High-flow nasal oxygen therapy (HFNO), and non-invasive ventilation (NIV) have been used to treat early acute hypoxemic respiratory failure due to SARS-CoV-2-induced pneumonia. In this sense, the PROSPERO trial1 found that HFNO did not reduce the rate of intubation or the mortality rate in emergency wards. However, after conducting a systematic review and a meta-analysis, Ferreiro et al. found that, compared to standard oxygen therapy, the use of non-invasive oxygenation strategies was associated with a lower risk of death.2 The guidelines established by our society for the management of SARS-CoV-2-induced pneumonia recommend using HFNO3,4 instead of NIV (except for selected cases).
One of the main justifications for this recommendation is based on the statement that “NIV can generate aerosols and facilitate the spreading of the virus”; in this sense, the study conducted by Fowler et al. found no significant association between both variables. Although the analysis conducted by Raboud et al. found differences in a percentage comparison that used the chi-square test, the logistic regression model confirmed the following as independent predictors: the exposure of the eyes and mucous membrane to the patient’s bodily fluids (OR = 7.34; P = .001), a patient’s APACHE II score ≥ 20 (OR = 17.05; P = .0009), a patient’s P/F ratio ≤ 59 (OR = 8.65; P = .001), and being present while an ECG (OR = 3.52; P = .002), and intubation (OR = 2.79; P = .004) are being performed.5,6
Based on the hypothesis that, in selected cases, the use of HFNO can improve the disease progression of patients with respiratory failure admitted to the ICU, a total of 79 patients hospitalized in the COVID unit of the critical care medicine section (CCMS) between March through May 2020 were retrospectively reviewed. The study was completed with an analysis of the cost-effectiveness ratio of the respiratory therapy to treat hypoxemic respiratory failure due to SARS-CoV-2-induced pneumonia (see electronic supplementary data).
Data were obtained from a registry of patients with COVID from the CCMS after receiving the approval from the local research ethics committee and obtaining the informed consent of the patient or his legal representative
In 12 patients the clinical suspicion of SARS-CoV-2-induced disease could not be microbiologically confirmed, which is why these patients were excluded from the analysis. The main clinical and epidemiological characteristics of the cohort of patients are shown on Table 1 of the electronic supplementary data.
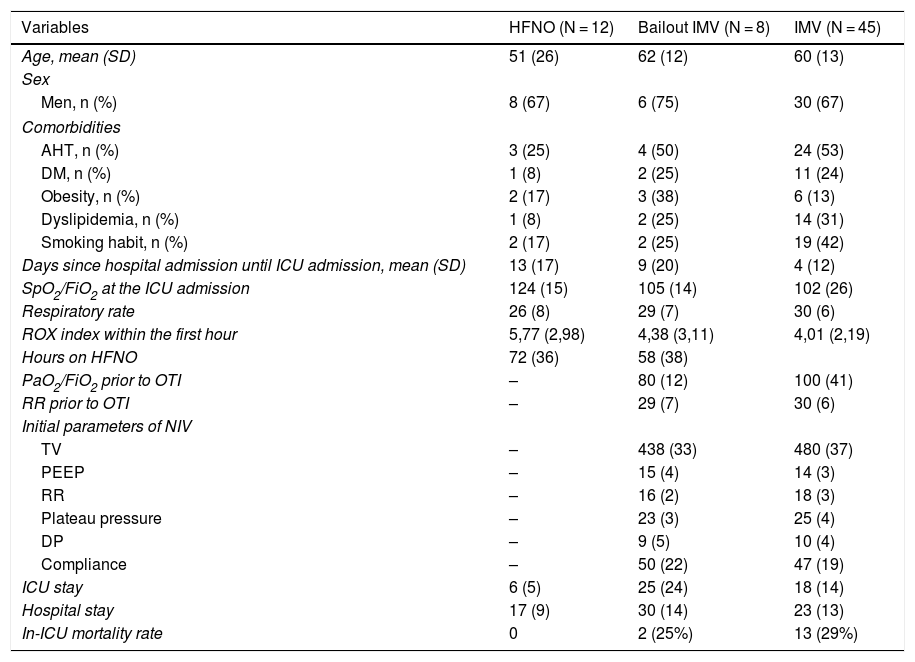
Main differences between patients on initial treatment of high-flow nasal oxygen, early non-invasive ventilation, and patients with bailout non-invasive ventilation.
| Variables | HFNO (N = 12) | Bailout IMV (N = 8) | IMV (N = 45) |
|---|---|---|---|
| Age, mean (SD) | 51 (26) | 62 (12) | 60 (13) |
| Sex | |||
| Men, n (%) | 8 (67) | 6 (75) | 30 (67) |
| Comorbidities | |||
| AHT, n (%) | 3 (25) | 4 (50) | 24 (53) |
| DM, n (%) | 1 (8) | 2 (25) | 11 (24) |
| Obesity, n (%) | 2 (17) | 3 (38) | 6 (13) |
| Dyslipidemia, n (%) | 1 (8) | 2 (25) | 14 (31) |
| Smoking habit, n (%) | 2 (17) | 2 (25) | 19 (42) |
| Days since hospital admission until ICU admission, mean (SD) | 13 (17) | 9 (20) | 4 (12) |
| SpO2/FiO2 at the ICU admission | 124 (15) | 105 (14) | 102 (26) |
| Respiratory rate | 26 (8) | 29 (7) | 30 (6) |
| ROX index within the first hour | 5,77 (2,98) | 4,38 (3,11) | 4,01 (2,19) |
| Hours on HFNO | 72 (36) | 58 (38) | |
| PaO2/FiO2 prior to OTI | – | 80 (12) | 100 (41) |
| RR prior to OTI | – | 29 (7) | 30 (6) |
| Initial parameters of NIV | |||
| TV | – | 438 (33) | 480 (37) |
| PEEP | – | 15 (4) | 14 (3) |
| RR | – | 16 (2) | 18 (3) |
| Plateau pressure | – | 23 (3) | 25 (4) |
| DP | – | 9 (5) | 10 (4) |
| Compliance | – | 50 (22) | 47 (19) |
| ICU stay | 6 (5) | 25 (24) | 18 (14) |
| Hospital stay | 17 (9) | 30 (14) | 23 (13) |
| In-ICU mortality rate | 0 | 2 (25%) | 13 (29%) |
AHT, arterial hypertension; DM, diabetes mellitus; DP, driving pressure; HFNO, high-flow nasal oxygen; NIV, non-invasive ventilation; OTI, orotracheal intubation.
Two patients received oxygen supply using conventional techniques without having to escalate the respiratory therapy during their ICU stay, 45 (67%) received invasive mechanical ventilation immediately while 20 (30%) were treated with HFNO at the beginning (Table 1). The early parameters of all the patients treated with HFNO were 60 L of air flow and 90% of FiO2 followed by FiO2 titration to achieve SpO2 > 95%.
Patients treated with HFNO had remained in the hospital ward prior to their CCMS admission more time compared to patients treated with IMV (invasive mechanical ventilation) (11 [17] days vs 4 [12]; P = .06). A tendency was found towards the use of IMV in patients with smoking habits (42% vs 20%; P = .09). During the ICU admission, the SpO2/FiO2 ratio was significantly higher (115.52 [14.64] vs 102.53 [26.41]; P = .04).
A downward tendency in the ROX index was indicative of a failed HFNO. In 8 patients (40%) bailout IMV was needed. In this group, IMV was started, on average, 58 h (38) after starting the HFNO in older patients, with more associated comorbidities, an elevated ROX index within the first hour of HFNO at the ICU, and with a significantly lower SpO2/FiO2 ratio (P = .01).
In the analysis of the cost-effectiveness ratio when both therapeutic strategies were compared (see figure 1 of the supplementary data), the probability that the experimental strategy was more effective was 0.956, although this never reached statistical significance:
Different proportions: median = 0.175; 95%CI = –0.028 to 0.351. This corresponds to a number needed to treat (NNT) of 6 patients.
The optimal decision was the HFNO strategy followed by IMV in cases of failed HFNO. However, the ICER amounts to €219 294 for every ICU discharge.
Our data prove that patients treated with HFNO at admission had previously spent more time at the hospital ward. This may be indicative that these were patients with an initially less serious disease progression, which may have led to delays in the application of the most adequate therapies. In this sense, it has been confirmed that delays from HFNO until intubation are associated with a higher mortality rate in critically ill patients.7
In the pediatric population with pneumonia, the HFNO has a higher mortality rate compared to bubble CPAP.8,9 However, in the adult population, results are more heterogeneous. In this sense, in a post hoc subgroup analysis (patients with PaO2/FiO2 < 200), the FLORALI-REVA trial confirmed that the rate of intubation was lower in patients treated with HFNO compared to those treated with NIV or standard oxygen without any adjustments for multiple comparisons being made or using a time-dependent variable model (HFNO and NIV were interchangeable) with the possibility of overadjustment.7
Our data show that failed HFNO and further bailout IMV are associated with longer ICU stays, which does not have any repercussions in the in-ICU mortality rate of these patients. Our analysis shows that these patients are bound by the cost analysis.10 There is no doubt that the implications of selecting a therapeutic strategy with an ICER > €200 000 for every ICU discharge should be analyzed in terms of how it impacts the budget; especially in the pandemic and economic deceleration times we are living under.
In conclusion, our data cannot confirm the initial hypothesis that considered HFNO as an effective therapy in the management of hypoxic respiratory failure due to SARS-CoV-2 infection in the ICU setting. And not only that, it seems that a more solid analysis should be conducted to confirm the economic impact of such strategy regarding the cost-effectiveness ratio.
Please cite this article as: González-Castro A, Cuenca Fito E, Fernandez-Rodriguez A, Escudero Acha P, Rodríguez Borregán JC, Peñasco Y. Oxigenoterapia de alto flujo en el tratamiento de la neumonía por sindrome respiratorio agudo grave por coronavirus tipo 2. Med Intensiva. 2022;46:105–107.