To evaluate the rate of thrombosis, bleeding and mortality comparing anticoagulant doses in critically ill COVID-19 patients.
DesignRetrospective observational and analytical cohort study.
SettingCOVID-19 patients admitted to the intensive care unit of a tertiary hospital between March and April 2020.
Patients201 critically ill COVID-19 patients were included. Patients were categorized into three groups according to the highest anticoagulant dose received during hospitalization: prophylactic, intermediate and therapeutic.
InterventionsThe incidence of venous thromboembolism (VTE), bleeding and mortality was compared between groups. We performed two logistic multivariable regressions to test the association between VTE and bleeding and the anticoagulant regimen.
Main variables of interestVTE, bleeding and mortality.
Results78 patients received prophylactic, 94 intermediate and 29 therapeutic doses. No differences in VTE and mortality were found, while bleeding events were more frequent in the therapeutic (31%) and intermediate (15%) dose group than in the prophylactic group (5%) (p<0.001 and p<0.05 respectively). The anticoagulant dose was the strongest determinant for bleeding (odds ratio 2.4, 95% confidence interval 1.26–4.58, p=0.008) but had no impact on VTE.
ConclusionsIntermediate and therapeutic doses appear to have a higher risk of bleeding without a decrease of VTE events and mortality in critically ill COVID-19 patients.
Evaluar la incidencia de eventos trombóticos, sangrado y mortalidad comparando diferentes regímenes de anticoagulación en pacientes ingresados en unidades de Cuidados Intensivos (UCI) por COVID-19.
DiseñoEstudio de cohortes retrospectivo observacional y analítico.
ÁmbitoPacientes con COVID-19 ingresados en una UCI de un hospital terciario entre marzo y abril del 2020.
PacientesSe incluyó a un total de 201 pacientes de UCI ingresados por COVID-19. Los pacientes se categorizaron en 3 grupos en función de la dosis de anticoagulación más alta recibida durante el ingreso: profiláctica, intermedia y terapéutica.
IntervencionesSe comparó la incidencia de eventos trombóticos, hemorragia y mortalidad entre los grupos. Se realizaron 2 regresiones logísticas multivariables para comprobar la asociación entre los eventos trombóticos y el sangrado con el régimen anticoagulante.
Principales variables de interésEventos trombóticos, sangrado y mortalidad.
ResultadosDe los pacientes incluidos, 78 recibieron dosis profilácticas, 94 intermedias y 29 terapéuticas. No se encontraron diferencias en los eventos trombóticos y la mortalidad entre grupos, mientras que los sangrados fueron más frecuentes en el grupo de dosis terapéutica (31%) e intermedia (15%) que en el grupo de dosis profiláctica (5%) (p <0,001 y p <0,05, respectivamente). El régimen anticoagulante fue el mayor determinante de sangrado (odds ratio 2,4;, intervalo de confianza del 95%, 1,26-4,58; p=0,008) pero no tuvo ningún impacto en los eventos trombóticos.
ConclusionesLas dosis intermedias y terapéuticas parecen tener un mayor riesgo de sangrado sin una disminución de los eventos trombóticos ni la mortalidad en pacientes de UCI con COVID-19.
The coronavirus disease 2019 (COVID-19) pandemic is responsible for high intensive care unit (ICU) admission rates and high mortality.1–3 COVID-19 is also associated to a high risk of venous thromboembolism (VTE) and a significant coagulopathy that correlates with disease severity.1,3,4 In this sense, high D-dimer levels, fibrinogen and C-reactive protein (CRP), as well as prolonged prothrombin time (PT), have been associated with increased need of ICU admission and worse outcomes.1,4,5 Previous studies have described a rate of VTE in ICU patients on standard-dose VTE prophylaxis that varies between 7.6% and 69%.2,5,6 Recent studies have suggested a decreased mortality associated with anticoagulant treatment in severe COVID-19.4,7,8 Consequently, the Italian Medicines Agency and some thrombosis Medical Societies recommend the use of intermediate-dose VTE prophylaxis in selected patients with major risk of thrombosis (ICU patients, weight over 80kg or high D-dimer levels).9–11
Remarkably, Al-Samkari and colleagues found that elevated D-dimer levels at admission predicted not only thrombotic complications, critical illness and death, but also bleeding complications.5 Due to these findings, it has been postulated that the elevated D-dimer levels, thrombotic manifestations and bleeding events are related to a coagulation system activation in the setting of severe inflammation and that local thrombi both in the lungs and other organs, rather than emboli from peripheral veins, appear to be the hallmark of severe COVID-19.12 In fact, post-mortem studies have highlighted marked pathological changes involving the lung microvasculature, including disseminated micro-thrombi and significant haemorrhagic necrosis,13 which suggest that the diffuse bilateral pulmonary inflammation observed in COVID-19 is associated with a novel pulmonary-specific vasculopathy termed pulmonary intravascular coagulopathy.1
In consequence, considering that therapeutic doses of heparin are not indicated for treatment of other types of thrombotic microangiopathies, higher than prophylactic doses of heparin may not be more effective preventing VTE and can yield an increased risk of bleeding (especially in critically ill patients who have major thrombosis risk, but also high bleeding risk,14 thereby worsening their prognosis.
Until now, there is a lack of evidence for the risk-benefit of intermediate or therapeutic doses in ICU patients with COVID-19 infection. The aim of this study was to evaluate the rate of thrombosis, bleeding and mortality comparing prophylactic, intermediate and therapeutic doses of anticoagulation in critically ill COVID-19 patients.
Patients and methodsPatients and data collectionWe conducted an observational and analytical retrospective cohort study at a university hospital in Barcelona. All patients consecutively admitted to the ICU of the Hospital between March 1 and April 30, 2020, aged>18 years old and with confirmed diagnosis of COVID-19 infection (defined as a positive SARS-CoV-2 reverse-transcriptase polymerase chain reaction test by nasopharyngeal/oropharyngeal swab or sputum specimen) were included. Patients without a confirmed COVID-19 infection were excluded. Patient’ data were obtained retrospectively and included: demographics, relevant comorbidities, invasive and non-invasive mechanical ventilation, bleeding events, venous thrombotic events, anticoagulation administered and mortality during the hospitalization. We also included inflammatory laboratory parameters (D-dimer, CRP, lactate dehydrogenase [LDH] and ferritin) at admission and at time of bleeding and thrombotic events.
The local institutional ethics committee approved the study, waiving the need for informed consent from individual patients because of its retrospective nature (HCB/2020/0273).
Anticoagulant dosePatients were categorized in three groups depending on the highest anticoagulant dose they received during the hospitalization, administered for at least 2 days, prior to a VTE confirmed event: prophylactic, intermediate and therapeutic anticoagulation. The choice of dosing strategy followed the local thrombosis prophylaxis protocol for COVID-19 patients. According to this protocol, prophylactic doses were recommended for all COVID-19 patients who require hospital admission. Intermediate or therapeutic doses were recommended for patients with body weight>80kg, patients with an added risk factor for VTE (cancer, previous VTE, thrombophilia, recent surgery, pregnancy or use of estrogens) or patients who had a persistent high D-dimer level (>3000ng/ml) dissociated from other inflammatory parameters (CRP, ferritin). The decision of using intermediate or therapeutic dose was taken by the medical team responsible for the patient. Table 1 summarizes the different doses used.
Doses of heparin administered in the three anticoagulation regimens.
| Prophylactic dose | Intermediate dose | Therapeutic dose | ||||
|---|---|---|---|---|---|---|
| CrCL>30ml/min | CrClr<30ml/min | CrCl>30ml/min | CrCl<30ml/min | CrCl>30ml/min | CrCl<30ml/min | |
| Enoxaparin (sc) | 40mg/24h | 20mg/24h | If>80kg: 60mg/24hIf other condition: 1mg/kg/24h | If >80kg: 40mg/24hIf other condition: 0.5mg/kg/24h | 1.5mg/kg/24hor1mg/kg/12h | 1mg/kg/24h |
| Tinzaparin (sc) | 4500IU/24h | 4500IU/24h | If >90kg: 50UI/kg/24hIf other condition: 75UI/kg/24h | If >90kg: 50IU/kg/24hIf other condition: 75IU/kg/24h | 175IU/kg/24h | 175IU/kg/24h |
| Bemiparin (sc) | 3500IU/24h | 2500IU/24h | 5,000UI/24h | 3500IU/24h | 115IU/kg/24h | 85IU/kg/24h |
| Fondaparinux (sc) | 2.5mg/24h | 1.5mg/24h | 5mg/24h | 2.5mg/24h | <50kg: 5mg/24h51–100kg: 7.5mg/24h>100kg: 10mg/24h | No recommended |
Abbreviations: MG: milligrams; IU: international units; kg: kilograms; h: hours. Sc: subcutaneous; mg: milligrams; Crcl: calculated creatinine clearance.
Bleeding events were graded according to the International Society of Thrombosis and Haemostasis definition, being categorized as major bleedings those with the following criteria: (1) Fatal bleeding, and/or (2) Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or (3) Bleeding causing a fall in haemoglobin level of 20g/L) or more, or leading to transfusion of two or more units of whole blood or red cells.15
Pulmonary embolism (PE) and deep vein thrombosis (DVT) were confirmed with a CT pulmonary angiogram (CTPA) and Doppler ultrasound respectively when there was a clinical suspicion. Synchronously diagnosed DVT and PE in the same patient were considered one VTE event.
Statistical analysisContinuous variables are presented as mean with standard deviation (SD), while categorical or binary data are shown as frequencies and proportions. To compare the demographics, clinical characteristics, incidence of VTE, bleeding and mortality depending on the anticoagulant regimen we used the chi-squared or Fisher's exact tests for categorical data, and the ANOVA test for quantitative continuous data. Pairwise comparisons were performed with chi-squared or Fisher's exact tests for categorical data and with Student's t-test and corrected by Bonferroni test when continuous data was analyzed.
Initial values on admission (CRP, LDH, ferritin and D-dimer) were compared in patients with and without thrombotic complications; as well as in patients with and without bleeding complications using the Mann–Whitney U test.
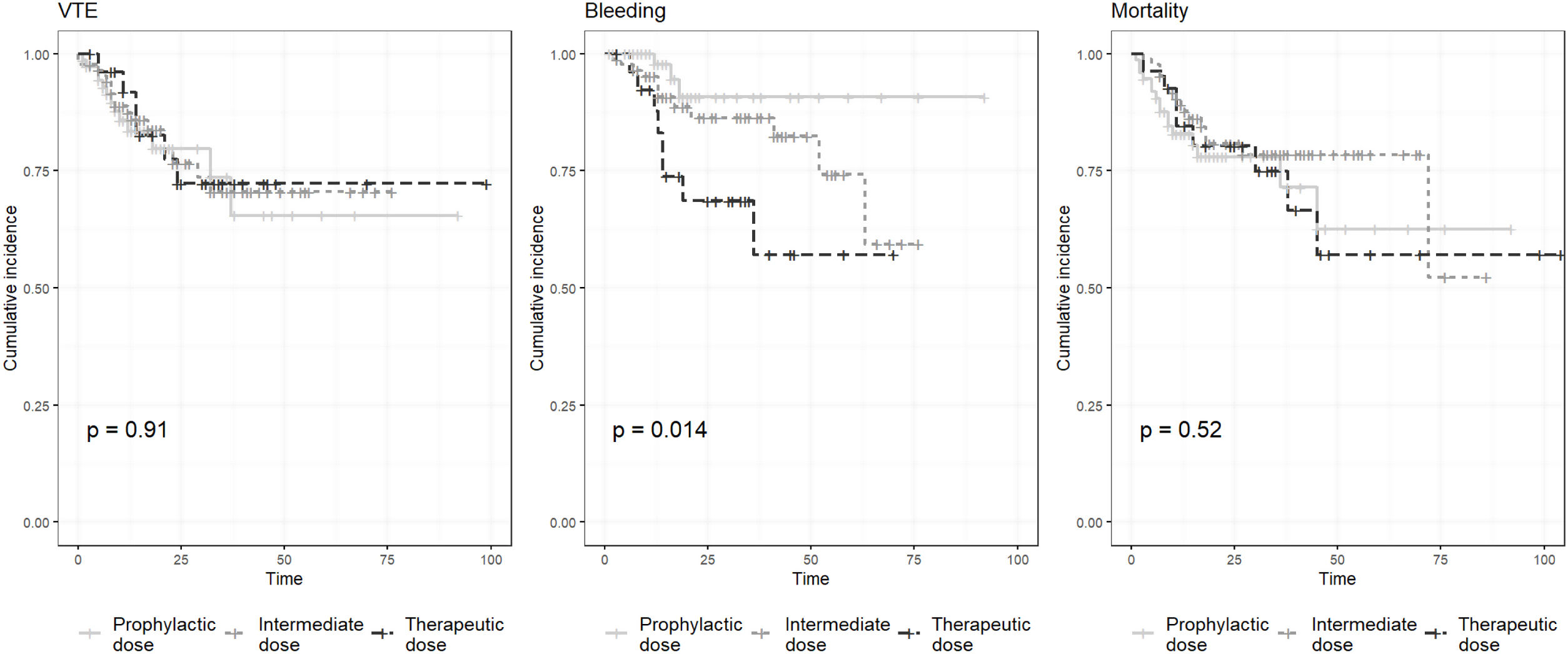
We performed 3 Kaplan–Meier curves, using the Log-rank test, in order to compare the cumulative incidence of VTE, bleeding and mortality between groups (prophylactic, intermediate and therapeutic anticoagulant doses).
We performed a logistic multivariable regression to test the association between VTE or bleeding and clinical characteristics as well as the anticoagulant regimen. The anticoagulant regimen was stratified in 3 groups (prophylactic, intermediate or therapeutic) using the prophylactic group as the reference one. The dependent variables were VTE and bleeding complication. The independent variables were age, sex, obesity, presence of comorbidity, moderate-severe renal impairment, invasive mechanical ventilation (IMV), D-dimer levels on admission and the anticoagulant category (prophylactic, intermediate or therapeutic doses). Two-sided p<0.05 was considered statistically significant for all analyses. Analyses were performed using SPSS v22.
ResultsBaseline patient characteristicsA total of 201 patients were included. Their mean age was 62 years (SD 12.9) and 142 (71%) were males. 82% of the patients had at least one comorbidity, the most frequent ones being: hypertension (54%), dyslipidaemia (33%), diabetes (19%), pulmonary disease (17%) and heart disease (17%). 15 patients (7.5%) were on therapeutic anticoagulant treatment before the admission: 10 (67%) for atrial fibrillation, 3 (20%) for VTE, 1 (6%) for mechanical aortic valve prosthesis and 1 (6%) for Antiphospholipid syndrome. From these, 13 received therapeutic dose during admission, 1 received intermediated dose and 1 received prophylactic dose. The mean follow-up from ICU admission to hospital discharge or death was 25.4 days (SD 20.5).
Seventy-eight patients (39%) received prophylactic doses, 94 (47%) intermediate doses and 29 (14%) therapeutic doses of anticoagulation, which consisted of low-molecular weight heparin in all patients.
Venous thromboembolismIn our cohort, 41 patients (20%) had a VTE complication, PE being the most frequent one (83%), followed by DVT (15%). PE was mainly peripheral (79%). The mean time between ICU admission and a VTE event was 12.6 days (SD 12.8). On admission, D-dimer levels were significantly higher in the VTE group compared to the non-VTE group (4361ng/mL [SD 9,600] vs 1754ng/mL [SD 3,374]; p=0.015). No significant differences in CRP, ferritin and LDH were found. At the time of VTE diagnosis, the mean D-dimer was 7511ng/ml (SD 4,633), CRP 3.35mg/dl (SD 4.64), ferritin 1161ng/ml (SD 874) and LDH 373U/L (SD 159).
BleedingThe incidence of bleeding was 13% and of major bleeds 8%. The most frequent localizations of all bleeds were central nervous system (CNS) (21%), muscular haematoma (21%), retroperitoneal (17%) and gastrointestinal bleeding (17%). The mean time between ICU admission and the first bleeding event was 14.7 days (SD 12.6). When we compared the inflammatory parameters on admission in patients with and without bleeding complications, we did not find differences between groups in any parameter (D-dimer, CRP, ferritin and LDH). At the time of bleeding, the mean of the blood parameters was D-dimer 4445ng/ml (SD 3,391), CRP 4.86mg/dl (SD 7), ferritin 1875ng/ml (SD 1,257) and LDH 410U/L (SD 235).
Mechanical ventilationA total of 112 patients (56%) required IMV and 31 (15%) needed non-invasive mechanical ventilation. There were no statistically significant differences in the anticoagulant regimens between patients who required IMV and the ones who did not (p=0.25). There were no significant differences in VTE between subjects with or without IMV (23% vs 17%, p=0.29). Subjects requiring IMV presented more bleeding events than patients who did not (20% vs 6%, p=0.004). Bleedings in those requiring IMV were mostly major bleeds (59%), being the most frequent sites of them the CNS (25%), muscular haematoma (25%) and retroperitoneal (20%).
MortalityThe overall in-hospital mortality was 20%, out of which 80% were related with COVID-19 complications. In-hospital mortality was higher in patients with VTE than in those without VTE, but there was not a statistically significant difference (10% vs 24%; p=0.054). There were also no differences in the mortality rate between patients with and without bleeding complications (26% vs 20%, p=0.46).
Clinical impact of anticoagulation regimen on the risk of VTE, bleeding and mortalityThe characteristics of the patients depending on the anticoagulant dose received (prophylactic, intermediate o therapeutic dose) are shown in Table 2.
Characteristics of the patients depending on the anticoagulant dose received.
| Prophylactic dose(n=78) | Intermediate dose(n=94) | Therapeutic dose(n=29) | |
|---|---|---|---|
| Gender, male n (%) | 56 (72%) | 65 (69%) | 21 (72%) |
| Age in years, mean (SD) | 59.5 (13.6) | 62.4 (12.5) | 68.1 (9.6)a,b |
| Obesity, n (%) | 3 (4%) | 19 (20%)c | 3 (10%) |
| Smoker, n (%) | 7 (9%) | 2 (2%) | 2 (7%) |
| Comorbidities, n (%) | 62 (80%) | 76 (81%) | 27 (93%) |
| Immunosuppression, n (%) | 7 (9%) | 10 (11%) | 0 (0%) |
| Need of IMV, n (%) | 39 (50%) | 54 (57%) | 19 (66%) |
| Need of vasoactive support, n (%) | 40 (51%) | 37 (39%) | 14 (48%) |
| VTE, n (%) | 14 (18%) | 21 (22%) | 6 (21%) |
| Bleeding, n (%) | 4 (5%) | 14 (15%)d | 9 (31%)a |
| Major, n (%) | 2 (3%) | 8 (9%) | 6 (21%)c |
| CNS | 1 (25%) | 1 (13%) | 3 (50%) |
| Retroperitoneal | 1 (25%) | 2 (25%) | 1 (17%) |
| Gastrointestinal | 0 (0%) | 5 (63%) | 2 (33%) |
| Non-major, n (%) | 2 (3%) | 6 (6%) | 3 (10%) |
| Mortality, n (%) | 17 (22%) | 17 (18%) | 8 (28%) |
Abbreviations: PE: pulmonary embolism; DVT: deep venous thrombosis; IMV: invasive mechanical ventilation; VTE: venous thromboembolism; CNS: central nervous system.
There were no differences between groups in sex, comorbidities, immunosuppression, requiring IMV or vasoactive support. Mortality rate was also not significantly different among groups (22% vs 18% vs 28%, respectively; p=0.49).
Patients receiving intermediate dose had higher body weight than patients on standard dose (p=0.002). Patients on therapeutic doses were older than patients on prophylactic dose (p=0.013).
The incidence of VTE in patients receiving prophylactic dose was 18%, in those receiving intermediate dose was 22% and in those with therapeutic dose was 21%, without statistically significant differences between them (p=0.8).
Bleeding events were more frequent in the intermediate (15%) and therapeutic doses (31%) groups than in the prophylactic dose group (5%) (p=0.03 and p=0.001 respectively). Major bleedings were more frequent in patients with therapeutic dose (21%) than in those with prophylactic dose (3%) (p=0.004). The most frequent bleeding location was the CNS (50%) in the therapeutic dose group and the gastrointestinal bleeding (62.5%) in intermediate dose group.
When we compared patients receiving prophylactic versus intermediate and therapeutic doses, there were no differences in mortality (22% vs 20%, p=0.86) or VTE (18% vs 22%, p=0.59), but there was a significant difference in overall bleeding rates (5% vs 19%, p=0.006) and in major bleeding events (3% vs 11%; p=0.03).
We performed 3 Kaplan–Meier curves in order to analyze the differences in cumulative incidence of VTE, bleeding and mortality (Fig. 1). We found statistical significant differences between groups in bleeding events (p=0.014), but not in VTE (p=0.91) and mortality (p=0.52).
Finally, to assess whether the anticoagulant dose and other clinical characteristics influence the risk of VTE and bleeding, a logistic regression analysis was performed (Table 3). In the analysis, only the male sex was associated with VTE. Regarding bleeding complications, the therapeutic anticoagulant regimen was the strongest determinant for bleeding (OR, 5.93; 95% CI, 1.55–22.7). We also found that those with IMV have a higher risk of bleeding (OR, 4.25; 95% CI, 1.47–12.29). The other clinical characteristics did not influence the bleeding risk in our model.
Logistic multivariable regressions.
| Venous thromboembolism | Bleeding | |||
|---|---|---|---|---|
| Clinical characteristics | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value |
| Age | 1.03 (0.99–1.06) | 0.11 | 1.03 (0.99–1.09) | 0.17 |
| Male Sex | 0.38 (0.16–0.94) | 0.03 | 0.43 (0.15–1.24) | 0.12 |
| Obesity | 2.19 (0.72–6.65) | 0.16 | 1.28 (0.32–5.03) | 0.73 |
| Comorbidities | 0.77 (0.28–2.15) | 0.62 | 1.31 (0.32–5.37) | 0.7 |
| Renal impairment | 0.45 (0.05–3.95) | 0.47 | 0.7 (0.07–6.82) | 0.76 |
| IVM | 1.55 (0.75–3.24) | 0.24 | 4.25 (1.47–12.29) | 0.008 |
| Anticoagulant dose | ||||
| Prophylactic vs intermediate doses | 1.13 (0.51–2.51) | 0.77 | 3.1 (0.94–10.45) | 0.064 |
| Prophylactic vs therapeutic doses | 0.87 (0.29–2.68) | 0.81 | 5.93 (1.55–22.72) | 0.009 |
Abbreviations: IMV: invasive mechanical ventilation; CI: confidence interval; vs: versus.
The aim of our study was to assess the rates of VTE, bleeding and mortality in critically ill COVID-19 patients and explore if higher doses of anticoagulation (intermediate and therapeutic) can offer a clinical benefit to these patients without increasing the bleeding risk. Our results show that patients receiving intermediate or therapeutic doses of heparin appear to have a higher risk of bleeding without a decrease of VTE events and mortality.
Patients were divided in three groups depending on the maximum anticoagulant doses they received during the hospitalization prior to a VTE confirmed event. Regarding clinical characteristics, patients in the intermediate dose group were most frequently obese, which was previously expected, as in our local protocol patients with>80kg must receive higher anticoagulant doses. Patients of the intermediate and therapeutic anticoagulant group were older than the ones of the prophylactic group. These results may be explained by the fact that older people usually present higher D-dimer levels than young people16 and, in consequence, due to the indication of higher anticoagulant doses when D-dimer levels were>3000ng/ml, older people could have been more susceptible to receive higher doses.
We found an overall VTE rate of 20%, which is consistent with previous data in the literature.2,5,6 PE was the most frequent form of presentation (80%), but it is important to remark that 80% of them were peripheral, which might not have the same clinical relevance as a central PE. When we explored the prevalence of VTE in the different anticoagulant groups, we did not find significant differences. Taking into account a type I error rate of 0.05, an event rate of VTE of 0.20 and a minimum detectable effect of VTE between groups of 0.15, we estimate a probability of type II error of 0.22 in this analysis. These results are very similar to the ones obtained in a recent published meta-analysis that compares the rate of VTE and bleeding with different intensity of anticoagulation17 and are also in consonance with de recent published INSPIRATION Randomized Trial, which compares intermediate dose versus standard dose prophylactic anticoagulation in patients with COVID-19 admitted to the ICU.18
In contrast to numerous reports assessing the high prevalence of VTE, only few studies have reported bleeding rates. These rates vary between 3 and 7.6% using prophylactic doses,5,7 and between 1.9 and 21.4%17,19–21 with higher anticoagulant doses.
Our results show a high bleeding rate (13%), including a high rate of major bleedings (8%), especially in patients receiving therapeutic anticoagulant doses. These results differ from those obtained by Mattioli et al.,19 who described lower rates (1.9% of major bleeding) in a cohort of 105 hospitalized patients with intermediate dose. Nevertheless, it is important to note that while these were not strictly ICU patients, the authors report that 6.7% of the patients needed transfusion of red blood cells, which can signal that the rate of overall haemorrhage was higher. A higher incidence, and more similar to our results, has been reported by Kessler and colleagues,20 who reported 6% of major bleeding in patients with intermediate doses and a 4.6% of fatal bleeding with full anticoagulant doses, which is not insignificant. Pesavento et al.21 also described a higher rate of relevant bleeding events (Hazard ratio [HR] 3.89; 95% CI, 1.9–8) in patients treated with (sub) therapeutic doses of anticoagulants instead of prophylactic doses.
We did not found statistically significant differences in bleeding between the intermediate and the therapeutic anticoagulant group (p=0.06), despite it seems to be clinically relevant (15% vs 31% respectively).
To confirm our results, we performed a multivariable analyses that showed a significant association between the therapeutic anticoagulant doses and bleeding events (OR, 5.93; 95% CI, 1.55–22.7), but not with the intermediate doses, despite it seems to be a clinical impact (OR, 3.1; 95% CI 0.94–10.45). We also found an association between the need of IMV and bleeding, which is consistent with the results obtained with Paranjpe et al.7 who describe higher bleeding rates in intubated patients than in non-intubated patients. This association may be in relation to disease severity. In contrast, we did not find any association between the anticoagulant regimen and VTE events.
We found no differences in mortality rates between groups, contrary to the results obtained by Jonmarker S et al.,8 who described a 67% decreased risk of death the first 28 days among those who received high vs low dose prophylaxis, without a major rate of bleeding. These differences can be partially explained because in our study, we followed patients until hospital discharge (not only the first 28 days), so we registered later bleeding events and mortality related with complications due to large hospitalization period. In contrast, our results are consistent with dose obtained in the INSPIRATION Randomized Trial18 and with those described in a pre-print article based on a recent international multiplatform randomized clinical trial, which concludes that therapeutic anticoagulation did not improve hospital survival in sever Covid-19 patients compared with standard doses.21
According to our results, a significant increase in bleeding rates overall, and especially in patients with intermediate (15%) and therapeutic (31%) anticoagulant doses, should raise awareness of this complication in COVID-19 critically ill patients. This high bleeding rates suggest that the presence of organ failure, coagulopathy and need for invasive interventions may increase the risk of bleeding on their own, and be further aggravated by adding high anticoagulant doses.
Nevertheless, due to the fact that higher anticoagulant doses are probably given to patients that are at highest risk of VTE, the absence of differences of VTE between groups could mean that with higher doses we may be preventing these patients of a VTE event that, otherwise, could have happened. In this sense, when suspected, the confirmation of the VTE events radiologically is important to adequate as soon as possible the anticoagulant dose.
Overall, it seems that intermediate anticoagulant doses may present a better benefit-risk profile in critically ill COVID-19 patients than the prophylactic and therapeutic doses, being able to prevent VTE events with lowest bleeding rates than therapeutic doses.
In this sense, there appears to be a need for large prospective randomized clinical trials to establish the real risk-benefit of higher than prophylactic dose in critically ill COVID-19 patients.
LimitationsThe present study has some limitations and the results must be interpreted with caution. First, this is a retrospective study, so it lacks the rigour of a prospective randomized study. Second, as it was a non-interventional study, treatment strategies were conditioned by the clinical practice. Third, there were fewer patients in the therapeutic anticoagulant dose group than in the prophylactic and intermediate groups (29 versus 94 and 78 respectively), limiting statistical power. Finally, we did not have reliable data on the use of aspirin and were therefore unable to include this variable in the logistic regression analysis.
ConclusionOur results show a significantly higher risk of bleeding and of major bleedings in patients receiving intermediate and therapeutic anticoagulant doses without a decrease in VTE events and mortality among critically ill COVID-19 patients.
Author contributionsC. Gabara was involved in data collection, data analysis and interpretation and drafting the article.
B. Solarat was involved in data collection and critical revision of the article.
P. Castro was involved in data collection and critical revision of the article.
S. Fernández was involved in data collection and critical revision of the article.
J. Badia was involved in data collection and critical revision of the article.
D. Toapanta was involved in data collection and critical revision of the article.
S. Schulman was involved in critical revision of the article.
J.C. Reverter was involved in data collection and critical revision of the article.
A. Soriano was involved in data collection and critical revision of the article.
J. Moisés was involved in the conception, design of the work, critical revision of the article.
J. Aibar was involved in the conception, design of the work, critical revision of the article.
All authors read, revised and approved the final manuscript.
FundingThis work has been funded by Contractes Clínic de Recerca “Emili Letang-Josep Font” 2020 (CG).
Conflict of interestsThe authors do not report conflicts of interest related to this article.
A. Almuedo, J.R. Alonso, R. Andrea, F. Aziz, E. Barbeta, X. Borrat, E. Bragulat, I. Carmona, M. Castellà, O. De Diego, M. Farrero, J. Fernández, C. Ferrando, M. Ferrer, M. Forga, F. Fuentes, E. Guasch, Mª Hernández-Tejero, A. Jacas, P. Leyes, T. López, JA. Martínez, G. Martínez-Palli, R. Mellado, J. Mercadal, G. Muñoz, J. Muñoz, R. Navarro, JM Nicolás, J. Ortiz, E. Poch, M. Pujol, E. Quintana, E. Reverter, I. Rovira, P. Ruiz, E. Sandoval, S. Schneider, F. Seguí, O. Sibila, D. Soy, M. Suárez, A. Téllez, A. Torres, X. Urra, H. Ventosa.