Intracranial hypertension (ICH) has a negative influence on the prognosis of severe cranioencephalic trauma (SCET). In 10–15 per cent of the cases the ICH does not respond to conventional therapeutics, is considered “refractory”, and associates high mortality.1,2 Refractory ICH has no validated therapy.1 The only available options are: barbiturates, hypothermia, deep hyperventilation or decompressive craniectomy (DC), none of which has proven to be effective conclusively.1,2
Why should we perform a DC then?
The DC increases the capacity of the cranial cavity in order to be able to tolerate volume increases of cerebral parenchyma that are proportional to the size of the DC.3,4 Depending on when it is performed we can distinguish 2 different types: “primary”, usually during the evacuation of an acute subdural haematoma associated to brain swelling; and “secondary”, when the traditional measures for ICH control fail, the DC may be bifrontal or frontotemporoparietooccipital, whether unilateral or bilaterally.1,3,4 The DC is considered beneficial for the management of malignant infarctions in the medial cerebral artery, while for the management of SCET its utility is controversial,1,3,4 although it is confirmed that reduces intracranial pressure (ICP), increases the cerebral perfusion pressure, the cerebral blood flow, and brain oxygenation, while reducing the therapeutic effort needed to control ICH.1,3,4
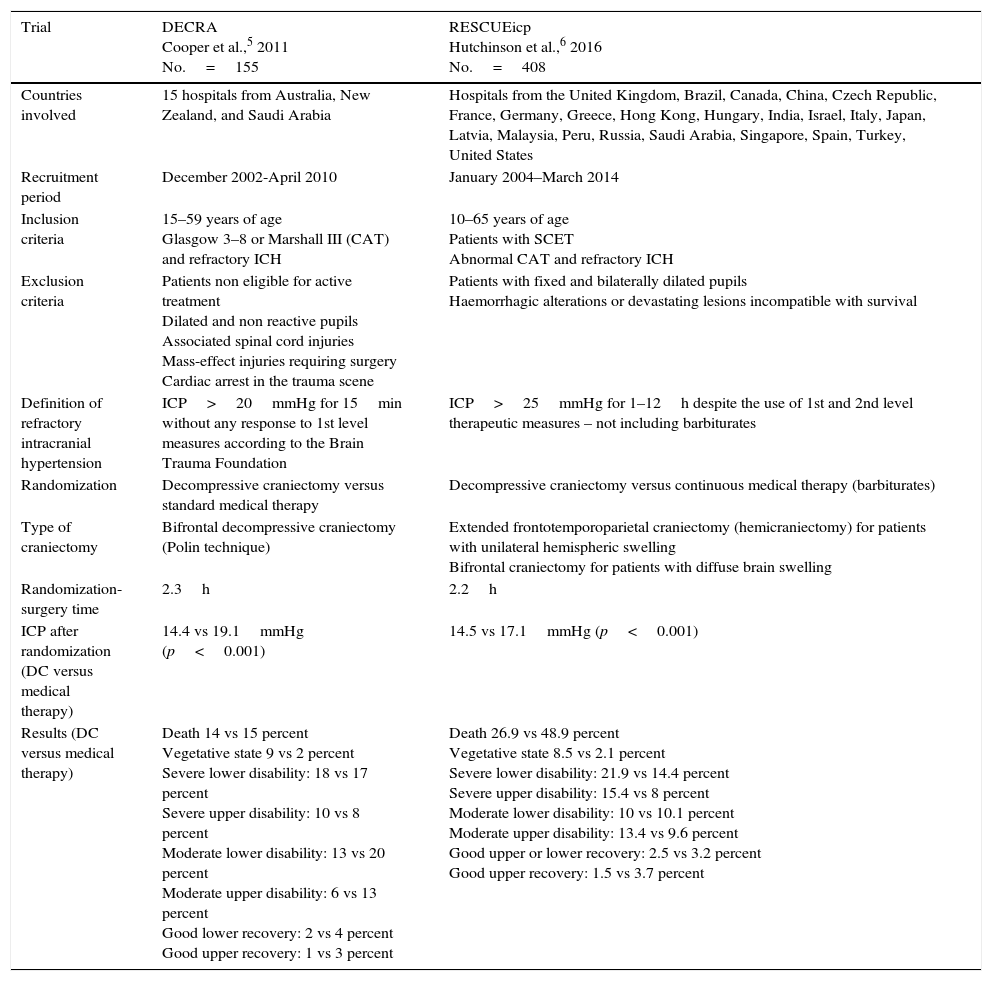
Cochrane does not advocate for its routine performance to reduce the chances of poor results in adults with SCETs, and refractory ICH.7 One meta-analysis conducted confirmed that 47 per cent of the patients treated with DC achieved favourable results 6 months after the event.8 Recently, 2 prospective randomized controlled trials – DECRA,5 and RESCUEicp6 tried to improve the scientific evidence available today. Table 1 shows the characteristics from both trials.
Main characteristics of the trials under analysis.
| Trial | DECRA Cooper et al.,5 2011 No.=155 | RESCUEicp Hutchinson et al.,6 2016 No.=408 |
|---|---|---|
| Countries involved | 15 hospitals from Australia, New Zealand, and Saudi Arabia | Hospitals from the United Kingdom, Brazil, Canada, China, Czech Republic, France, Germany, Greece, Hong Kong, Hungary, India, Israel, Italy, Japan, Latvia, Malaysia, Peru, Russia, Saudi Arabia, Singapore, Spain, Turkey, United States |
| Recruitment period | December 2002-April 2010 | January 2004–March 2014 |
| Inclusion criteria | 15–59 years of age Glasgow 3–8 or Marshall III (CAT) and refractory ICH | 10–65 years of age Patients with SCET Abnormal CAT and refractory ICH |
| Exclusion criteria | Patients non eligible for active treatment Dilated and non reactive pupils Associated spinal cord injuries Mass-effect injuries requiring surgery Cardiac arrest in the trauma scene | Patients with fixed and bilaterally dilated pupils Haemorrhagic alterations or devastating lesions incompatible with survival |
| Definition of refractory intracranial hypertension | ICP>20mmHg for 15min without any response to 1st level measures according to the Brain Trauma Foundation | ICP>25mmHg for 1–12h despite the use of 1st and 2nd level therapeutic measures – not including barbiturates |
| Randomization | Decompressive craniectomy versus standard medical therapy | Decompressive craniectomy versus continuous medical therapy (barbiturates) |
| Type of craniectomy | Bifrontal decompressive craniectomy (Polin technique) | Extended frontotemporoparietal craniectomy (hemicraniectomy) for patients with unilateral hemispheric swelling Bifrontal craniectomy for patients with diffuse brain swelling |
| Randomization-surgery time | 2.3h | 2.2h |
| ICP after randomization (DC versus medical therapy) | 14.4 vs 19.1mmHg (p<0.001) | 14.5 vs 17.1mmHg (p<0.001) |
| Results (DC versus medical therapy) | Death 14 vs 15 percent Vegetative state 9 vs 2 percent Severe lower disability: 18 vs 17 percent Severe upper disability: 10 vs 8 percent Moderate lower disability: 13 vs 20 percent Moderate upper disability: 6 vs 13 percent Good lower recovery: 2 vs 4 percent Good upper recovery: 1 vs 3 percent | Death 26.9 vs 48.9 percent Vegetative state 8.5 vs 2.1 percent Severe lower disability: 21.9 vs 14.4 percent Severe upper disability: 15.4 vs 8 percent Moderate lower disability: 10 vs 10.1 percent Moderate upper disability: 13.4 vs 9.6 percent Good upper or lower recovery: 2.5 vs 3.2 percent Good upper recovery: 1.5 vs 3.7 percent |
The DECRA trial assessed the effectiveness of DC versus the optimal medical therapy. Although the DC was able to control the ICH and minimized the days on mechanical ventilation and ICU stay, it was not able to improve the results.5 However, this study reaffirmed the capacity of the DC to control the ICH and reduce the therapeutical effort as the shorter ICU stays and mechanical ventilation courses confirmed. With doubts we claim that the DECRA trial needed 8 years to recruit 155 patients from 15 different centres. The recruitment rate (4.5 per cent) limits the study to a selected subpopulation. Its population size is, therefore, insufficient to detect 10 per cent differences between groups; it would have been needed to study 321 patients randomized on a 1:1 basis.9 Although the baseline characteristics of both groups were well balanced, the presence of bilateral non-reactive pupils was greater in the DC group (27 versus 12 per cent). This is important because after adjusting for this variable, no significant differences were seen in the results obtained 6 months after the trauma. Another consideration here is the definition of ICH (ICP>20mmHg for more than 15min) since most intensivists or neurosurgeons do not perform DCs under such circumstances. The technique used was incomplete. Since the falx cerebri was not dissected, the space needed to allow the expansion of the anterior brain simply was not there. Lastly, the result assessment using the dichotomized GOS-E is controversial and according to Murray et al., it should take into account the subject's clinical conditions prior to randomization.10
The RESCUEicp trial assessed the secondary type of DC compared to continuous medical treatment with barbiturates in patients with SCET and ICH refractory to 1st and 2nd level therapeutical measures.6 Six months after the SCET, mortality was lower in the DC group, but the rates of vegetative state, and serious disability rose, while the percentage of moderate disability and good recovery were similar in both groups.6 The size of the sample was adequate and populations were well balanced. The average interval of time elapsed between randomization and surgery was correct. The presence of ICH was confirmed whenever the ICP exceeded 25mmHg, yet despite the use of 1st and 2nd level therapeutical measures from 1 to 12 hours. The ICP was better controlled and the ICH had a shorter duration in the DC group (p<0.001).
The setbacks would be an inclusion rate of 20.36 per cent after 10 years recruiting. There are not ICP data prior to randomization. The medical therapeutics was established “at the discretion of the treating medical team” in a heterogeneous way and without a uniform protocol. There are no data on the level of therapeutical intensity prior to randomization and the therapeutical protocol was modified during the clinical trial. Some aspects that were not fully clarified may have confounded the results; the management of cerebral perfusion pressure is an example of this. In the protocol it is recommended to maintain the cerebral perfusion pressure figures>60mmHg as opposed to what the actual guidelines say. Sixty-three per cent of the patients underwent bifrontal decompressions, however, the final results have not been adjusted based on the technique used. From the mechanical point of view of brain tissue, bifrontal DCs have different implications from hemispheric DCs. On the other hand, 37 per cent of the subjects from the medical group crossed to the DC group. It does not say when, why or what technique was used
The dichotomized GOS-E was used for result assessment. Despite the complexity of the statistical analysis, when taking a look at result assessment from the RESCUEicp, when the GOS-E was traditionally discriminated at 12 months in the favourable group, in the patients included in the categories “moderate upper and lower disability”, and “good upper and lower recovery” the surgical group (32 versus 28.5 per cent) showed a clear trend towards better results.
Both these trials show that the DC controls the ICH, with no benefits on the final results. The SCET is one dynamic heterogenous entity where controlling the ICP is important but not enough to improve the results. The conclusions of these two trials are not applicable to the primary DC or hemicraniectomy – procedures usually conducted in countries that have a large number of SCETs and limited resources of neuromonitoring. Doubts and controversies still remain. The ongoing Rescue-ASDH trial4 is investigating the effectiveness of primary DCs for the management of patients with acute subdural haematomas. If we take into consideration the available evidence, the secondary bifrontal DC should not be considered a useful therapy for the management of refractory ICH. When it comes to uni or bilateral hemispheric DC, there are no conclusive data, but we believe that it may be considered an option in certain selected cases (diffuse lesions type iii or iv).
Conflicts of interestsThe authors declare no conflicts of interest whatsoever.
Please cite this article as: Godoy DA, Moscote Zalazar LR, Rubiano A, Muñoz-Sánchez Á, Lubillo S, Murillo-Cabezas F. Craniectomía descompresiva secundaria para el manejo de la hipertensión endocraneanal refractaria en el traumatismo craneoencefálico grave. Luces y sombras de los estudios recientes. Med Intensiva. 2017;41:487–490.