We report the case of a 58-year-old female patient with a history of limited systemic scleroderma (CREST) under chronic treatment with prednisone 5 mg/24 h and rituximab (last dose administered in May 2023). She received four doses of the mRNA vaccine against SARS-CoV-2 (the last one in March 2022).
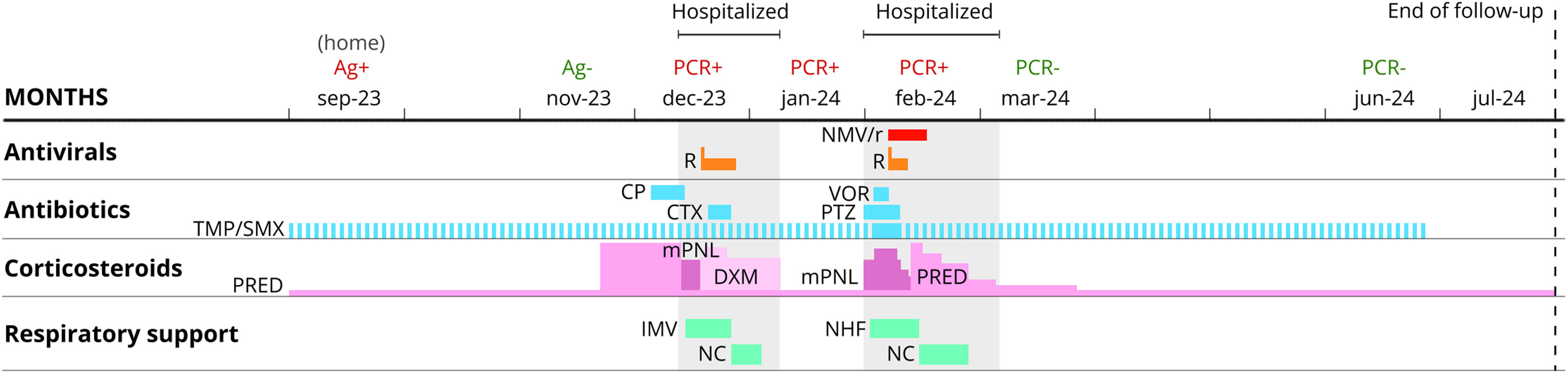
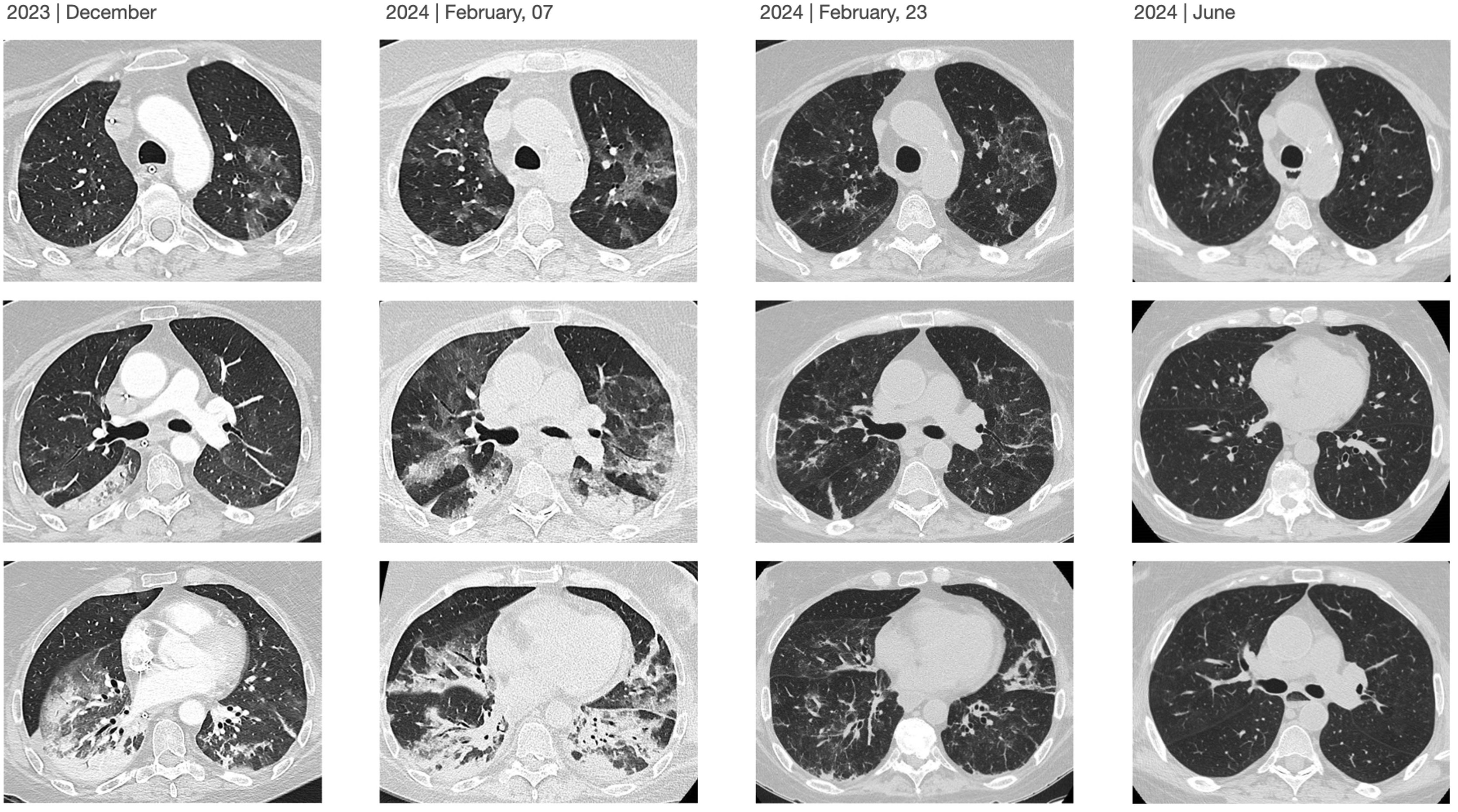
The patient experienced a mild episode of COVID-19 in September 2023. She was evaluated one month later due to dyspnea with minimal exertion, classified as grade 3 on the modified Medical Research Council (mMRC) scale. A scheduled high-resolution chest Computed Tomography (CT) revealed patchy ground-glass opacities and consolidations with air bronchograms in the left lower lobe, showing an inverted halo sign suggestive of cryptogenic organizing pneumonia (CON). Prednisone 60 mg/24 h and cefditoren were prescribed (Fig. 1). The patient's condition deteriorated, resulting in hospitalization in the Intensive Care Unit (ICU) due to hypoxemic respiratory failure requiring invasive mechanical ventilation. Supplementary studies revealed a positive polymerase chain reaction (PCR) test for SARS-CoV-2 from a nasopharyngeal sample with cycle threshold (Ct) values of 34-36-29, negative for other viruses, while microbiological analysis of bronchoalveolar lavage (BAL) was negative for bacterial, fungal and mycobacterial cultures, as well as for PCR tests targeting community-acquired and opportunistic pneumonia, and for the galactomannan antigen. Serological testing showed positive SARS-CoV-2 IgG, and there was a generalized reduction in T and B lymphocyte subpopulations in peripheral blood (0 × 1/µL for CD + 19) along with hypogammaglobulinemia G. The echocardiography was normal, and pulmonary artery CT angiography ruled out pulmonary embolism. Based on these results, empirical antibiotic treatment was discontinued, and targeted therapy with remdesivir (initial dose of 200 mg, followed by 100 mg/24 h for 10 days) and dexamethasone 8 mg for ten days with subsequent tapering was started. The patient showed a favourable outcome, with removal of mechanical ventilation and subsequent discharge home without the need for oxygen therapy. Follow-up chest CT scans also demonstrated radiological improvement (Fig. 2).
Clinical evolution and treatments. Abbreviations. CP, Cefditoren Pivoxil; CTX, Ceftriaxone; DXM, dexamethasone; IMV, invasive mechanical ventilation; mPNL, methylprednisolone; NC, nasal cannula; NHF, nasal high-flow cannula; NMV/r, nirmatrelvir/ritonavir; PCR, polymerase chain reaction test; PRED, prednisone; PTZ, Piperacillin/tazobactam; R, remdesivir; TMP/SMX, trimethoprim/sulfamethoxazole (dashed blue line shows prophylaxis); VOR, voriconazole.
Three weeks later, the patient was readmitted to the ICU due to worsening respiratory failure, but she tolerated high-flow nasal oxygen support well. High-resolution chest CT revealed an increase in patchy ground-glass opacities in the upper lobes, with some perilobular areas showing an inverted halo sign, suggestive of CON. Treatment with piperacillin-tazobactam and methylprednisolone 1 mg/kg (three doses of 3 mg/kg) was initiated, but there was no clinical improvement. New supplementary studies revealed a positive PCR for SARS-CoV-2 in BAL, with cycle threshold (Ct) values of 31-34-26, while other microbiological tests were negative. The lymphocyte subpopulation ratio in the BAL (CD4/CD8) was below normal (0.2), with no eosinophilia observed. Considering these findings, the possibility of persistent SARS-CoV-2 infection in a severely immunocompromised patient was considered and it was decided to initiate dual treatment with remdesivir for 5 days (loading dose of 200 mg, followed by 100 mg/24 h) and nirmatrelvir/ritonavir (NMV/r) 300 mg/100 mg every 12 h for 10 days. The patient showed significant improvement, returned to the general ward five days after starting dual antiviral therapy without any adverse effects, and was discharged home without the need for oxygen therapy. A nasopharyngeal PCR test for SARS-CoV-2 was negative at discharge. After four months of outpatient follow-up with no new hospitalizations, the patient presented with mMRC class 1 dyspnea, a baseline saturation of 97% without oxygen therapy, a negative PCR test for SARS-CoV-2 from a nasopharyngeal sample, and significant radiological improvement on chest CT.
We describe the case of a severely immunocompromised patient (chronic treatment with corticosteroids and rituximab, significant reduction in peripheral blood T and B lymphocytes, and hypogammaglobulinemia) who presented with critical hypoxemic respiratory failure and suspected persistent SARS-CoV-2 infection. Following dual therapy with remdesivir for 5 days and an extended course of NMV/r for 10 days, the patient showed a very favorable clinical response, with no relapses and SARS-CoV-2 PCR testing negative. Immunity alterations (e.g., immunosuppression, hematologic malignancies) have been directly linked to various complications in the context of SARS-CoV-2 infection. These complications may include prolonged viral shedding, persistent SARS-CoV-2 infection, failure to seroconvert, and an increased risk of reinfection.1–3 The literature employs various terms to describe these issues—such as persistent SARS-CoV2 infection, not resolved infection, prolonged COVID, relapse, or reactivation of COVID-19—without a clear consensus on their definitions, diagnostic criteria, and therapeutic management. Machkovech HM et al. propose that persistent SARS-CoV-2 is an unresolved infection, where the definition may include the persistent detection of SARS-CoV-2 for at least 30 days after symptom onset in patients with different clinical presentations: asymptomatic/paucisymptomatic, remitting and relapsing, or chronic symptoms (progressive respiratory and/or systemic). The clinical expression may change in the same patient over time. They note that while immunocompromised conditions present an individual risk for persistent infection, it can also occur in individuals without known immunocompromised.4 Diagnostic challenge may, in some cases, be further complicated by other clinical conditions that can impact the patient's clinical status and whose significance is sometimes hard to gauge, as was the case in our patient (e.g., the presence of cryptogenic organizing pneumonia), although these conditions might also be related to persistent viral infections.5 It is crucial to rule out other complications related to SARS-CoV-2, such as thrombotic events, bacterial infections, fungal infections, or other opportunistic infections.
Regarding the therapeutic approach for patients with suspected persistent SARS-CoV-2 infection and immune alterations, current evidence is limited. An optimal treatment may provide not only individual benefits but also global benefits (e.g., by limiting the virus's opportunities to generate and transmit novel variants); however, the impact of increased antiviral pressure is unknown and may pose a challenge.1,4 Different strategies have been reported, including the co-administration of antivirals with antibody-based therapies (such as convalescent plasma, specific immunoglobulins, or nonspecific immunoglobulins in cases of hypogammaglobulinemia), repetition or extension of antiviral treatment cycles, and the use of various combinations of antivirals.4 Case reports and case series involving patients with haematological conditions and/or those undergoing immunosuppressive therapy (e.g., anti-CD20 therapies) show that the combination of antivirals (remdesivir, nirmatrelvir/ritonavir or molnupiravir) and antibody-based therapies may improve virological and clinical responses.5–12 In the series by Mikulska et al., 73% (16/22) of patients exhibited a positive response.12 However, despite these favorable results, some patients require a second course of antiviral treatment. These combinations are usually well tolerated but should be carefully monitored, as adverse events have been reported.12 In our case, we opted for dual antiviral therapy with remdesivir (an adenosine analogue) and extended nirmatrelvir/ritonavir (a 3CLpro protease inhibitor) to minimize the risk of remdesivir resistance by combining two distinct mechanisms of action.13
COVID-19 infection guidelines specify the use of corticosteroids during the initial phase and highlight the potential harm when used outside of this indication. Their administration in cases of persistent SARS-CoV-2 infection remains controversial.1 Although they were prescribed to our patient for possible organizing pneumonia, their use in the weeks following the initial infection is debated, as corticosteroids may contribute to reduced viral clearance, persistent infection, and/or an increased risk of secondary infections (e.g., invasive fungal infection).1,5,7
We cannot determine how the clinical outcome might have differed if we had chosen one of the other therapeutic alternatives (e.g., administration of nonspecific immunoglobulins was indicated due to her hypogammaglobulinemia). The ability to confirm or rule out antiviral resistance (e.g., remdesivir resistance due to de novo V792I mutations) could influence the choice of antiviral strategy; however, such testing was not available in our case.13 We acknowledge the challenge in assessing the impact of other treatments administered, such as corticosteroids or antibiotics. Despite this, a favorable clinical response was observed following the initiation of antiviral therapy. We believe that in this immunosuppressed patient, early antiviral treatment during the initial phase of mild COVID-19 should have been considered due to her high risk, as it might have reduced the likelihood of later complications. A consistent definition of persistent SARS-CoV-2 needs to be agreed, and well-designed studies are required to provide clearer evidence on the optimal therapeutic regimen for this specific patient subgroup.
FundingThis study has not obtained funding for its completion.
We thank Dr. Rafael León López for his contributions in the management of the patient and in the writing of this manuscript.