Bronchiolitis (BR) is the most common cause of admission to the Pediatric Intensive Care Unit (PICU).1 Although no specific recommendations referred to critically ill children are available, the main clinical practice guides advocate supportive treatment and consider most diagnostic and pharmacological interventions to be futile.2,3 In developing countries such as Colombia, the epidemiological information on patients with BR admitted to intensive care is limited. The present study was carried out to compare the characteristics, interventions and clinical outcomes of infants with BR admitted to three Colombian PICUs pertaining to a Latin American collaborative network, and to compare their care outcomes in the Latin American context. For this purpose we compared our data against those of another 23 Units in the region (5 countries), based on the Latin American Pediatric Network (Red Pediátrica de Latinoamérica [LARed]) of which we form part.4
During the period between 1 January and 31 December 2018, a total of 886 children with BR were entered in the registry. To the effects of our analysis, we excluded 132 infants with diagnoses at discharge different from BR or with previous bronchopulmonary disease conditions. The final study sample thus consisted of 754 patients.
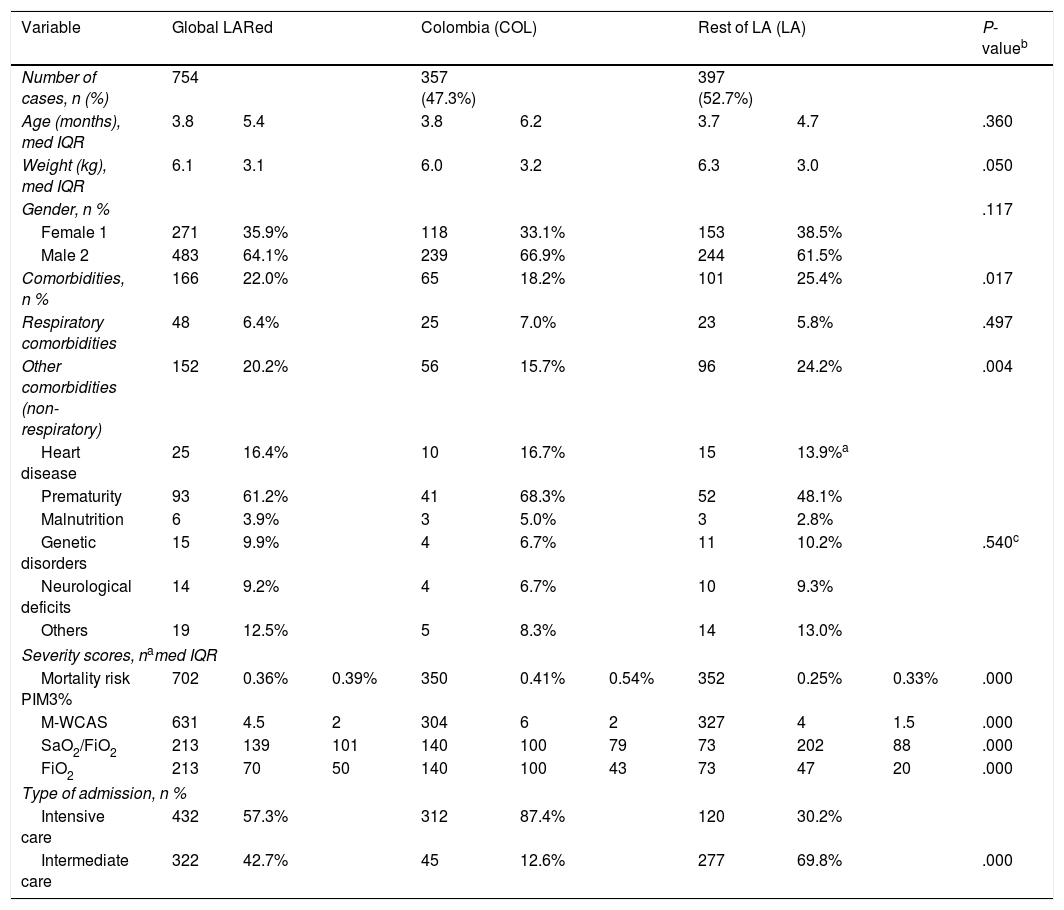
Table 1 shows the demographic characteristics and data referred to non-respiratory comorbidities, and offers a comparative analysis of Colombian Units (COL) and Latin American Units (LA). Of note is the absence of comorbidities in most cases, and the COL group showed fewer non-respiratory comorbidities (15.7 vs. 24.2%; P = .004), with no statistically significant differences according to the type of comorbidity – though the capacity to detect relative differences of >25% was low.
Comparison of the demographic characteristics, risk factors and severity at the time of admission.
| Variable | Global LARed | Colombia (COL) | Rest of LA (LA) | P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases, n (%) | 754 | 357 (47.3%) | 397 (52.7%) | |||||||
| Age (months), med IQR | 3.8 | 5.4 | 3.8 | 6.2 | 3.7 | 4.7 | .360 | |||
| Weight (kg), med IQR | 6.1 | 3.1 | 6.0 | 3.2 | 6.3 | 3.0 | .050 | |||
| Gender, n % | .117 | |||||||||
| Female 1 | 271 | 35.9% | 118 | 33.1% | 153 | 38.5% | ||||
| Male 2 | 483 | 64.1% | 239 | 66.9% | 244 | 61.5% | ||||
| Comorbidities, n % | 166 | 22.0% | 65 | 18.2% | 101 | 25.4% | .017 | |||
| Respiratory comorbidities | 48 | 6.4% | 25 | 7.0% | 23 | 5.8% | .497 | |||
| Other comorbidities (non-respiratory) | 152 | 20.2% | 56 | 15.7% | 96 | 24.2% | .004 | |||
| Heart disease | 25 | 16.4% | 10 | 16.7% | 15 | 13.9%a | ||||
| Prematurity | 93 | 61.2% | 41 | 68.3% | 52 | 48.1% | ||||
| Malnutrition | 6 | 3.9% | 3 | 5.0% | 3 | 2.8% | ||||
| Genetic disorders | 15 | 9.9% | 4 | 6.7% | 11 | 10.2% | .540c | |||
| Neurological deficits | 14 | 9.2% | 4 | 6.7% | 10 | 9.3% | ||||
| Others | 19 | 12.5% | 5 | 8.3% | 14 | 13.0% | ||||
| Severity scores, named IQR | ||||||||||
| Mortality risk PIM3% | 702 | 0.36% | 0.39% | 350 | 0.41% | 0.54% | 352 | 0.25% | 0.33% | .000 |
| M-WCAS | 631 | 4.5 | 2 | 304 | 6 | 2 | 327 | 4 | 1.5 | .000 |
| SaO2/FiO2 | 213 | 139 | 101 | 140 | 100 | 79 | 73 | 202 | 88 | .000 |
| FiO2 | 213 | 70 | 50 | 140 | 100 | 43 | 73 | 47 | 20 | .000 |
| Type of admission, n % | ||||||||||
| Intensive care | 432 | 57.3% | 312 | 87.4% | 120 | 30.2% | ||||
| Intermediate care | 322 | 42.7% | 45 | 12.6% | 277 | 69.8% | .000 | |||
COL: Colombian Units; FiO2: fraction of inspired oxygen; LA: Latin American Units; med IQR: median and interquartile range; n %: absolute number and percentage in column; PIM3%: mortality risk according to the Pediatric Index of Mortality 3 scale; M-WCAS: respiratory severity according to the modified Woods-Downes scale; SaO2: arterial oxygen saturation.
On assessing severity, we recorded a low median probability of mortality based on the PIM3 (Pediatric Index of Mortality 3) score (0.36%; interquartile range [IQR] 0.39) and moderate breathing difficulty according to the modified Woods-Downes Clinical Asthma Score (M-WCAS) (M-WCAS 4.5; IQR 2) – though these scores were comparatively higher in the COL group. In those cases where oxygenation could be evaluated (use of defined FiO2systems), impairment was seen to be greater in the COL group: median SaO2/FiO2100; IQR 79) versus median SaO2/FiO2202; IQR 88) in the LA group (P < .0001).
Upon admission to the PICU, the patient transfer respiratory support measures were seen to be diverse. A total of 51.4% of the patients were admitted to the Unit with respiratory support based on conventional oxygen therapy; 37.9% were admitted with high-flow nasal cannulas (HFNCs); 2.4% were admitted with continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) support; and 7.0% were admitted with invasive mechanical ventilation (IMV). On individually comparing each type of therapy, the COL group only showed a significantly lesser use of conventional nasal cannulas (10.6 vs. 54.7%; P = .0004). With regard to the rest of the systems, although relative differences of >25% were observed, the low statistical power was unable to confirm significance.
A high incidence of respiratory syncytial virus (RSV) was observed, with some significant differences between regions. In effect, a higher frequency of RSV and metapneumovirus was recorded in the LA group, while negative viral panels and parainfluenza virus were more common in the COL group. Antibiotic use was high (56.3%), despite a low declared suspicion of bacterial infection in 70 cases (9.3%) and even lesser confirmation based on culture or serological testing. High frequency of use and great variability were also observed on analyzing the use of coadjuvant drug therapies: while the COL group used significantly more antibiotics (66.7 vs. 46.9%; P < .0001), adrenaline (14.3 vs. 2.0%; P = .0007) and hypertonic therapies (27.5 vs. 10.8%; P < .000), the LA group used more antiviral agents (0.3 vs. 2.5%; P = .01), corticosteroids (3.4 vs. 17.9%; P < .0001) and bronchodilators (9.0 vs. 68.8%; P < .0001).
A significant difference was observed in relation to the frequency of IMV as initial support and maximum support in both the COL group (initial: 52.7%; maximum: 56.9%) and the LA group (initial: 9.07%; maximum: 19.4%). Variability was also recorded in relation to the other respiratory support systems, though no statistically significant differences were observed.
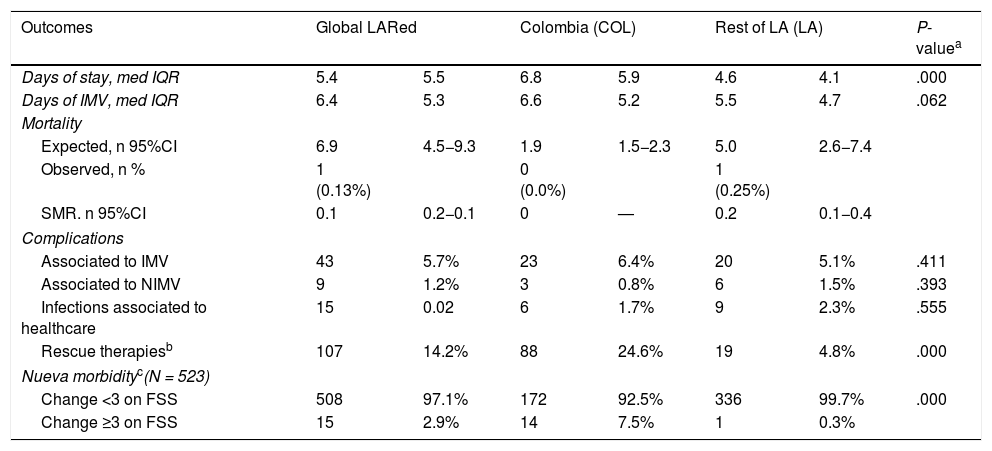
Overall, mortality (absolute and standardized according to the PIM3) was low. The COL group was characterized by longer stay in the PICU, a greater use of rescue therapies (neuromuscular relaxants, prone decubitus, high-frequency ventilation) and greater residual morbidity defined as an increase of ≥3 points on the Functional Status Score (FSS) at discharge (Table 2).
Comparison of outcomes.
| Outcomes | Global LARed | Colombia (COL) | Rest of LA (LA) | P-valuea | |||
|---|---|---|---|---|---|---|---|
| Days of stay, med IQR | 5.4 | 5.5 | 6.8 | 5.9 | 4.6 | 4.1 | .000 |
| Days of IMV, med IQR | 6.4 | 5.3 | 6.6 | 5.2 | 5.5 | 4.7 | .062 |
| Mortality | |||||||
| Expected, n 95%CI | 6.9 | 4.5−9.3 | 1.9 | 1.5−2.3 | 5.0 | 2.6−7.4 | |
| Observed, n % | 1 (0.13%) | 0 (0.0%) | 1 (0.25%) | ||||
| SMR. n 95%CI | 0.1 | 0.2−0.1 | 0 | — | 0.2 | 0.1−0.4 | |
| Complications | |||||||
| Associated to IMV | 43 | 5.7% | 23 | 6.4% | 20 | 5.1% | .411 |
| Associated to NIMV | 9 | 1.2% | 3 | 0.8% | 6 | 1.5% | .393 |
| Infections associated to healthcare | 15 | 0.02 | 6 | 1.7% | 9 | 2.3% | .555 |
| Rescue therapiesb | 107 | 14.2% | 88 | 24.6% | 19 | 4.8% | .000 |
| Nueva morbidityc(N = 523) | |||||||
| Change <3 on FSS | 508 | 97.1% | 172 | 92.5% | 336 | 99.7% | .000 |
| Change ≥3 on FSS | 15 | 2.9% | 14 | 7.5% | 1 | 0.3% | |
COL: Colombian Units; LA: Latin American Units; med IQR: median and interquartile range; n %: absolute number and proportion; 95%CI: 95% confidence interval; SMR: standardized mortality ratio, calculated as observed mortality with respect to expected mortality; FSS: Functional Status Scale; IMV: invasive mechanical ventilation; NIMV: noninvasive mechanical ventilation (including high-flow nasal cannula).
Our results indicate that both the epidemiological profile and the type of therapies and clinical outcomes of infants admitted to Colombian PICUs due to BR differ from those of their Latin American peers. The data show the variability of practices to be a frequent phenomenon in Colombia.
Of note is the observation that the patients in the COL group were in more serious condition at the time of admission. This could be explained by the geographical setting (over 1500 m above sea level), where altitude could have an important influence upon hypoxemia.5
Although the suspicion (and confirmation) of bacterial coinfection was low, we recorded a high use of antibiotics, which would go against the recommendations of the clinical practice guides referred to children with BR.6 The indication of antibiotics in patients admitted to the PICU remains the subject of debate among pediatric intensivists. The antibiotic indication rate in our series was below that reported by some large series in other parts of the world, where the proportion of antibiotic prescription in infants admitted to the PICU ranges in practically all cases above 50% as reported by Shein et al.7
One recently described phenomenon is the variability of clinical, diagnostic and therapeutic practices in children with BR.8,9 Our findings evidence that this situation is common in Latin America. The great variability and high frequency of use of coadjuvant therapies (nebulizers, antibiotics, corticosteroids) seen in all the Latin American regions analyzed is a matter of concern. There is a dissociation between the general treatment recommendations warranted by the scientific evidence and actual clinical practice. For many healthcare professionals, supportive treatment might not be enough, and concern about “not doing anything” and “letting the situation evolve with monitoring alone” can result in an overuse of futile and sometimes deleterious therapies.10
The Colombian PICUs were characterized by a greater use of IMV and a lesser use of noninvasive therapies such as HFNC and BiPAP, compared with the LA group. The use of rescue therapies (e.g., prone decubitus and high-flow ventilation) in the patients subjected to IMV was higher in the COL group. This may be explained by the greater severity of the patients at the time of admission or may serve to alert us to the possible overuse (over-indication) of such invasive and rescue measures – particularly considering that a less invasive strategy affords similar outcomes in children with BR.9
In conclusion, significant differences were observed in the epidemiological profile and care outcomes in Colombia, with greater patient severity at the time of admission and the use of more invasive respiratory support measures, a longer PICU stay, and higher residual morbidity. This situation warrants the implementation of collaborative initiatives to reduce such variability, improve the quality of care and optimize resource utilization.
Financial supportThe present study received proprietary funding.
Thanks are due to all the professionals and families that participate in the Latin American Pediatric Network (Red Pediátrica de Latinoamérica [LARed]).
Dr. Analía Fernández, Hospital Durand, Buenos Aires, Argentina; Dr. Roberto Jabornisky and Dr. Silvina Muzzio, Hospital Juan Pablo II, Corrientes, Argentina; Dr. Evelin Cidral and Dr. Alejandro Mansur, Hospital Regional Olga Stucky de Rizzi, Reconquista, Argentina; Dr. Miguel Céspedes Lesczinsky and Dr. Zurama Velasco, Hospital Materno Infantil Boliviano Japonés, Trinidad, Bolivia; Dr. Regina Grigolli Cesar, Hospital Infantil Sabará, San Pablo, Brazil; Dr. Pablo Cruces and Dr. Tamara Cordova, Hospital El Carmen, Maipú, Santiago, Chile; Dr. Diego Aranguiz Quintanilla, Dr. Juan Sepúlveda and Dr. Ivette Padilla, Complejo Asistencial Dr. Víctor Ríos Ruíz, Los Ángeles, Chile; Dr. Alejandro Donoso, Dr. María José Núñez Sánchez; Dr. Adriana Wegner, Complejo Asistencial Dr. Sotero del Río, Santiago, Chile; Dr. Pietro Pietroboni Fuster, Hospital Regional, Antofagasta, Chile; Dr. José Rosales Fernández and Dr. Silvia Sanabria, Hospital Nacional de niños «Dr. Carlos Sáenz Herrera», Costa Rica; Dr. Araní Ferre, Dr. Magalí España, Dr. Andrea Iroa and Dr. Raul Navatta, Hospital Policial, Montevideo, Uruguay; Dr. Ema Benech and Dr. Mónica Carro, Sanatorio Círculo Católico, Montevideo, Uruguay; Dr. Alicia Fernández, Asociación Española, Montevideo, Uruguay; Dr. Nicolás Monteverde and Dr. Martha Carbonell, Médica Uruguaya, Montevideo, Uruguay; Dr. Bernardo Alonso, Dr. Alberto Serra and Lic. Fátima Varela, Sanatorio Casa de Galicia, Montevideo, Uruguay; Lic. Cristina Courtie, Dr. Javier Martínez and Dr. Krystel Cantirán, Hospital Militar, Montevideo, Uruguay; Dr. Loredana Matrai and Dr. Cecilia Mislej, Hospital Evangélico, Montevideo, Uruguay; Dr. Luis Castro, Sanatorio CAMDEL, Minas, Uruguay; Dr. Soledad Menta, Hospital Tacuarembó, Uruguay; Dr. Carolina Talasimov and Lic. María José Caggiano, Sanatorio COMECA, Canelones, Uruguay; Dr. Luis Pedrozo and Dr. Alejandro Franco, Hospital Salto, Uruguay; Dr. Luis Martínez Arroyo and Dr. Silvia Dubra, Sanatorio COMEPA, Paysandú, Uruguay; Dr. Ana Inverso, Dr. Nora Mouta, Dr. María Parada and Lic. Karina Etulain, Sanatorio Semm-Mautone, Maldonado, Uruguay.
The collaborators of the Latin American Pediatric Network (Red Pediátrica de Latinoamérica [LARed]) are named in Appendix 1.
Please cite this article as: Vásquez-Hoyos P, Pardo-Carrero R, Jaramillo-Bustamante JC, González-Dambrauskas S, Carvajal C, Diaz F, et al. Ingreso en cuidados intensivos debido a bronquiolitis grave en Colombia: ¿dónde nos encontramos en relación con el resto de Latinoamérica? Med Intensiva. 2021;45:e18–e21.