Oral anticoagulants are widely used to prevent and treat venous and arterial thrombosis. In recent years, there has been a transition from vitamin K antagonists (VKAs) to direct oral anticoagulants (DOACs) such as rivaroxaban, edoxaban, apixaban, and dabigatran. While the risk of bleeding is nearly 40% lower with DOACs compared to VKAs, it is still significant. In large population studies, the incidence of major bleeding ranges from 1.5% to 3% per year with DOACs,1 with a mortality rate due to major bleeding of 7.6% (95%CI, 6.5–8.7).2 In the United States alone, it is estimated that up to 6% of drug-related visits to the ER are associated with the use of DOACs.3
Importance of pharmacokinetics and monitoring of DOACs in patients with critical bleedingTo treat DOAC-related bleeding, it is of paramount importance to know their pharmacokinetics, clearance, and time elapsed since the last dose. DOACs reach peak levels between 2 and 4 h after being administered, their half-life ranges from 6 to 14 h in patients with normal renal function, and their anticoagulant effect disappears within 24 h, being reversal uncommon beyond that point in time. We should assess drug concentration using special tests such as ecarin clotting time (dabigatran) or anti-Xa chromogenic assays (factor Xa inhibitors). However, these tests are not always available in the labs at the ER, and we should mention that conventional coagulation tests do not exclude significant drug concentrations.4
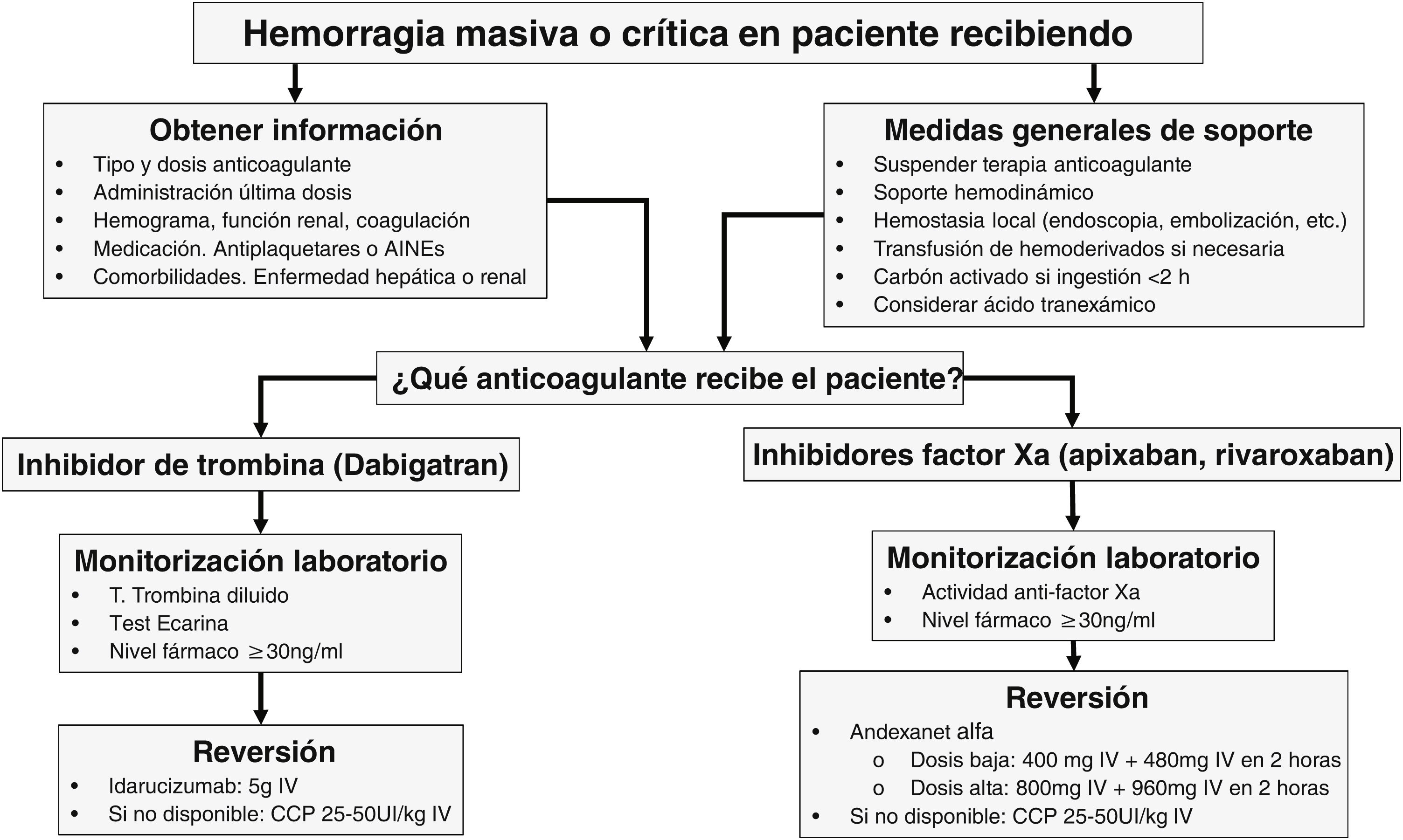
When to reverse?The phases to treat acute bleeding in patients on DOACs can be summarized by the “4R” rule: Review (last dose), Remove (activated charcoal), Repair (surgery, embolization), and Reverse (specific or prohemostatic agents).
The treatment of bleeding in patients on DOACs should be guided by its severity. In moderate-to-severe non-life-threatening bleeding (e.g., GI bleeding), the drug should be temporarily discontinued, while systemic (hemostatic and hemodynamic resuscitation) and local (endoscopy) supportive measures should be implemented. In cases of massive or life-threatening bleeding, in addition to these measures, drug reversal and clearance within the first 2 h after the intake should be considered if there is a clear clinical benefit and/or significantly high drug levels in blood. Reversal should also be considered in patients requiring urgent, non-deferrable surgery or at high risk of bleeding.5–7
How to reverse?Specific agents: these include idarucizumab and andexanet alfa, with others like ciraparantag, currently in the pipeline.
- •
Idarucizumab (Praxbind®): this is a humanized monoclonal antibody that binds to dabigatran in a 1:1 ratio, immediately neutralizing its anticoagulant effect after its IV administration, thus achieving a rapid normalization of the ecarin time. The dose is 5 g, administered as 2 vials of 2.5 g every 5 min–10 min. Its half-life is 45 min, and it requires close clinical and laboratory monitoring, and the possibility of an additional dose if bleeding persists. The drug was approved after the results of the REVERSE-AD trial of 503 patients with severe bleeding (GI, intracranial, or traumatic) were published.8 Idarucizumab completely reversed the anticoagulant effect of dabigatran 4 h after its administration, with bleeding cessation observed in 68% of the patients treated, a mortality rate of 14%, and a thromboembolic event (TE) rate of 5% 30 days after treatment. For all these reasons, idarucizumab is considered the first-line therapy for dabigatran reversal.
- •
Andexanet alfa (Andexxa®): it is a modified variant of factor Xa that lacks enzymatic activity but binds to factor Xa inhibitors with high affinity. It has been approved by the FDA exclusively for the reversal of severe bleeding due to the anticoagulant effect of apixaban and rivaroxaban based on randomized clinical trials but not for urgent surgery. It is administered as an IV bolus of 400 mg or 800 mg, depending on the dose and time since the last intake, followed by the infusion of 4–8 mg/min for 120 min. A phase 3 trial (ANNEXA-4) was conducted among 352 patients with severe bleeding, showing a 92% reduction in activity after infusion.9 At 30 days, 10% of the patients developed TEs. Based on this data, the FDA, and the European Medicines Agency (EMA) have approved its use for patients with severe bleeding due to rivaroxaban and apixaban. However, its use in the routine clinical practice is still controversial due to its high cost and meta-analyses that have not shown any differences compared to the prothrombin complex concentrate (PCC).10
Non-specific agents: these include 4-factor PCC (25–50 IU/kg), activated PCC, and recombinant factor VIIa. These are not considered reversal agents but rather hemostasis restorers. Results from comparing the PCC and specific antidotes against DOACs in a meta-analysis of 34 trials with 3135 patients demonstrated a nearly 80% efficacy rate with no significant differences being reported. Therefore, the PCC is considered a reasonable option to restore the anticoagulant effect of factor Xa inhibitors,10 with an incidence of TE around 4%. While other hemostatic agents have been used adjuvantly or as supportive measures in patients with severe bleeding, there are no clinical data on the clinical efficacy of factor VIIa and tranexamic acid in patients on DOACs.
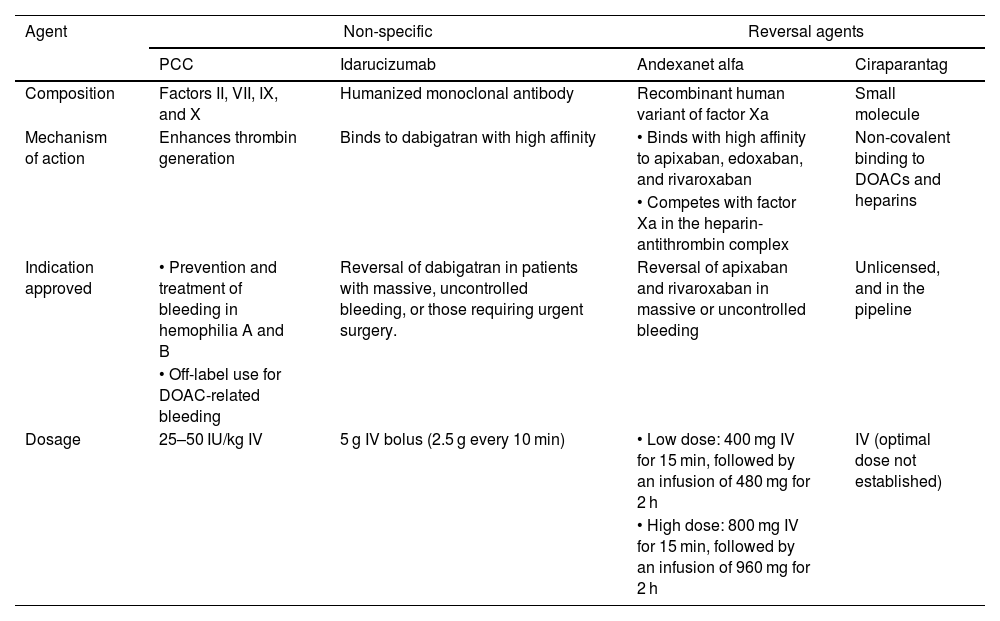
Table 1 illustrates the characteristics of agents for DOAC reversal, while Fig. 1 shows a treatment algorithm used for the management of severe bleeding associated with these anticoagulant agents.
Characteristics of DOAC reversal agents.
| Agent | Non-specific | Reversal agents | ||
|---|---|---|---|---|
| PCC | Idarucizumab | Andexanet alfa | Ciraparantag | |
| Composition | Factors II, VII, IX, and X | Humanized monoclonal antibody | Recombinant human variant of factor Xa | Small molecule |
| Mechanism of action | Enhances thrombin generation | Binds to dabigatran with high affinity | • Binds with high affinity to apixaban, edoxaban, and rivaroxaban | Non-covalent binding to DOACs and heparins |
| • Competes with factor Xa in the heparin-antithrombin complex | ||||
| Indication approved | • Prevention and treatment of bleeding in hemophilia A and B | Reversal of dabigatran in patients with massive, uncontrolled bleeding, or those requiring urgent surgery. | Reversal of apixaban and rivaroxaban in massive or uncontrolled bleeding | Unlicensed, and in the pipeline |
| • Off-label use for DOAC-related bleeding | ||||
| Dosage | 25–50 IU/kg IV | 5 g IV bolus (2.5 g every 10 min) | • Low dose: 400 mg IV for 15 min, followed by an infusion of 480 mg for 2 h | IV (optimal dose not established) |
| • High dose: 800 mg IV for 15 min, followed by an infusion of 960 mg for 2 h | ||||
DOACs, direct oral anticoagulants; PCC, prothrombin complex concentrate.
With the addition of DOACs, clinicians will end up treating patients with severe bleeding due to this therapy in their routine clinical practice. Current recommendations are based on limited randomized clinical trials and expert opinions, with different strategies yet to be compared. The severity of bleeding dictates swift reversal, relying on idarucizumab for dabigatran and andexanet alfa for oral factor Xa inhibitors, apixaban and rivaroxaban. However, if unavailable, non-specific strategies involving the PCC should be used. Clinical trials are still needed to determine whether specific agents are safer and more effective than non-specific agents to reverse DOAC-related critical bleeding.
Conflicts of interestNone declared.
FundingNone.