Multiorgan failure remains one of the leading causes of late morbidity and mortality after severe trauma. In the early phase, it is related with an uncontrolled hyper-inflammation state, whereas in the late phase (>72h), septic complications play a major role. We review the underlying pathophysiology, the evaluation with different scales and the clinical factors associated with multiorgan failure, as well as potential treatment options.
El fallo multiorgánico tras el trauma grave constituye una de las principales causas de morbimortalidad tardía en este grupo de pacientes. En su fase precoz, es consecuencia de un estado de hiperinflamación no controlado, mientras que en su presentación tardía (>72h) se relaciona principalmente con las complicaciones infecciosas. Se resumen los mecanismos fisiopatológicos implicados en su desarrollo, la valoración mediante diferentes escalas y los factores clínicos asociados, además de las potenciales opciones de tratamiento.
The development of multiorgan failure (MOF) after severe trauma is one of the leading causes of late mortality in such patients.1,2 The incidence of MOF varies between 7% and 66%, and despite recent advances in the pre- and in-hospital management of these patients, the disorder is associated to important mortality and prolonged hospital stay.1–5 Although MOF is recognized to be a dynamic process, agreement is lacking regarding its definition.6 The underlying physiopathology is moreover subject to debate, and involves the participation of different components of the immune and inflammatory systems.7,8
The present article reviews the current knowledge on the epidemiology and physiopathology of MOF, the scales used to evaluate the disorder, the clinical factors associated to the development of MOF, and the possible treatment options.
DefinitionWhile no uniform definition of post-trauma MOF has been established to date,6 a number of scales have been developed for evaluating patient respiratory, cardiovascular, hepatic, renal, neurological and coagulation function–including particularly the Denver, Marshall and Sequential-related Organ Failure Assessment (SOFA) scales. These instruments will be examined further below.
Despite the limitations imposed by the lack of a clear definition of MOF, there is agreement in regarding early MOF as multiorgan failure occurring in the first 72h after trauma (this representing approximately 40% of all cases), while late MOF is taken to be multiorgan failure occurring beyond day three post-trauma (60% of all cases).6
Epidemiological aspectsPost-trauma MOF is the leading cause of late mortality in severe trauma, accounting for 50–60% of all deaths in such patients.1–6 Those individuals that develop post-trauma MOF have a longer stay in the Intensive Care Unit (ICU). The associated mortality rate varies between 27% and 100%, and increases with the number of affected organs.6,8,9 As early as 1980, Fry et al.8 showed mortality to increase from 30% in the presence of single-organ dysfunction to 100% in the case of dysfunction affecting four organs. A study conducted in a single reference center for severe trauma in the United States, involving data prospectively collected over a period of 12 years, has found the incidence, duration and mortality of MOF to have decreased in recent years–this being related to advances and new treatment modalities in application to severe trauma patients, and to a decrease in the number of transfusions.1 A recent multicenter study including 1643 trauma patients reported a decrease in incidence of almost 50%–though the related mortality remains very high.2
The incidence of MOF is greater in closed trauma patients, and in these cases are associated to greater mortality than in penetrating trauma.1,6
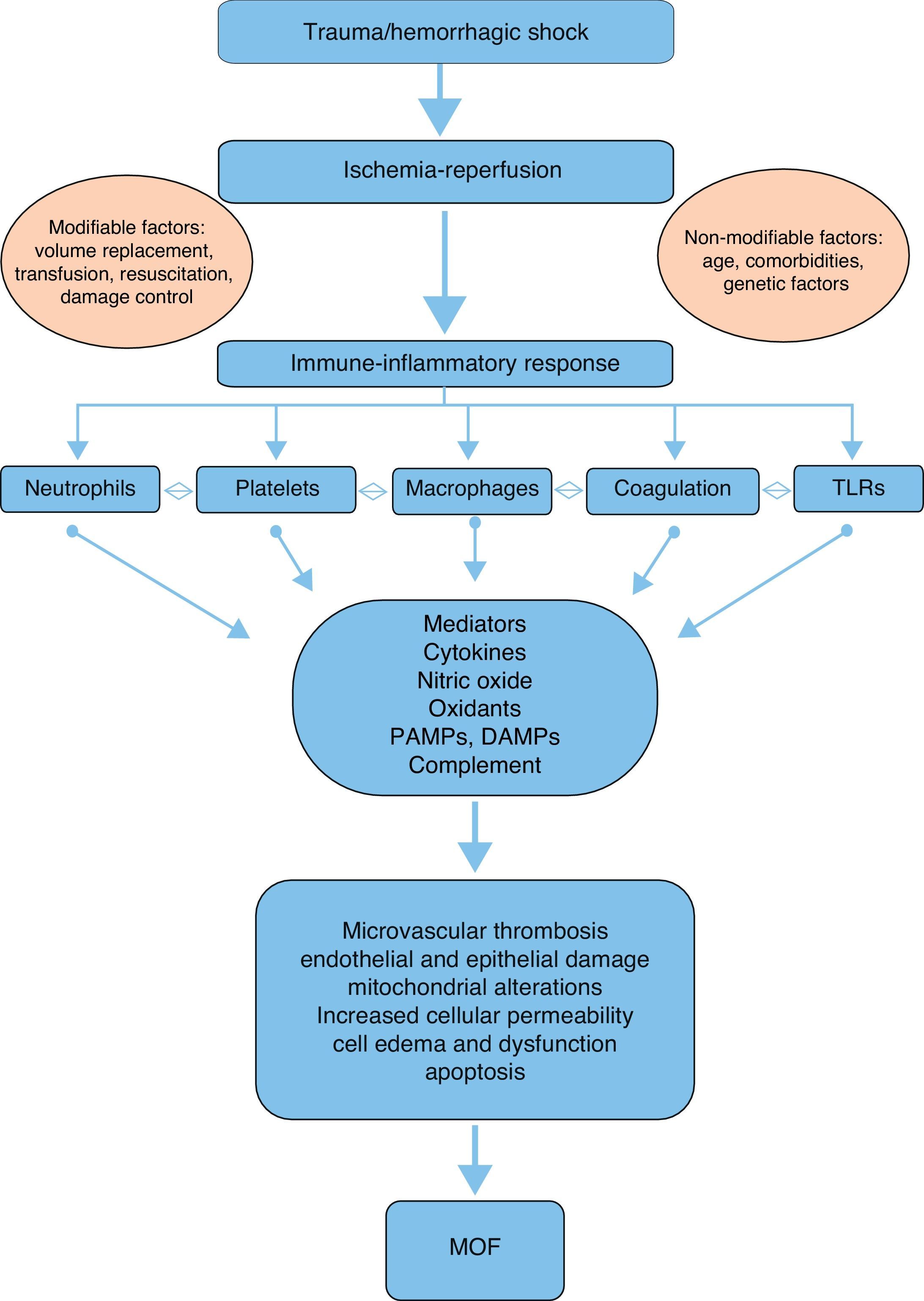
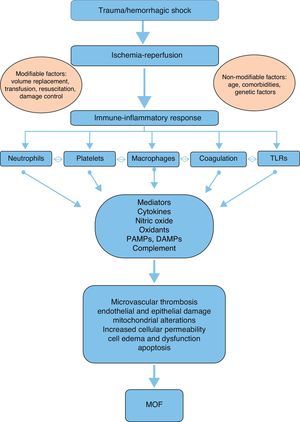
PhysiopathologyAlthough the physiopathology of MOF in severe trauma is not fully clear and has evolved over the last 30 years, it is currently accepted that the disorder is a bimodal phenomenon with two peaks of presentation, due to an alteration in the balance of the systemic inflammatory response followed by ischemia-reperfusion after hemorrhagic shock. This is in contrast to the early theories which pointed to generalized infection as the sole cause of MOF.6,9
In this regard, respiratory failure appears to play a key role in early MOF, manifesting in 99% of all cases and usually preceding heart dysfunction by a few hours, and liver and kidney dysfunction by about 5 days.10 In contrast, late MOF occurring beyond 72h after trauma requires a second “hit”,6 usually in the form of infection (mostly of pulmonary origin).10
A number of mediators and effectors can potentially intervene in the physiopathology and development of post-trauma MOF (Fig. 1):
Mediators- -
Cytokines: The balance between proinflammatory and antiinflammatory cytokines plays a key role in the maintenance of homeostasis. Following severe trauma there is an overproduction of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6 and IL-8 on the part of monocytes and macrophages. This constitutes part of the acute phase response, contributing to initiation and perpetuation of the local and systemic inflammatory response.7,11 TNF-α increases the production of nitric oxide (NO) and activates cyclooxygenase, resulting in an increased production and release of thromboxanes, prostaglandins and platelet activating factor (PAF)–with a consequent increase in procoagulant activity.7,12 In turn, IL-6, produced by different cells such as activated monocytes, macrophages, neutrophils and endothelial cells, plays an important role in the acute response, intervening in the production of C-reactive protein, procalcitonin, fibrinogen, α-1-antitrypsin and complement factors.6 In addition, IL-6 regulates the growth and differentiation of lymphocytes and activates natural killer (NK) cells and neutrophils. It is widely agreed that the measurement of IL-6 is a good indicator of trauma severity and outcome.13 Interleukin-8 participates in leukocyte recruitment, and facilitates the activation of these cells at the damage site. The levels of IL-8 are correlated to the development of acute respiratory distress syndrome (ARDS) following severe trauma.7 Of the different antiinflammatory cytokines, mention must be made of IL-10, which is synthesized by lymphocytes and monocytes. This cytokine fundamentally inhibits the production of TNF-α, IL-6 and IL-8.7
- -
Complement system: Activation of the complement system can occur after severe trauma via any of the known pathways (alternative, classical, lectin), generating biologically active peptides that play a key role through different mechanisms7,14: the elimination of invasive pathogens via opsonization and phagocytosis (C3b, C4b), leukocyte chemotaxis (C3a, C5a), and pathogen destruction through the membrane attack complex (C5b-9). In addition, the anaphylotoxins C3a, C4a and C5a attract phagocytes and polymorphonuclear cells (PMNs) toward the damage site15,16 and induce the degranulation of mast cells, basophils and eosinophils.7,14 Experimental and clinical studies have demonstrated that complement activation takes place both locally at the damage site and systemically after trauma.14 The plasma C3 levels are correlated to the severity of trauma and to the infectious complications and mortality after trauma.16
- -
Pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs) and alarmins: PAMPs are inflammatory molecules related to activation of the innate immune system in cases of infection, while the alarmins constitute their equivalents in the absence of infection and in the presence of tissue damage.14 Such molecules include the heat-shock proteins, annexins, defensins, HMGB1 and S100. Both families have been grouped into a larger family known as DAMPs, which are recognized by the immune system through multiligand receptors such as the toll-like receptors (TLRs).17 However, their precise role in the development of MOF remains to be fully clarified.14
- -
Neutrophils: Polymorphonuclear cells, monocytes, macrophages, dendritic cells and natural killer cells play a relevant role in the cellular immune response after trauma.6–9,18 Neutrophils are attracted to the damage site by cytokines such as IL-8, and participate in the defense and debridement of the damaged tissue. In addition, these cells participate in the activation of molecules such as TNF-α, IL-8, platelet activating factor and anaphylotoxin (C5a), leading to hyper-inflammation and PMN activation and recruitment, and contributing to the development of systemic inflammatory response syndrome and MOF.6–8,19 This mobilization of PMNs results in neutrophilia three hours after injury, which represents a “vulnerability window” during which a second “hit” can give rise to MOF.6,20 In those patients that develop MOF, neutrophilia is followed by neutropenia 6–12h after trauma.6 Moreover, the PMNs induce endothelial and epithelial damage through the overregulation of adhesion molecules in the cells, and this situation results in permeability changes and cellular edema, with cellular dysfunction.7
- -
Ischemia-reperfusion: This phenomenon develops in two phases following trauma: a first phase is characterized by the temporary privation of nutrients and oxygen due to the lack of blood supply, resulting in a switch from aerobic to anaerobic metabolism at cellular level. The second phase is characterized by the restoration of blood flow and oxygenation to the ischemic tissue.7 In the ischemic phase, hypoxemia implies a low production and high consumption of adenosine triphosphate, which is degraded to adenosine diphosphate. This in turn is degraded to inosine and hypoxanthine,21 triggering alterations in permeability with an increase in intracellular sodium that causes cell edema and membrane disruption.7,22 In addition, the depletion of adenosine triphosphate causes cell damage mediated by alterations in the concentrations of cytosolic calcium.23,24 The reperfusion phase is of greater importance in relation to tissue damage and MOF than the ischemia phase. In the reperfusion phase, hypoxanthine is degraded to xanthine and finally to uric acid through the mediation of xanthine oxidase, with the production of superoxide anions (O2+−) from the available oxygen. Such anions are moreover reduced to hydrogen peroxide (H2O2) and to hydroxyl ions (OH2+−) through the mediation of superoxide dismutase. These free radicals enhance the alterations in intracellular calcium homeostasis and induce lipid peroxidation, membrane disintegration and deoxyribonucleic acid alterations – with apoptosis and cell necrosis as the end result.22
- -
Intestinal hypothesis: The alterations in the structure and permeability of the intestinal mucosa play a key role as a source of bacterial products and antiinflammatory mediators that penetrate the systemic circulation and damage different organs. Two basic factors may contribute to this phenomenon: intestinal stasis allowing bacterial overgrowth, and ischemia-reperfusion, which increases intestinal permeability. However, the precise mechanisms involved have not been well established.6
As has been mentioned, there is no uniform definition of post-trauma MOF.6 However, a number of scales have been developed for evaluating patient respiratory, cardiovascular, hepatic, renal, neurological and coagulation function. The most widely used scales are summarized below.
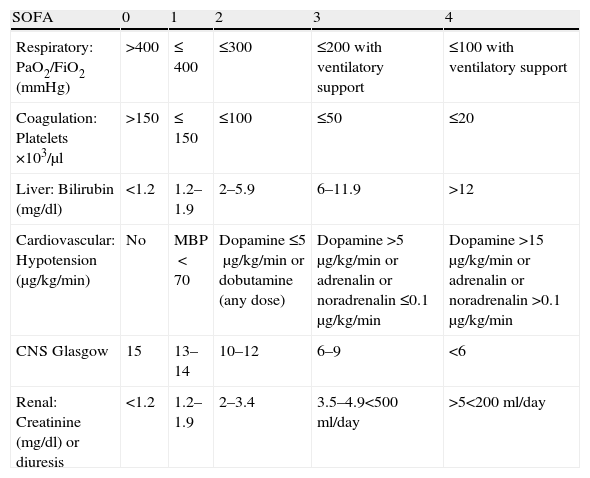
Sequential-related Organ Failure Assessment (SOFA)The SOFA was developed in 1994 under the auspices of the European Society of Intensive Care Medicine as an objective tool for quantitatively assessing organ dysfunction or failure over time, and thus also for evaluating patient response to treatment.23 The SOFA scores 6 organs from 0 to 4 according to the degree of dysfunction in each of them (Table 1). Multiorgan failure is defined as the alteration of two or more organs with a score of ≥3.23 Although the initial aim of the scale was to evaluate morbidity, a good correlation has been observed between the maximum score obtained and mortality. In this regard, the mortality rate is over 90% in those patients presenting a total score of over 15 (with a specificity of almost 99%).24
SOFA scale.
| SOFA | 0 | 1 | 2 | 3 | 4 |
| Respiratory: PaO2/FiO2 (mmHg) | >400 | ≤ 400 | ≤300 | ≤200 with ventilatory support | ≤100 with ventilatory support |
| Coagulation: Platelets ×103/μl | >150 | ≤ 150 | ≤100 | ≤50 | ≤20 |
| Liver: Bilirubin (mg/dl) | <1.2 | 1.2–1.9 | 2–5.9 | 6–11.9 | >12 |
| Cardiovascular: Hypotension (μg/kg/min) | No | MBP<70 | Dopamine ≤5μg/kg/min or dobutamine (any dose) | Dopamine >5μg/kg/min or adrenalin or noradrenalin ≤0.1μg/kg/min | Dopamine >15μg/kg/min or adrenalin or noradrenalin >0.1μg/kg/min |
| CNS Glasgow | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Renal: Creatinine (mg/dl) or diuresis | <1.2 | 1.2–1.9 | 2–3.4 | 3.5–4.9<500ml/day | >5<200ml/day |
Glasgow: Glasgow coma score; MBP: mean blood pressure; CNS: central nervous system.
Antonelli et al.25 evaluated the SOFA in trauma patients and found non-survivors to have poorer respiratory, cardiovascular and neurological scores, particularly during the first 5 days of admission. In this context, neurological function is the most complicated dimension to evaluate in certain cases, due to both the potential interferences of sedating drugs and the presence of traumatic brain injury. The SOFA has recently been used by a German group in the largest series of trauma patients published to date, with the assessment of MOF in 31,154 patients.26 The authors recorded an incidence of MOF of 32%, which suggests that the SOFA may overestimate the incidence of post-trauma MOF. The scale, developed at European level, is used in the Spanish national trauma registry (RETRAUCI) for assessing the incidence of MOF.27
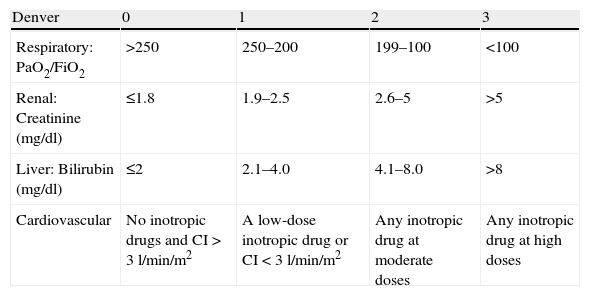
Denver scaleIn 1987, the Denver group for the first time developed a scale for assessing MOF.28 This instrument evaluates four systems, each of which are scored from 0 to 3 (Table 2). Organ failure is defined by a score of over 0, while MOF is defined as the failure of two or more organs, with a total score of ≥4 as determined 48h after trauma.28 The Denver scale has been revised on several occasions since its introduction, and some of the original parameters have received modifications–fundamentally in relation to the respiratory and cardiovascular dimensions. The current version of the scale evaluates the parameters described in Table 2.
Denver scale.
| Denver | 0 | 1 | 2 | 3 |
| Respiratory: PaO2/FiO2 | >250 | 250–200 | 199–100 | <100 |
| Renal: Creatinine (mg/dl) | ≤1.8 | 1.9–2.5 | 2.6–5 | >5 |
| Liver: Bilirubin (mg/dl) | ≤2 | 2.1–4.0 | 4.1–8.0 | >8 |
| Cardiovascular | No inotropic drugs and CI>3l/min/m2 | A low-dose inotropic drug or CI<3l/min/m2 | Any inotropic drug at moderate doses | Any inotropic drug at high doses |
CI: cardiac index.
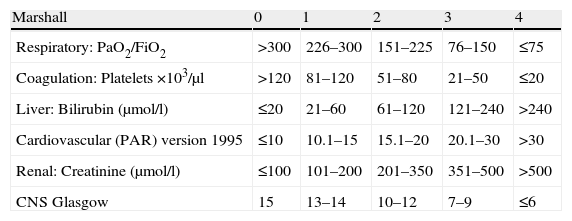
In the 1990s, Marshall et al. presented a new scale for assessing MOF.29 The authors evaluated 6 systems, each of which were scored from 0 to 4 (Table 3). The parameters are always recorded at the same time of day (the first value in the morning). The Marshall scale does not consider specific values as indicators of MOF as such; rather, it establishes degrees of severity defined by the observed mortality. Thus, no mortality is observed in patients with a score of 0, while the mortality rate is 25% in those with a score of 9–12; 50% in those with a score of 13–16; 75% in those with a score of 17–20; and 100% in the case of a score of over 20. A good correlation is observed between the score and hospital stay. In the same way as the SOFA, the Marshall scale evaluates neurological status. In this case, a Glasgow coma score of 15 is assumed in sedated patients. On the other hand, because of the difficulty of defining the optimum parameter for assessing patient hemodynamics, a new variable was introduced in the updated version of 1995: the pressure adjusted heart rate (PAR). This variable is the ratio between the product of heart rate and central venous pressure, and the mean blood pressure (PAR=HR×CVP/MBP).
Marshall scale.
| Marshall | 0 | 1 | 2 | 3 | 4 |
| Respiratory: PaO2/FiO2 | >300 | 226–300 | 151–225 | 76–150 | ≤75 |
| Coagulation: Platelets ×103/μl | >120 | 81–120 | 51–80 | 21–50 | ≤20 |
| Liver: Bilirubin (μmol/l) | ≤20 | 21–60 | 61–120 | 121–240 | >240 |
| Cardiovascular (PAR) version 1995 | ≤10 | 10.1–15 | 15.1–20 | 20.1–30 | >30 |
| Renal: Creatinine (μmol/l) | ≤100 | 101–200 | 201–350 | 351–500 | >500 |
| CNS Glasgow | 15 | 13–14 | 10–12 | 7–9 | ≤6 |
HR: heart rate; Glasgow: Glasgow coma score; MBP: mean blood pressure; PAR: pressure adjusted heart rate; CVP: central venous pressure; CNS: central nervous system.
PAR=HR×CVP/MBP.
Bilirubin conversion factor: 1mg/dl=17.1μmol/l.
Creatinine conversion factor: 1mg/dl=88.4μmol/l.
Some studies have compared the different scales described above. A German study analyzing the performance of the Goris, Marshall and Denver scales found the latter instrument to offer the best performance, with a sensitivity of 81% and a specificity of 88%.30 The authors highlighted that the Denver scale does not include more complex evaluations such as aspartate transaminase levels (GOT) or PAR, which had a scant impact upon the calculations made. Some years later, the Denver group compared their scale with the Marshall scale in patients of their severe trauma database and excluding pure traumatic brain injury cases and patients with an extracranial Abbreviated Injury Score of under 2. The receiver operator characteristic (ROC)-based analysis yielded similar results for both scales, though the Denver scale had greater specificity and a greater capacity to evaluate patient response to treatment.31 Different studies in turn have compared the SOFA with the Marshall scale, though not in specific trauma patient groups.
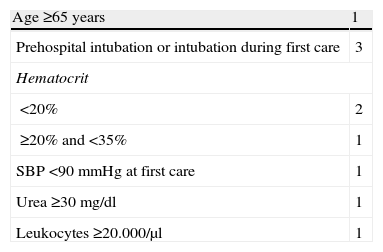
A new scale has recently been developed: the Denver Emergency Department Trauma Organ Failure Score (Denver ED TOF Score).32 This is the first scale designed to allow early prediction (within the first 4h after trauma) of the development of MOF in the first 7 days of admission. The instrument includes pre-hospital variables and parameters collected during first care in the emergency room (Table 4). The score ranges from 0 to 9, and an increased risk of MOF is associated to higher scores, with excellent calibration and discrimination. The Denver ED TOF Score could be useful not only in identifying patients in early stages of MOF but also as a screening tool for patient transfer to a reference center. Its main limitation is lesser patient severity (median Injury Severity Score [ISS] 9, with an interquartile range of 4–16), since it was developed from secondary analysis of a database not designed for this purpose, and the lack of external validation.
Predictors of severe multiorgan failure after traumaDifferent parameters have been associated to an increased risk of developing MOF after trauma. Those most frequently cited in the literature are commented below:
- -
Age: In recent years there has been an increase in the age of patients with trauma. A number of studies have identified patient age as an independent risk factor for the development of MOF, with the definition of different cutoff points–generally 55 or 65 years of age.1,5,26,33
- -
Gender: Previous studies had not considered the male gender to be a risk factor for the development of post-trauma MOF. However, in the recent German registry, the male gender (73.1% of all patients) was identified as a risk factor in the multivariate analysis.26 This observation could be mediated by the existence of higher IL-6 levels in males following severe trauma.34
- -
Severity of trauma: The severity of trauma as assessed by the ISS has consistently been cited as a risk factor1,2,4,26,33–the most widely used cutoff point being a score of 24. However, some authors believe that the ISS has lost usefulness as a predictor of MOF, since it may underestimate severity in patients with several injuries in a given anatomical area. In fact, the ISS has been compared with the New Injury Severity Score (NISS) as a predictor of the development of MOF in a limited population of patients,35 with the NISS being found to offer superior performance. However, while this new scale is attractive, its use has not become widespread in the major trauma registries.
- -
Traumatic brain injury: Among the different body regions analyzed in the ISS, a score for the head on the Abbreviated Injury Scale of ≥3 is related to an increased risk of developing MOF,27,34 in the same way as an initial Glasgow coma score of ≤8.26
- -
Blood products: It is well known that blood products contain abundant mediators that act as immune modulators. As a result, their use is associated to an increased incidence of nosocomial infections, MOF and mortality. Many studies have shown that the transfusion of more then 6 red cell concentrate units in the first hours is an independent risk factor for the development of MOF.1,26,33 Moreover, the age of the concentrates is also associated to an increased risk of MOF.36 The transfusion of fresh frozen plasma likewise constitutes a risk factor for the development of acute respiratory distress syndrome and MOF,37 though not so the transfusion of platelets.
- -
Coagulation disorders and thrombocytopenia: Coagulation disorders and platelet count are well known to play a prognostic role in relation to post-trauma MOF. The platelet count in the first hours following admission has recently been established as an independent predictor of MOF using different cutoff points and times after trauma: counts below 80,000/μl to 150,000/μl between admission and the first 24h have been evaluated. Furthermore, those patients who continue to present low platelet counts after several days have a poorer prognosis.38
- -
Hemodynamic status and lactate: The hemodynamic situation upon admission, evaluated on the basis of shock parameters such as systolic blood pressure (≤90mmHg), and base deficit or lactate concentration have also been identified as risk factors.1,26 Not only the initial concentration is of prognostic importance in the case of lactate, but also the time elapsed until the concentration normalizes (assuming the levels do return to normal).39
- -
Other factors that have been proposed as possible predictors of the development of MOF are the minimum bilirubin levels or the peak creatinine concentrations in the first 24h. Obesity might also play a role. However, the evidence supporting these factors is much more limited.
Although there is no specific treatment for MOF once it has become established, the incidence of the disorder is considered to have decreased in recent years thanks to advances in the general management of critical trauma patients and the early correction of shock.1,2,6,26 Considering the results recently published by the German group, it seems less clear whether the associated mortality rate has also decreased in recent years.26 Some strategies referred to the early management of these patients appear to contribute to avoid the development of MOF.1,6 Such strategies include the following:
- -
Cardiorespiratory support: Initially, the recommendations for the management of patients with traumatic shock focused on obtaining parameters indicative of supranormal oxygen transport. However, in recent years there have been changes in the general approach to the treatment of these patients, oriented toward permissive hypotension and the correction of hypothermia, coagulopathy and acidosis.40,41 In this way the use of crystalloids is reduced and the incidence of abdominal compartmental syndrome, MOF and death is lowered.42 It should be mentioned that the presence of traumatic brain injury obliges us to seek higher arterial pressure levels.
- -
Damage control surgery and debridement of infected tissues: The first concern is to control the bleeding, avoiding prolonged interventions, in an attempt to not worsen the acidosis and coagulopathy. In addition, this helps limit the release of inflammatory mediators in the acute phase.22 Likewise, the debridement of infectious foci, with the removal of non-viable tissue, the use of vacuum systems and second-step interventions contribute to reduce the infectious complications which play a very important role in late MOF.
- -
Volume replacement and blood products: This issue remains subject to controversy, though the current tendency is to administer lesser crystalloid volumes. Furthermore, since it is known that the transfusion of blood products is significantly associated to the development of MOF,1,26,33 a more restrictive approach is advised in order to avoid unnecessary transfusions. However, as has been mentioned, this subject remains open to debate.
- -
Others: Different treatment measures used in critical patients in general, such as protective mechanical ventilation, adequate nutritional support, the management of hyperglycemia with insulin or adrenal gland replacement therapy, while controversial in the general population of patients in the ICU, could play a role in lowering the incidence of post-trauma MOF.
To summarize, post-trauma MOF remains an important cause of morbidity-mortality in severe trauma patients. Because of the different definitions used, the true incidence of MOF remains unclear. The RETRAUCI registry, auspiced by the Trauma and Neurointensive Care work group of the SEMICYUC, will help define the incidence in our setting. The prognosis of MOF has improved in recent years, probably as a result of advances in the general management of critical trauma patients. While certain non-modifiable factors such as age and comorbidities among trauma patients imply a poorer prognosis, the application of uniform protocols referred to resuscitation, damage control, sedation and volume replacement appears to have mitigated their negative effects.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Llompart-Pou JA, Talayero M, Homar J, Royo C, grupo de trabajo de Trauma y Neurointensivismo de SEMICYUC. Fallo multiorgánico en el paciente con trauma grave. Med Intensiva. 2014;38:455–462.