Severe burn patients are one subset of critically patients in which the burn injury increases the risk of infection, systemic inflammatory response and sepsis. The infections are usually related to devices and to the burn wound. Most infections, as in other critically ill patients, are preceded by colonization of the digestive tract and the preventative measures include selective digestive decontamination and hygienic measures. Early excision of deep burn wound and appropriate use of topical antimicrobials and dressings are considered of paramount importance in the treatment of burns.
Severe burn patients usually have some level of systemic inflammation. The difficulty to differentiate inflammation from sepsis is relevant since therapy differs between patients with and those without sepsis. The delay in prescribing antimicrobials increases morbidity and mortality. Moreover, the widespread use of antibiotics for all such patients is likely to increase antibiotic resistance, and costs. Unfortunately the clinical usefulness of biomarkers for differential diagnosis between inflammation and sepsis has not been yet properly evaluated.
Severe burn injury induces physiological response that significantly alters drug pharmacokinetics and pharmacodynamics. These alterations impact antimicrobials distribution and excretion. Nevertheless the current available literature shows that there is a paucity of information to support routine dose recommendations.
Los pacientes con quemaduras graves son un subgrupo de pacientes críticos en los que la lesión por quemadura aumenta el riesgo de infección, de respuesta inflamatoria sistémica y de sepsis. Las infecciones suelen estar relacionadas con los dispositivos y la quemadura. La mayoría de las infecciones, al igual que en otros pacientes críticos, están precedidas por la colonización del tracto digestivo y de medidas preventivas que incluyen la descontaminación digestiva selectiva y las medidas de higiene. La escisión precoz de las quemaduras profundas y el uso adecuado de los antimicrobianos tópicos y apósitos se consideran de suma importancia en el tratamiento de las quemaduras.
Los pacientes con quemaduras graves suelen tener un cierto nivel de inflamación sistémica. La dificultad para diferenciar inflamación de sepsis es relevante debido a que la terapia difiere entre los pacientes con y sin sepsis. El retraso en la prescripción de antimicrobianos aumenta la morbimortalidad. Además, el uso generalizado de antibióticos en todos estos pacientes es probable que aumente la resistencia a estos y los costes. Desafortunadamente, la utilidad clínica de biomarcadores para el diagnóstico diferencial entre inflamación y sepsis aún no ha sido adecuadamente evaluada.
La lesión por quemadura severa induce una respuesta fisiológica que altera significativamente la farmacocinética y farmacodinámica de los fármacos. Estas alteraciones afectan a la distribución y excreción de los antimicrobianos. Sin embargo, la literatura disponible actual muestra que hay una escasez de información para apoyar las recomendaciones de dosis rutinarias.
Critically ill burn patients are more susceptible that other critically ill patients to acquire infections as traditionally reported in infection surveillance systems surveillance in the Intensive Care Units (ICU).1 This increased susceptibility has been attributed to four facts: a non-specific immunosuppressive state induced by burns, frequent use of invasive devices (tracheal intubation, intravascular catheters, urinary catheters), loss of skin protection related to burn injury and in some cases respiratory injury from smoke inhalation. In addition surgery carried out in areas with bacterial contamination is associated with transient bloodstream infection caused by the flora colonizing burn wounds.
Another characteristic to tackling infections in burn patients is the low value of clinical criteria, i.e. fever, and biomarkers to differentiate systemic inflammatory response syndrome (SIRS) from sepsis.2 The severe burn patient, i.e. burns >20% of the body surface, in adults, usually shows signs of inflammation without a proven infection. This difficulty differentiating inflammation form infection can lead in some cases to excessive use of antibiotics with associated costs and the possibility to select resistant flora. On the other hand delay in the administration of appropriate antibiotics may be associated with increased morbidity and mortality.
The pharmacokinetics and pharmacodynamics (PK/PD) of antimicrobials are other differentiating factors of burn patients over other critically ill patients. The antibiotic volume of distribution are often very high, especially in the first two weeks after injury because the accumulated oedema during resuscitation, and the increase in glomerular filtration rate.3
Finally it should be noted that recommendations on the clinical management of severe burn patients, including prevention and treatment of infections are almost always supported by the expert opinion and the assumption that critically ill burn patients should treated similarly to other critically ill patients.4 There is little relevant clinical research to support an adequate level of evidence for any specific recommendation in this population group.
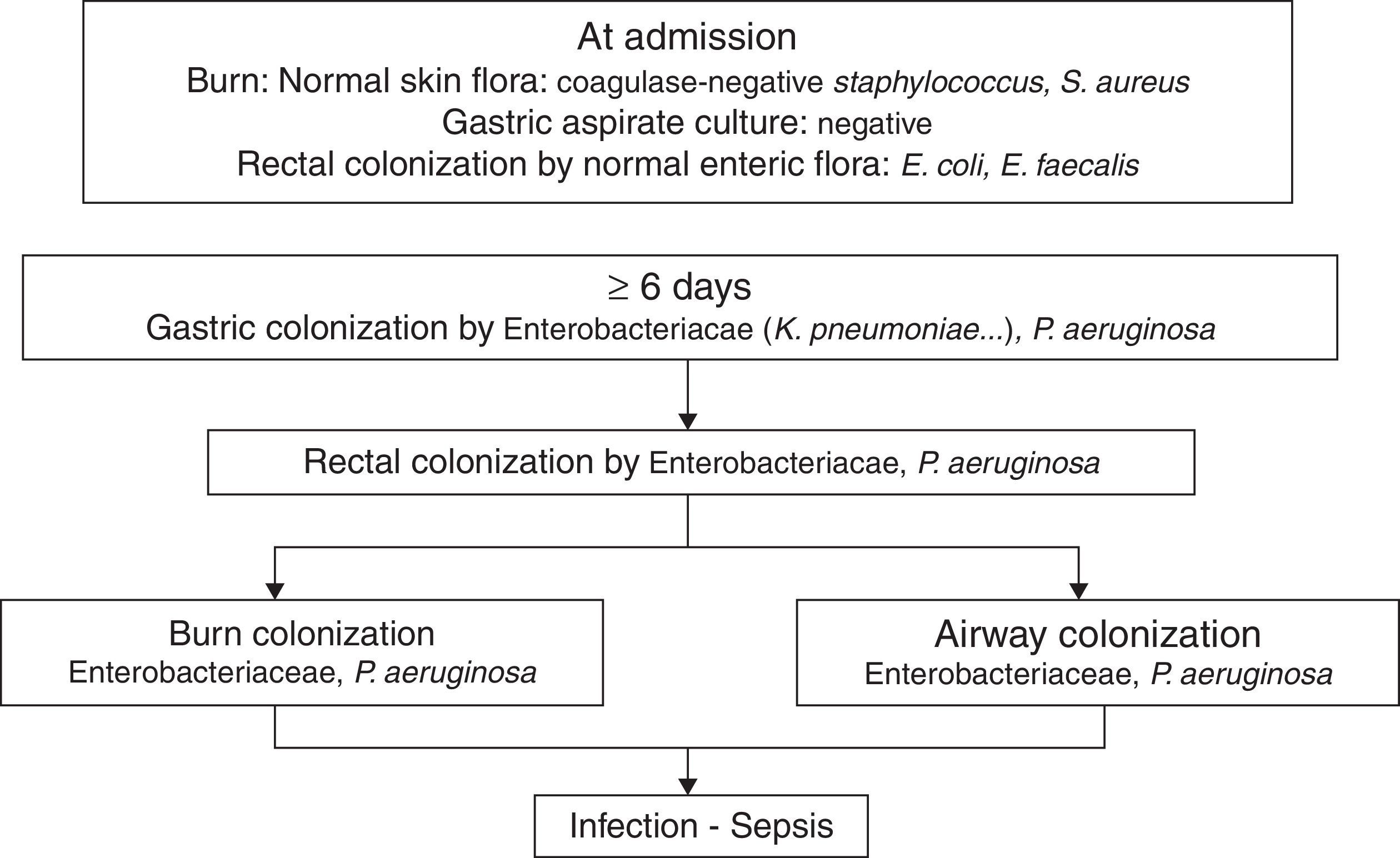
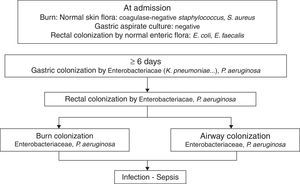
PathogenicityIn 1979, van Saene et al.5 in a prospective study of 32 patients showed that the flora that colonizes the digestive tract of patients often infects the burn patient. More recently Barret et al.6 studied digestive, respiratory and burn wounds colonization in 30 burn children treated in an ICU with a nurse/patient ratio 1.5:1 without strict preventative measures beyond those recommended for contact with biological fluids. At ICU admission, digestive and skin flora was the flora usually carried by healthy subjects: Escherichia coli, Enterococcus spp. in rectum and Staphylococcus epidermidis in skin. After 6–7 days this flora is replaced by Enterobacteriaceae and Pseudomonas aeruginosa acquired in the ICU, that colonized burn wounds and respiratory system later (Fig. 1). In other studies, non-fermenting Gram-negative bacilli and methicillin-resistant Staphylococcus aureus are part of ICU acquired flora.7
The chronology of bacterial colonization/infection in critically burn patients.
This pattern of colonization-infection has been previously described in critically ill patients8 and shows two characteristics:
- 1.
The flora colonizing and sometimes infecting critically ill patients changes during ICU stay. At admission patients without previous illnesses potentially pathogenic microorganisms (PPM) carried in digestive tract and skin are similar to those usually carried by healthy subjects. Later that flora is replaced by the UCI acquired flora. The digestive tract of other patients is the most important reservoir.
- 2.
Ninety nine percent of infections in critically ill patients, including severe burn patients are caused by PPM previously isolated in the digestive tract of the patient. They are considered endogenous.8,9
Selective digestive decontamination (SDD) is a strategy to prevent infections in critically ill patients.10 It was initially designed for the prevention ventilator-associated pneumonia, but subsequently it has been shown to be effective to prevent aerobic Gram-negative bacilli bloodstream infection,11 and control outbreaks of resistant flora.12,13 It is the only infection preventative measure that has consistently shown to reduce mortality in critically ill populations.10
The rationale for the SDD to prevent infection is to avoid or eradicate the carrier state of oropharyngeal and gastrointestinal PPM.8 The protocol of the SDD includes a short course of systemic antibiotics (cefotaxime), the use of nonabsorbable antimicrobial oral paste and digestive solution (polymixin, tobramycin and amphotericin B o nystatin) and performing surveillance rectal and pharyngeal cultures, to monitor the effectiveness of nonabsorbable antimicrobials7 It should be underlined that the administration of nonabsorbable antimicrobials not always achieves decontamination of the digestive tract.
This practice has proven to reduce mortality of critically ill patients [OR 0.79 (CI 95% 0.68 to 0.89)],10 the incidence of pneumonia [OR 0.35 (95% CI 0.29 to 0.41)],9 and the incidence of aerobic gram-negative bloodstream infections [OR 0.39 (95% CI 0.24 to −0.63)].11
The use of DDS has been evaluated in severe burn patients in one observational study14 and in one randomized controlled clinical trial.7
Mackie et al.14 compared a group of consecutive patients with burns >30% total body surface area (TBSA) conventionally treated for two years with 31 similar patients treated with SDD in the following two years. Mortality in the SDD group was 7% and 23% in the standard group [OR=0.11 (95% CI 0.01 to 0.93)]; the incidence of pneumonia was 6% and 29% and the incidence of bloodstream infection was 3% and 26%, respectively.
In the randomized controlled clinical trial7 107 patients with TBSA burns >20% were included. The SDD treated group showed a significant decrease in mortality compared to the placebo group [RR 0.25 (95% CI 0.08 to 0.76)] and hospital mortality [RR 0.28 (CI 95% 0.10 to 0.80)]. The incidence of pneumonia was reduced, 30.8 per 1000 days of mechanical ventilation in the placebo group and 17.0 per 1000 days of mechanical ventilation in the SDD group (p=0.03). It was observed that the use of nonabsorbable antimicrobial against Gram negative bacilli can increase the incidence of Gram positive carriers, i.e. methicillin-resistant S. aureus. The administration of enteral vancomycin controlled the growth of methicillin-resistant S. aureus safely without the appearance of vancomycin-resistant Enterococcus sp.15
Therefore, selective digestive decontamination has proven useful and safe in controlling infections and reducing mortality in severe burn patients as has been widely described in critically ill patients.
Ventilation associated pneumoniaIn patients with burns ≥20% of body surface, inhalation injury incidence is >37%.16 This condition is associated with a high incidence of pneumonia. Another risk factor of pneumonia in severe burn patients is related with mechanical ventilation, even in the absence of inhalation injury, to treat respiratory failure and to keep patients deeply sedated for long periods. Overall the incidence of ventilator-associated associated pneumonia in burn patients is three times higher than in patients in a medical-surgical ICU.1
In a cohort study of 56 patients with TBSA≥20%,9 the incidence of pneumonia was 31.3 episodes per 1000 days of mechanical ventilation in the subgroup of patients who suffered inhalation injuries. Ninety-five percent of the episodes of pneumonia were caused by microorganisms that were previously colonized the digestive tract, oropharynx and/or rectum. Fifty seven percent of patients develop early-onset pneumonia by microorganisms colonizing the patient oropharynx on admission to the ICU: S. aureus, Streptococcus pneumoniae and Haemophilus influenzae. The pneumonia that appeared later (median 16 days), were caused by microorganisms acquired in the ICU and were always preceded by an episode of early-onset pneumonia.
Bloodstream infectionShupp et al.17 conducted a retrospective case-control study of all patients in the National Burn Repository between 1981 and 2007. They included 3931 cases and 7862 controls randomly selected from the same database16 and adjusted by year of injury and percentage of TBSA burned. The microorganisms most frequently isolated from blood cultures were Gram positive cocci; the subgroup of not specified microorganisms was the largest, followed by S. aureus (32%). Among the Gram negative bacilli, P. aeruginosa (35%) was the most frequently isolated. Mortality showed a paradoxical effect: in patients with <50% TBSA bloodstream infection was associated with increased mortality, whereas in patients with ≥50% TBSA the mortality was lower in patients with bloodstream infection that in controls. The adjustment for potential confounders (age, sex, inhalation injury) did not modify this paradoxical finding for which the authors found no explanation. These findings bring in question the effect of bloodstream infection in mortality in severe burn patients.
Bloodstream infections associated with intravascular catheters in burn patients have some peculiarities in relation to other critically ill patients. They are more common18 and its incidence is associated with the proximity of the insertion site of the catheter to burn wound.
Ramos et al.19 reported a cumulative incidence of catheter-related bloodstream infection in 20 patients: when the burn wound was within an area of 25cm2 around the catheter insertion site the incidence of bloodstream infection was 27% but when the distance was greater the incidence was 6%. According to these observations it seems advisable to insert intravascular catheters away from burn wounds and bloody surfaces (grafts, donor sites) when possible.
In general, experts recommend changing intravascular catheters with a scheduled frequency, i.e. 5–7 days in patients with ≥20% TBSA.4 However, no clinical trials support this practice.
According to the available evidence, it seems advisable to prevent intravascular catheter-related bloodstream infection with the following manoeuvres:
- 1.
Insert the catheter away, if possible, from the burn wounds or the bloody, grafted or donor areas.
- 2.
Follow the same best practices for critically ill patients.20
- 3.
Assess scheduled shift when the catheter is near or within the burned area.
- 4.
Assess replacing the catheter with guide without changing the insertion, in order to preserve vascular access for possible future insertions.
In burn patients the predominant pathogenic microorganisms are aerobic Gram-negative bacilli, and S. aureus.17 Thus, it is therefore advisable that the empirical treatment of suspected bloodstream infection must include systemic antibiotics to cover this flora.
BurnsBurn wound infection is a severe complication in burn patient, increasing the degree of burn wound depth, healing delay, grafts loss and sepsis in cases where bacterial invasion occurs subdermal.21
The current management of deep burns is based on early excision of burned tissue, coverage with autograft, homograft or skin substitutes, and on preventing colonization/infection with topical antimicrobial treatment. The recommendation of early excision, between 1 and 7 days, is mainly based on two assumptions: the burned tissue is prone to infection and, even without being infected, it promotes the production of proinflammatory molecules associated with multiorgan failure.22,23 But the effectiveness of early excision in reducing mortality or morbidity has not been properly evaluated. One meta-analysis of 6 early excision clinical trials24 shows that trials have insufficient sample size, poor methodological quality, and it just seems to be a trend to reduce mortality only in patients without inhalation injury.
Given that the effectiveness of early excision has not been adequately evaluated in critically ill burn patients, topical application of antimicrobial that effectively prevents burn wound infections allows to delay surgery in high risk patients (high transfusion needs, frequent perioperative multiorgan failure). It seems appropriate to individually assess the benefits and risks to set the timing and the area(s) to be excised in each interventions. In practice the attitude of the early excision and prevention of infection varies considerably between countries and, within countries, between centres.25,26
The American Burn Association has established standardized definitions of burn wound infections.27 All types of infection, except from impetigo, are associated with fever and/or leukocytosis and/or thrombocytopenia.
- -
Burn wound impetigo which is defined as the loss of epithelium in areas previously re-epithelialized: grafts, wounds healed by secondary intention and donor sites. It may be associated or not with systemic inflammatory symptoms.
- -
Open burn-related surgical wound that includes both excised or donor area is characterized by the presence of purulent exudate with positive culture, often accompanied by loss of grafts or synthetic skin preparations.
- -
Burn wound cellulitis is characterized by the presence of erythema beyond that expected in the burn wound or in the donor area, usually with other signs of local inflammation as oedema, pain, heat and, less frequently, lymphangitis.
- -
Invasive infection in unexcised burn wounds is characterized by discoloration of the unexcised eschar and local signs of infections. It may be associated to multiple organ failure and bloodstream infection. This type of burn wound infection is rarely seen in Spanish ICU due to early excision and use of topical antibiotics.
Other rare infections related to deep burns are fasciitis and myositis.28
The value of biopsy culture for the diagnosis of burn wound infection was proposed McManus et al.29 They compared the results of quantitative cultures of burn wound biopsy with histopathologic results 200 burn patients. The “gold standard” of burn wound infection was histopathologic evidence of invasion by microorganisms within underlying healthy tissue. Growth of the cultures with values <105CFU/g of tissue were not accompanied by histopathologic evidence of infection. Values ≥105CFU/g showed histopathologic evidence of infection in only 36% of cases. Thus the biopsy cultures of burn wound have little value for the diagnosis of infection. The histopathologic examination is not a routinely performed in clinical practice except when suspected invasive fungal infection.
The diagnosis of burn wound infection is usually based on clinical criteria and the treatment is guided by the results of cultures of burn wound exudates and blood.
Topical antimicrobialsFrom the time the burn occurs until healing local cures are needed to assess the evolution of the burn wounds, the grafts, the skin substitutes and the donor surfaces. These cures are often accompanied by routine showers in appropriate baths25 and the application of antimicrobial dressing in order to prevent bacterial or fungal colonization and possible infection.
The choice of topical antimicrobial dressing varies widely across countries. In this article we will discuss the most commonly used antimicrobials in our medium.
- –
Silver sulfadiazine. It is the most widely used topical antiseptic in the treatment of burn wounds worldwide. It is an insoluble white, not painful cream with antimicrobial activity against a large number of microorganisms (S. aureus, Enterobacterias, P. aeruginosa, Candida albicans.). It has poor penetration of eschar. It produces a pseudoeschar to interact with burn wound exudate and it is easily removed The main side effect is called early postburn leukopenia, reaching minimum values around 2000 white cells/μl30 that reverses spontaneously without removing sulfadiazine.31 Silver sulfadiazine must be applied every 12 or 24h.
- –
Cerium nitrate – silver sulphadiazine. Cerium is an element with in vitro and antimicrobial activity and low toxicity. The addition to silver sulfadiazine potentially increases the antimicrobial activity of silver sulphadiazine. The use of cerium nitrate–silver sulphadiazine changes the burn eschar into a dry leathery crust, which does not spontaneously separate from the burn wound and acts as a physical barrier. There has been also hypothesized that cerium nitrate reduces immune suppression because it has a very high binding affinity with the toxin formed by thermal energy in the burned skin, a molecule that contributes to SIRS after burn injury.32
- –
Silver containing dressings.33 In recent years, several new silver impregnated dressings have been developed. Acticoat consists of two layers of high-density polyethylene net with a layer of rayon/polyester gauze in between which has been impregnated with nanocrystalline silver. When the dressing contacts the wound exudate causes the release of silver ions steadily. Its antiseptic spectrum is similar to the silver nitrate, and has the advantage of allowing prolonged time between cures up to 48–72h, which has a positive effect on wound healing and nursing time consumption.
- –
Occlusive cures. These sheets are placed over the burn wound until wound healing. Therefore, they are used in non-surgical superficial dermal burns. Silver Aquacel consists of sodium carboxymethylcellulose to which silver ions have been incorporated, while Biobrane a synthetic bilaminar membrane without antiseptic activity. If the burn progress to deeper thickness the material does not adhere and must be removed, partially or completely. Biobrane spontaneously separates from the healed wound
Other less common alternatives are the use of chlorhexidine 0.5% creams and hydrocolloids with antibiotics or antifungals, at 0.5% effective against microorganisms isolated from burn wound exudate cultures.
Finally it should be remembered that despite its widespread use, effectiveness of silver sulfadiazine and silver-impregnated dressings have not been adequately evaluated in clinical trials.34
Systemic inflammatory response and sepsis. BiomarkersPatients with extensive burns, “by definition, already have SIRS”.2 Thus, differential diagnosis between SIRS and sepsis is frequently difficult. This phenomenon migh6t be in part responsible for withholding, delaying, or overusing antimicrobial treatment in critically ill burn patients. Obviously in the presence of shock, early antibiotic treatment is indicated. In other cases, antibiotics may be unnecessary, expensive and may increase antibiotic resistance of ICU flora.
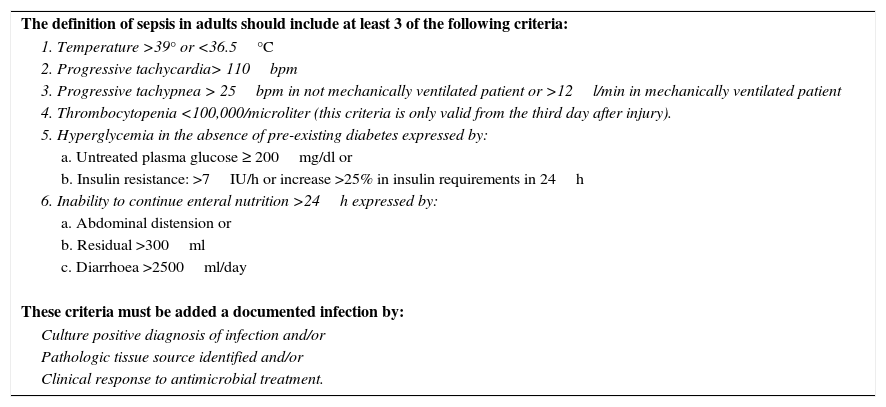
In a Consensus Conference the American Burn Association proposed the standardized diagnostic criteria as a “gold standard” to establish a uniform classification in all diagnostic studies and clinical trials (Table 1).2 The value of these criteria has not been evaluated in clinical practice.
Diagnostic criteria for sepsis in burn patients.2
| The definition of sepsis in adults should include at least 3 of the following criteria: |
| 1. Temperature >39° or <36.5°C |
| 2. Progressive tachycardia> 110bpm |
| 3. Progressive tachypnea > 25bpm in not mechanically ventilated patient or >12l/min in mechanically ventilated patient |
| 4. Thrombocytopenia <100,000/microliter (this criteria is only valid from the third day after injury). |
| 5. Hyperglycemia in the absence of pre-existing diabetes expressed by: |
| a. Untreated plasma glucose ≥ 200mg/dl or |
| b. Insulin resistance: >7IU/h or increase >25% in insulin requirements in 24h |
| 6. Inability to continue enteral nutrition >24h expressed by: |
| a. Abdominal distension or |
| b. Residual >300ml |
| c. Diarrhoea >2500ml/day |
| These criteria must be added a documented infection by: |
| Culture positive diagnosis of infection and/or |
| Pathologic tissue source identified and/or |
| Clinical response to antimicrobial treatment. |
The diagnostic utility of the biomarkers most frequently used in the differential diagnosis of SIRS form infection in burn patients (C-reactive protein, procalcitonin) has been evaluated in a systematic review of six studies.35 The small sample size of the studies and the inconsistent results can not recommend routine use of biomarkers in the differential diagnosis of inflammation and sepsis. However some authors of included studies suggested that procalcitonin levels greater than 2.5ng/ml or 3ng/ml favours the diagnosis of sepsis and therefore the early use of systemic antibiotics, which would always be indicated in patients with shock. The same authors consider that the leukocytes count or C-reactive protein levels are not useful to differentiate SIRS form sepsis.
Pharmacokinetics/pharmacodynamicsSevere burn injury results in a multifaceted physiological response that significantly alters drug PK/PD. This response includes initially hypovolemia, increased vascular permeability, increased interstitial hydrostatic pressure, vasodilation and hypermetabolism. These physiological changes impact the distribution and excretion of drugs (increased volume of distribution, increase or decrease of total drug exposure), thus varying the therapeutic effect “in vivo” of drug.
There is a consensus that the pathophysiological changes that occur after the burn wound, including organ dysfunction (acute renal failure, liver dysfunction) and alterations in fluid and electrolyte balance, impact the PK/PD and consequently can modify drug administration, dose and administration frequency, to maintain therapeutic levels.4
A recent review,36 summarized the literature on the PK/PD of antibiotics and antifungals in burn patients, providing suggestions for dosing. Beta-lactams, carbapenems, aminoglycosides, vancomycin, daptomycin, linezolid and colistin were reviewed.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to acknowledge Dr. Miguel Angel de la Cal for his support.