Thiopental is a barbituric anaesthetic drug used in the management of refractory epileptic seizures and intracranial high blood pressure. It has multiple adverse events such as low blood pressure; myocardial depression; immunodepession; an extended depression of consciousness due to accumulations after the infusion; and ischaemia, among others.1,2 However, the altered concentration of sodium is not an adverse effect reported in the technical label. We wish to inform on the finding of false thiopental-induced hypernatremia due to the interaction of this drug with our core laboratory analyzer. Thus, the goal of our study is to analyze the concordance and correlation between the natremia obtained in two (2) different analyzers in patients treated with sodium thiopental from an index case.
The index case of our observation was a two-year-old child hospitalized in our unit with clinical manifestations of refractory seizures who required the initiation of one continuous perfusion of sodium thiopental. Right from the beginning of the infusion we observed initially mild hypernatremias (151mEq/L 6h after starting the infusion) that progressed upward up to 164mEq/L that were inconsistent with the values shown by the gasometer even though the levels of IV sodium supplied had been reduced, but without presence of polyuria or other criteria of diabetes insipidus (DI). The initial dose was 1.5mg/kg/h, but it was titrated upward to a maximum of 4mg/kg/h.
After withdrawing the drug, the levels of natremia went back to normal, and we were able to conduct one retrospective study based on a review of the clinical histories of all children who received sodium thiopental from 2009 to 2015, in order to analyze all possible inconsistencies between the natremia found in our core lab (NaB)—Dimension Vista 1500, Siemens Healthcare Diagnostics, Newark, USA—and the natremia found in the gasometer (NaG)—ABL800 Flex, Radiometer GmbH, Copenhagen, Denmark.
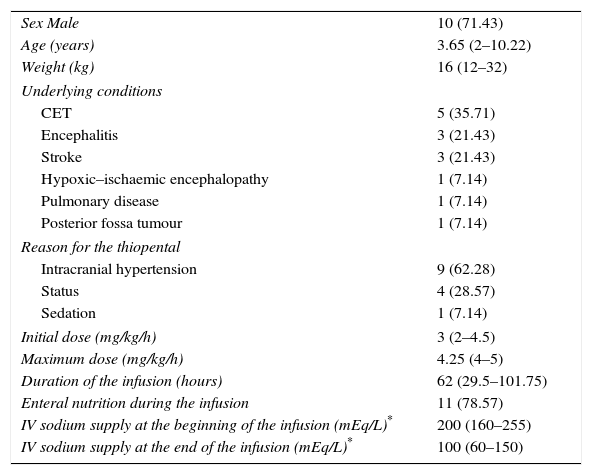
Several sociodemographic variables were considered together with characteristics from the episodes reported, the doses of thiopental, the sodium supplied during the infusion, and the levels of natremia 48h after the administration obtained by the analyzer (NaB) and the gasometer (NaG) (Table 1). Since the samples analyzed through these two methods were not obtained simultaneously, and in an attempt to avoid the influence of IV sodium supply, the pairs of values obtained with time intervals<12h were selected as long as the same infusion and clinical situation was maintained, and with time intervals<2h whenever any of these parameters had changed.
Sociodemographic characteristics of patients and episodes recorded.
| Sex Male | 10 (71.43) |
| Age (years) | 3.65 (2–10.22) |
| Weight (kg) | 16 (12–32) |
| Underlying conditions | |
| CET | 5 (35.71) |
| Encephalitis | 3 (21.43) |
| Stroke | 3 (21.43) |
| Hypoxic–ischaemic encephalopathy | 1 (7.14) |
| Pulmonary disease | 1 (7.14) |
| Posterior fossa tumour | 1 (7.14) |
| Reason for the thiopental | |
| Intracranial hypertension | 9 (62.28) |
| Status | 4 (28.57) |
| Sedation | 1 (7.14) |
| Initial dose (mg/kg/h) | 3 (2–4.5) |
| Maximum dose (mg/kg/h) | 4.25 (4–5) |
| Duration of the infusion (hours) | 62 (29.5–101.75) |
| Enteral nutrition during the infusion | 11 (78.57) |
| IV sodium supply at the beginning of the infusion (mEq/L)* | 200 (160–255) |
| IV sodium supply at the end of the infusion (mEq/L)* | 100 (60–150) |
The qualitative variables are expressed as absolute frequency (relative). The results from the numerical variables are expressed as mean (interquartile range).
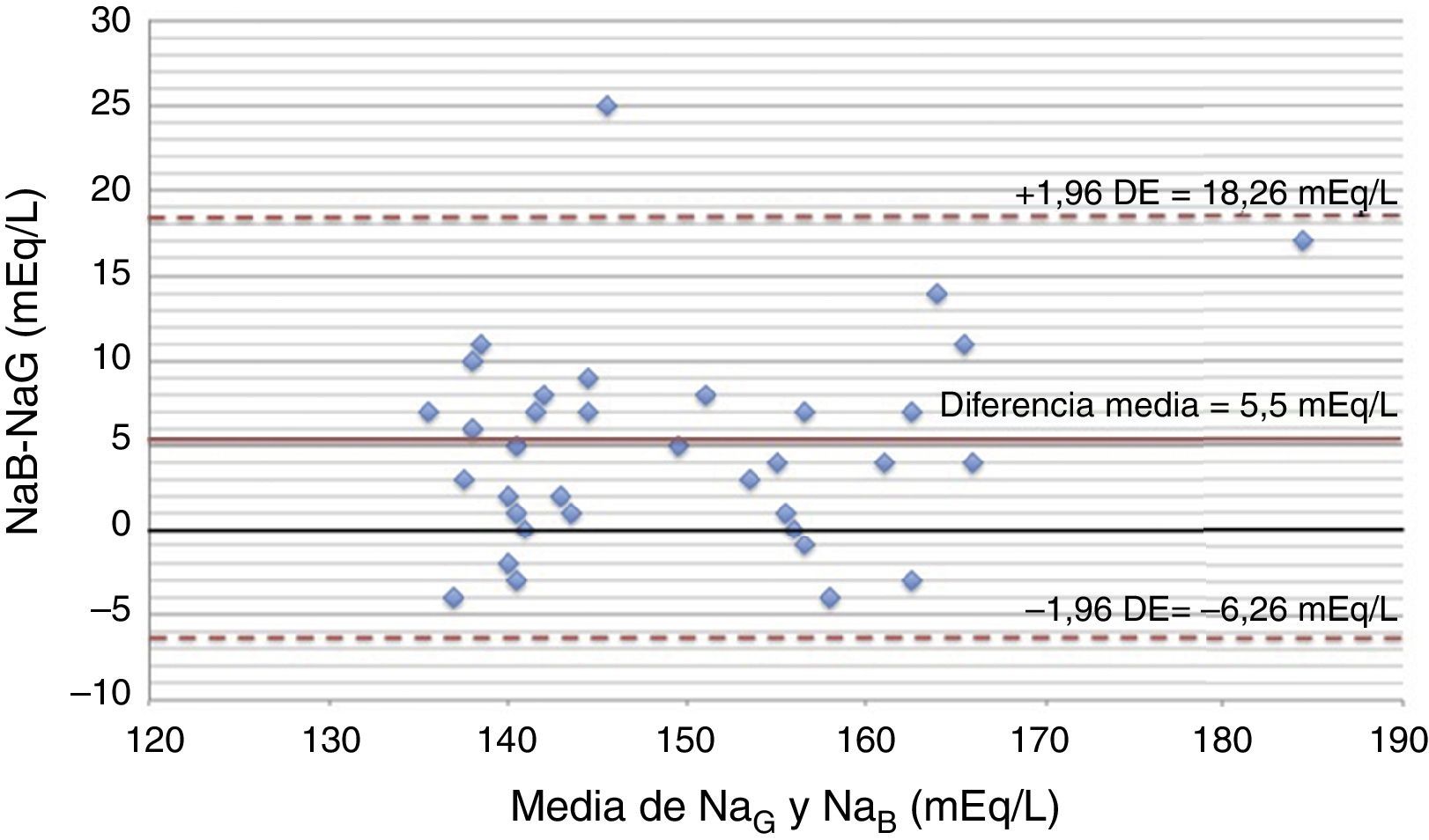
The quantitative variables are expressed as mean and standard deviation (normal distribution), or mean and interquartile range (IQR) (for the rest). For the comparison of the levels of natremia obtained through both of the aforementioned methods, the Student's t test and the Mann–Whitney's U test were used when appropriate. The steady-state interclass correlation coefficient was estimated, and the Bland–Altman diagram was built. The p value was established in 0.05.
The study was assessed and approved by the corresponding Ethics Committee from the Provincial Research.
Fourteen (14) patients received thiopental in the period analyzed; nine (9) of them due to intracranial hypertension; four (4) due seizures; and one (1) case due to difficult sedation. Thirty-five (35) pairs of measurements were analyzed 28/35 during the infusion. The NaG average was 148.7±11.65mEq/L, and the NaB average 154.78±12.04mEq/L (p<0.001). The difference mean during the infusion was 5.5mEq/L (1.5–8.75), and before the infusion, 1mEq/L (−3 to 2), p=0.017. The interclass correlation coefficient was 0.77 (p<0.001) when we looked at the pairs of values during the infusion. The concordance limits in the Bland–Altman diagram (Fig. 1) were −6.26, 18.26. In fifty (50) per cent of the pairs of measurements, the difference was>5mEq/L (17.9 per cent between 5 and 7.5mEq/L; 14.3 per cent between 7.5 and 10mEq/L; 17.9 per cent>10mEq/L), with a maximum difference of 25mEq/L. The mean dose of thiopental was 4mg/kg/h (2–5). The differences between both methods were more significant when doses>4mg/kg/h were used (7mEq/L [2–11] versus 5mEq/L [1–7], p=0.12). In all patients the IV sodium supplies were reduced as a consequence of hipernatremia.
In the patients who received osmolar therapy after the withdrawal of thiopental, the goal was to maintain the levels of natremia in blood between 145 and 150mEq/L as part of the management of the underlying condition. This is why it makes sense that after the withdrawal of thiopental, the state of normonatremia was not reached in these patient. However, in the four (4) patients who did not receive osmolar therapy after the withdrawal of thiopental, the hipernatremia disappeared.
In order to assess the measurement differences between both methods—gasometry an core lab analyzer, we analyzed thirty (30) pairs of values in patients who did not receive thiopental and obtained a difference of 1.5±0.75mEq/L (p=0.71), and a Pearson correlation coefficient of 0.87.
One of the main limitations of this study is its retrospective nature, which is why the pairs of compared values were not collected simultaneously. This is why we picked the analytics based on the time interval elapsed between both pairs. Most patients received isotonic serum as a neuroprotective strategy, or hypertonic serum for the management of brain swelling, and intracranial hypertension. For this reason, the levels of natremia that were slightly high can be considered consistent with the therapy received and not lead to any initial changes of serum therapy; however, as we extend the infusion, the false hypernatremia becomes more evident, leading to the reduction of the IV sodium supply. The complications derived from the use of hypotonic serum in this type of patients are well known, and they favour the development of secondary brain swelling, and brain hypertension.3,4
The only study published so far is the one conducted by Feyen et al. who have been investigating the same adverse event associated to Siemens Dimension Vista analyzer from an index case that we have been investigating.5 These authors have been testing this discrepancy in vitro and have confirmed that the mechanism responsible for such a discrepancy is based on the interaction between the thiopental molecule and the polymeric membrane, which in turn alters its polarity and, consequently, the reading of the results based on different electric powers.5 Also there are two (2) cases of adult patients that have been presented in different congresses.6
This finding was brought to the attention of the Unit of Clinical Analyses and the manufacturer of the analyzer, since we are talking about an important error that may be detrimental to the security of the patients.
The false hypernatremia due to the interference between thiopental and the Siemens Dimension Vista analyzer is an important factor that may influence the therapeutic decisions made in neurocritical patients, and hence, lead to the administration of hypotonic fluids that may have deleterious effects such as brain swelling, and intracranial pressure.
The authors of this paper wish to thank Dr. De la Mota Ybancos, MD for his assistance to the patients included in the study.
Please cite this article as: García-Soler P, Amores-Torres M, Sanchiz-Cárdenas S, González-Gómez JM, Dayaldasani A, Milano-Manso G. Tiopental y falsa hipernatremia: compruebe su analizador. Med Intensiva. 2017;41:573–574.