a prophylactic measure in cases of severe pulmonary hypertension. Thus, Pereszlenyi et al.28 published their experience with 17 patients diagnosed with severe pulmonary hypertension subjected to ECMO in the intra- and postoperative period. In most patients the assist was implanted following anesthetic induction. In three cases the device could be removed after the intervention, while in 14 patients ECMO was suspended 12h after the operation. The mean stay in Critical Care was 12 days. The final results referred to survival were two deaths in the postoperative period (days 7 and 140), while the rest of the patients were still alive after one and a half years of follow-up.
The same group14 has published its experience from 2001 to 2006, with a total of 306 lung transplants. Of these patients, 147 received ECMO, and in 130 patients the device was implanted intraoperatively. The results showed a survival rate of about 74% in the ECMO group, which was not significantly greater than that recorded in the group requiring cardiopulmonary bypass.
Likewise, Ling-feng et al.29 recommend the use of ECMO instead of cardiopulmonary bypass, since it is associated with a lesser risk of bleeding, lesser primary graft dysfunction, and a lesser inflammatory response.
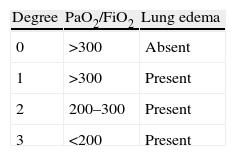
Extracorporeal membrane oxygenation in the postoperative periodECMO plays an important role in the postoperative period of LT. The presence of complications such as primary graft dysfunction (PGD), hyperacute rejection, and hemodynamic alterations are the main reasons for introducing ECMO. The latter is considered adequate for providing cardiorespiratory support in patients with pulmonary and cardiac dysfunction after transplantation, and once the conventional management options are found to be ineffective. PGD is defined by the presence of non-cardiogenic, reperfusion lung edema secondary to lung parenchymal alterations accompanied by an increase in pulmonary vascular resistances, a decrease in compliance, increased capillary permeability, alveolar-interstitial edema, and oxygenation alterations. If not quickly and adequately treated, the persistent hypoxemia can give rise to hemodynamic instability and multiorgan failure. Severe cases of dysfunction are usually accompanied by important hemodynamic alterations due to right ventricle failure that worsen the prognosis.6 Ischemia-reperfusion damage is the main determinant of PGD, though some data suggest that many other factors such as prolonged ischemia time, preservation quality, the need for abundant blood transfusions, prolonged bypass, etc., can exacerbate or induce PGD. Different degrees of dysfunction intensity can be observed (Table 2). Severe PGD is recorded in 2–4% of the patients after the operation, and ECMO is used either to allow recovery from organ damage or as a bridge to retransplantation.14,30
According to the International Society for Heart and Lung Transplantation, lung graft failure is responsible for one-third of the postoperative (30 days) mortality and for 15% of the deaths occurring in the first 3 months in LT patients31–though it also produces an important increase in postoperative morbidity.
Few studies have been published on the use of ECMO in situations of PGD.32 Undoubtedly, the reasons for such a low incidence in the use of ECMO after LT can be explained by: (a) improvements in preservation strategies, using fluids and added substances that avoid the presence and formation of free radicals–avoiding greater lung injury; (b) prospective availability of the cross-matching results (T and B cells); (c) the increasingly lesser use of intraoperative cardiopulmonary bypass; and (d) improvement in the mechanical ventilation techniques, using pulmonary protection measures, and with the increasingly widespread use of differential ventilation in cases of unilateral graft dysfunction.27
Primary pulmonary hypertension, chronic obstructive pulmonary disease and retransplantation are associated with an increased risk of PGD and the need for ECMO.33
Hartwig et al. published30 their results after the use of ECMO VV versus VA due to post-LT PGD. After 30 days the survival rate was 88% in the group of patients in which ECMO VV had been used. The authors found the complications to be greater, and survival lower (6%), in the patients requiring ECMO VA. The advantages of venovenous cannulation versus venoarterial cannulation have been described in many studies.30,34,35
It is interesting to know the long-term results referred to graft functionality and survival among patients who required ECMO because of PGD. Dahlberg et al.32 reported an acceptable middle-term survival rate in 16 patients treated with ECMO due to PGD after LT. The survival rate after 2 years was 46% versus 69% among the 172 patients who did not require ECMO. In this same study the authors found LT graft function as reflected by FEV1 to be 59±13% of the theoretical value in the ECMO group after 1 year, versus 60±15% of the theoretical value after 2 years.
The group supervised by Hartwig et al.36 has published its experience in patients requiring ECMO VV following transplantation because of PGD. The survival rates after one and 5 years were 64% and 49%, respectively. A total of 88% of the survivors who required ECMO remained free of bronchiolitis obliterans after 3 years, though graft function was considerably better in the group of patients who did not need ECMO in the postoperative period (FEV1 58% in the ECMO group versus 83% in the non-ECMO group).
The Pittsburgh group37 in turn has published its long-term results (15 years) referred to graft quality and survival in patients subjected to ECMO VV or ECMO VA. Although the mortality rate was higher among the patients requiring ECMO because of PGD in comparison with the patients who did not need such support, the authors also found that the survivors had graft functionality results similar to those of the patients who did not require ECMO. Likewise, they found no significant differences between the assist techniques used (ECMO VV versus ECMO VA).
The Wisconsin group38 also underscores the importance of using ECMO in PGD, with survival rates after 1 month, 1 year and 3 years of 74.6%, 54% and 36%, respectively.
Another of the early complications in LT is hyperacute rejection, which is produced by an antibody-mediated immune response to the graft. These antibodies are targeted to the HLA antigen of the donor. Hyperacute rejection is characterized by: (a) the development of lung edema and bleeding immediately after transplantation; (b) the presence of antibodies against antigens of the major histocompatibility complex (HLA I and II); (c) histological findings of interstitial neutrophilia and diffuse alveolar damage, sometimes accompanied by platelet and fibrin thrombi and small-vessel vasculitis; and (d) a rapidly progressing, fatal outcome.
From the clinical and radiological point of view, the characteristics of ischemia-reperfusion damage and hyperacute rejection are identical. It is therefore likely that cases of antibody-mediated rejection have been registered as primary graft failure. The group directed by Mason et al. extends the use of ECMO after LT to graft rejection, with an immediate postoperative survival rate of about 60%.39 Likewise, the Austrian group40 has published its experience with three patients suffering severe acute rejection. They temporarily used venoarterial and femoro-femoral ECMO, observing advantages in terms of hemodynamic and respiratory stability, and making it possible to reduce the need for aggressive mechanical ventilation. Two of the patients were still alive after 30 and 60 months of follow-up, with no bronchiolitis obliterans.
ComplicationsThe complications of ECMO have gradually decreased as a result of the technological improvements of the latest assist models, but problems nevertheless remain frequent.Hemorrhage: This can be produced by many factors, such as coagulation disorders caused by the continuous administration of heparin and platelet dysfunction41; alternatively, a defect in the surgical technique can produce bleeding at the cannulation site. Reoperation due to bleeding is not uncommon. Recently, a publication has been made of the experience gained using ECMO without anticoagulation, due to the risk of bleeding.42Thromboembolism: This is the result of clot formation in the extracorporeal circuit. It is essential to regularly inspect the circuit in search of clotting. Likewise, a rise in pressure gradient across the oxygenator suggests thrombus formation in this part of the extracorporeal circuit. In the event of signs of circuit clotting, the circuit must be replaced. Infection: This is one of the most frequent complications–hence the importance of prophylaxis and of the monitoring of signs of infection, in order to ensure adequate antibiotherapy as quickly as possible. Inadequate and late treatment of sepsis can give rise to multiorgan failure, which greatly complicates the management of patients subjected to ECMO. Smedira et al.43 recorded a 49% infection rate in 202 patients assisted with ECMO. Neurological complications: These complications are the most frequent primary cause of death according to the INTERMACS registry.44 They tend to be secondary to embolic or bleeding problems, and have devastating effects.45Vascular and mechanical complications: These problems involve particularly arterial rupture or dissection, ischemia of the lower extremity in which the cannula has been placed, phlegmasia or tension edema of the lower extremity secondary to obstruction of venous return along the venous femoral cannula, right ventricle rupture due to complications in placement of the cannula, tamponade, etc.46–49
ConclusionsAlthough ECMO is relatively little used in LT, and the literature moreover does not refer to large patient series, the technical improvements in the devices and the results obtained are causing the technique to become increasingly popular. Artificial devices offer pulmonary and circulatory support that contribute to lessen morbidity–mortality among selected patients during the different phases of the perioperative period. The use of ECMO is becoming increasingly consolidated in the different transplant groups, since there is presently less controversy regarding the use of ECMO-based cardiorespiratory assist procedures as an alternative for patients on the waiting list, applied during the intra- and postoperative periods of LT.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vicente R, et al. Oxigenador de membrana extracorpóreo en el trasplante pulmonar. Med Intensiva. 2013.