To determine the prognostic value of the Vasoactive-Inotropic Score (VIS) in patients with Takotsubo syndrome (TTS) complicated by cardiogenic shock (CS).
DesignRetrospective cohort analysis.
SettingMulticenter registry (RETAKO) of patients diagnosed with TTS between 2003 and 2022.
Patients or participantsA total of 1591 patients with TTS, of which 412 (26%) developed CS.
InterventionsPatients were managed according to clinical criteria, with VIS calculated based on the maximum doses of inotropic and vasoactive drugs administered within the first 24 h of CS diagnosis.
Main variables of interest30-day and 1-year mortality rates, VIS tertile classifications.
ResultsOf the patients who developed CS, 208 received inotropic support. Patients in the highest VIS tertile had significantly higher 30-day (HR 8.80, 95% CI 1.96−39.48; p = 0.005) and 1-year (HR 4.55, 95% CI 1.11−18.63; p < 0.035) mortality compared to the lowest tertile. High VIS was also linked to increased complications, including acute kidney injury, major bleeding, and the need for mechanical circulatory support. In-hospital mortality rates were 4% for the low tertile, 14% for the middle tertile, and 47% for the high tertile (p < 0.001).
ConclusionsVIS is associated with worse short- and long-term outcomes in TTS complicated by CS. Further research is needed to explore potential causal pathways, if any, and to optimize therapeutic strategies for these patients.
Determinar el valor pronóstico del Índice Vasoactivo-Inotrópico (VIS) en pacientes con síndrome de Takotsubo (TTS) complicado con shock cardiogénico (SC).
DiseñoAnálisis de cohorte retrospectivo.
ÁmbitoRegistro multicéntrico (RETAKO) de pacientes con diagnóstico de TTS entre 2003 y 2022.
Pacientes o participantesUn total de 1591 pacientes con TTS, de los cuales 412 (26%) desarrollaron SC.
IntervencionesLos pacientes fueron manejados según criterios clínicos, y el VIS se calculó a partir de las dosis máximas de fármacos inotrópicos y vasoactivos administradas en las primeras 24 horas tras el diagnóstico de SC.
Variables de interés principalsMortalidad a 30 días y a 1 año, estratificados por terciles de VIS.
ResultadosDe los pacientes que desarrollaron SC, 208 recibieron soporte inotrópico. Los pacientes en el tercil más alto de VIS presentaron una mortalidad significativamente mayor a 30 días (HR 8.80, IC 95% 1.96−39.48; p = 0.005) y a 1 año (HR 4.55, IC 95% 1.11−18.63; p < 0.035) en comparación con el tercil más bajo. Un VIS alto también se asoció con un aumento de complicaciones, incluyendo lesión renal aguda, sangrado mayor, y necesidad de soporte circulatorio mecánico. Las tasas de mortalidad hospitalaria fueron de 4% para el tercil bajo, 14% para el medio y 47% para el alto (p < 0.001).
ConclusionesEl VIS se asocia con peores resultados a corto y largo plazo en TTS complicado con SC. Se necesitan más estudios para aclarar la posible causalidad de esta correlación, si esta existe, y optimizar las estrategias terapéuticas para estos pacientes.
Cardiogenic shock (CS) is defined as end-organ hypoperfusion resulting from deficient cardiac output despite adequate preload, caused by a structural or functional cardiac dysfunction.1 Regardless of numerous evidence-based therapeutic advances in modern cardiovascular medicine, CS remains a life-threatening acute condition with an in-hospital mortality rate as high as 40–50%, a figure that has not diminished over the last two decades.2
Takotsubo syndrome (TTS) is characterized by acute transient left ventricular systolic dysfunction, typically triggered by emotional or physical stress, in the absence of culprit coronary artery disease. The classical etiopathogenic theory suggests that TTS is caused by massive sympathetic nervous system activation and subsequent catecholamine-induced myocardial injury.3,4 CS complicates 5–20% of TTS cases, contributing to a poorer prognosis.5–7 Short-term mortality rates of cardiogenic shock due to Takotsubo syndrome (CS-TTS), though not as high as in CS secondary to acute myocardial infarction, remain substantial, ranging between 10–15%.8
During the acute phase of CS, hemodynamic stabilization is typically pursued through the administration of vasoactive and inotropic medications, which adjust vascular tone and enhance myocardial contractility, providing a time window for recovering while addressing the underlying cause of CS development.9 However, these drugs frequently induce cardiac arrhythmias, exacerbate or initiate myocardial ischemia, and can lead to peripheral ischemia.10 Increasing vasopressor and inotrope doses has been shown to be associated with higher mortality in patients with CS.11,12 In the context of CS-TTS, a condition triggered by massive microvascular catecholamine release, inotropes can have additional specifical harmful effects, such as increasing in left ventricular outflow tract obstruction (LVOTO) or worsening of mitral regurgitation due to systolic anterior movement (SAM) of the mitral leaflets: this complexity makes management of CS in this population particularly challenging.13–15
In order to objectively quantify the degree of pharmacological hemodynamical support, several scoring systems have been proposed. In 1995, Wernowsky et al. numerically described the combined doses of dopamine, dobutamine, and epinephrine, assigning each drug a coefficient that gives them an arbitrary equivalent value in the calculation.16 This inotropic score (IS) was upgraded in 2010 by Gaeies et al., who incorporated the doses of dopamine, dobutamine, and epinephrine into the calculation, resulting in the Vasoactive-Inotropic Score (VIS).17 Several corrections of the VIS have been proposed, adding to the calculation levosimendan,18 phenylephrine,19 enoximone,20,21 and olprinone.22 Lastly, Belletti et al. suggested the use of a more comprehensive score that incorporates the doses of terlipressin, methylene blue, and angiotensin-II, in addition to the previously mentioned medications.23
The VIS was initially validated as an independent predictor of clinical outcomes after cardiac surgery in pediatric patients.24 Subsequently, the prognostic value of the VIS has been demonstrated in adult patients after cardiothoracic surgery25,26 and, more recently, in patients with CS of nonsurgical etiology.27,28 However, data are lacking on whether a higher level of pharmacological hemodynamic support is associated with higher mortality in CS-TTS. Therefore, the aim of this study was to describe the pharmacological hemodynamic support employed in CS-TTS and to evaluate its prognostic value as quantified by VIS, examining its association with both short- and long-term outcomes.
MethodsData sourceAll data were collected from the Spanish multicenter REgistry of TAKOtsubo syndrome (RETAKO), a voluntary observational study that enrolled patients from 23 centers in Spain. Its rationale and design have been previously described.29 Baseline patient characteristics, triggering factors, in-hospital course, procedures and therapies performed at the discretion of the attending physician, and short-and long-term outcomes were captured through a dedicated electronic case report form. The admission value of all vital signs, clinical measurements, and laboratory values was defined as either the first value recorded after hospital admission or the value recorded closest to hospital admission. All participants were treated in compliance with the principles of the Declaration of Helsinki, and provided signed informed consent for the utilization of personal data for research purposes. The study was approved by the Institutional Ethics Committee of Hospital Clínico San Carlos.
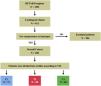
Study populationWe analyzed a database of consecutive unique adult patients >18 years admitted to the hospital with a diagnosis of TTS between January 1st, 2003, and December 31st, 2022. Inclusion in the RETAKO registry required a TTS diagnosis according to the Modified Mayo Clinic Criteria. CS was clinically diagnosed at each hospital, and for the purposes of this study it was defined by one of the following criteria: systolic blood pressure (SBP) < 90 mmHg for at least 30 min, use of vasoactive agents or mechanical support to maintain SBP > 90 mmHg, cardiac index < 2.2 L/min/m 2 in the absence of hypovolemia, each determined to be secondary to cardiac dysfunction. VIS was calculated as follows: dopamine dose (μg/kg/min) + dobutamine dose (μg/kg/min) + 100 × epinephrine dose (μg/kg/min) + 100 × norepinephrine dose (μg/kg/min) + 10,000 × vasopressin dose (U/kg/min) + 10 × milrinone dose (μg/kg/min) + enoximone dose (μg/kg/min) + 50 × levosimendan dose (μg/kg/min) + 25 × olprinone dose (μg/kg/min) + methylene blue dose (mg/kg/h) + 10 × phenylephrine dose (μg/kg/min) + 10 × terlipressin dose (μg/min) + 25 × angiotensin II dose (ng/kg/min). A local researcher from each participating center retrospectively extracted the dose of inotropic and vasoactive treatment used upon admission and the maximum dose during the first 24 h. Patients who did not receive vasoactive or inotropic support were excluded from the study. Based on the highest dose of each drug administered during the first 24 h, patients in the overall cohort were divided into three groups according to tertiles of maximum VIS.23 All included patients fulfilled the defined criteria for TTS and CS, as outlined in Fig. 1.
Statistical analysisThe primary end point was 30-day mortality and the secondary end point was 1-year mortality. Survival was assessed from hospital admission to death from any cause or last follow-up for censored patients.
The VIS group was treated as an ordinal variable to determine the risk associated with each of its categories. Summary statistics for continuous variables included mean and standard deviation, with groups compared by the analysis of variance or Kruskal-Wallis test, as appropriate. For categorical variables, number and percentage were used, with groups compared by the Pearson chi‐square test or with Fisher’s exact test, as appropriate. Kaplan–Meier 30-day and 1-year survival curves were plotted. The same method was used for the primary and the secondary end point.
Characteristics of survivors and non-survivors were compared using Cox proportional hazard regression models with estimation of hazard ratios (HR). Log-linearity was tested for quantitative variables. Correlations were assessed using Cramer’s V and Spearman’s rank correlation (Rho) for categorical and quantitative variables, respectively, and values above 0.50 were considered to indicate correlations. Firstly, univariable regression was performed for the variables that were identified to be possibly associated to worse outcomes based on clinical relevance, which were: age, male sex, diabetes, active neoplasm, left ventricular ejection fraction (LVEF), right ventricular failure, LVOTO, severe mitral regurgitation, acute kidney injury, and use of mechanical ventilation. Detailed variable definitions are reported in supplemental material. All covariates with p < 0.1 from the univariable analysis were included in the stepwise regression. Then, multivariable backwards stepwise Cox regression analysis (inclusion p < 0.05, exclusion p < 0.1) was performed to identify potential confounding variables and predictive factors associated with the outcome. Missing was <10% for all of the covariates (Supplemental Table 1). The proportional hazard assumption was assessed visually with graphical methods (Supplemental Fig. 1) and statistically using the Schoenfeld residual test. Calibration and discrimination of the final multivariable model were tested using the Akaike goodness-of fit test and the Harrell’s c-index.
Two‐tailed p values <0.05 were considered statistically significant. Statistical analysis was performed with Stata software version 17.0 (Statacorp).
ResultsClinical characteristicsCS was diagnosed in 412 (26%) of the 1591 patients included in the RETAKO registry. Of these, 199 (48%) patients did not receive any hemodynamic pharmacological support during the first 24 h from hospital admission, and 5 (1%) patients only received intravenous beta-blockers. The remaining 208 patients constituted the overall cohort (Fig. 1).
Baseline characteristics according to the VIS tertile are shown in Table 1. VIS ranged from 1 to 23 in the low tertile (T1), from 24 to 57 in the middle tertile (T2), and from 58 to 300 in the high tertile (T3). No significant differences were observed among the three groups in the mean age (68 ± 14), male sex (20%), smoker status (32%), arterial hypertension (57%), pulmonary disease (25%), and history of coronary artery disease (2%). In the high tertile, diabetes mellitus (34%, p = 0.001) and malignant neoplasia (7%, p = 0.038) were more prevalent. Although no significant differences in the type of TTS trigger were found among the three VIS groups, patients with a physical trigger had a distinct tail of extremely high VIS values, as evidenced by the upper points in the box plot of the Supplemental Fig. 2. Presentation with out-of-hospital cardiac arrest occurred more frequently in T2 and T3 compared to T1 (6% and 10% vs 1%, respectively; p = 0.034). Echocardiography revealed no significant differences in mean LVEF (33 ± 11%), rate of right ventricular failure (8%), LVOTO (24%), or severe mitral regurgitation (8%).
Baseline characteristics, admission vital signs, laboratory data, severity of illness scores and treatment characteristics during hospitalization.
| VIS 1−23 (n = 71) | VIS 24−57 (n = 69) | VIS > 57 (n = 68) | p | |
|---|---|---|---|---|
| Age, years | 69 ± 12 | 68 ± 16 | 69 ± 13 | 0.799 |
| Male sex | 14 (20) | 15 (22) | 12 (18) | 0.764 |
| Hypertension | 39 (55) | 40 (58) | 39 (57) | 0.771 |
| Diabetes | 8 (11) | 12 (17) | 23 (34) | 0.001 |
| Smoking | 23 (32) | 23 (33) | 20 (29) | 0.709 |
| Pulmonary disease | 14 (20) | 18 (26) | 19 (28) | 0.259 |
| Malignancies | 0 | 2 (3) | 5 (7) | 0.038 |
| Coronary artery disease | 1 (1) | 4 (6) | 0 | 0.610 |
| Charlson Comorbidity Index | 3.7 ± 1.9 | 4.0 ± 2.4 | 4.4 ± 2.3 | 0.141 |
| Stressful trigger | 0.662 | |||
| None | 11 (15) | 21 (30) | 16 (24) | |
| Emotional | 23 (32) | 9 (13) | 10 (15) | |
| Physical | 37 (52) | 39 (57) | 42 (62) | |
| Postcardiac arrest | 1 (1) | 4 (6) | 7 (10) | 0.034 |
| Admission echocardiography | ||||
| LVEF (%) | 35 ± 12 | 31 ± 10 | 32 ± 11 | 0.137 |
| Right ventricular failure | 4 (6) | 4 (6) | 8 (12) | 0.184 |
| Left ventricular outflow tract obstruction | 17 (24) | 15 (22) | 17 (25) | 0.888 |
| Severe mitral regurgitation | 2 (3) | 7 (10) | 8 (12) | 0.060 |
| Vital signs | ||||
| Systolic blood pressure, mmHg | 85 ± 13 | 86 ± 13 | 86 ± 17 | 0.600 |
| Diastolic blood pressure, mmHg | 50 ± 8 | 51 ± 8 | 52 ± 10 | 0.288 |
| Mean blood pressure, mmHg | 62 ± 9 | 63 ± 9 | 63 ± 11 | 0.548 |
| Heart rate, beats per minute | 102 ± 23 | 98 ± 24 | 104 ± 23 | 0.285 |
| Shock index score | 1.22 ± 0.3 | 1.15 ± 0.4 | 1.25 ± 0.4 | 0.467 |
| SCAI shock stage | <.001 | |||
| C | 68 (96) | 47 (68) | 14 (21) | |
| D | 2 (3) | 12 (17) | 25 (37) | |
| E | 1 (1) | 10 (14) | 29 (43) | |
| Laboratory data | ||||
| pH | 7.35 ± 0.11 | 7.32 ± 0.10 | 7.26 ± 0.16 | <.001 |
| Lactate at admission, mmol/L | 2.5 ± 1.0 | 3.3 ± 1.7 | 4.8 ± 2.5 | <.001 |
| Lactate peak at 24 h, mmol/L | 3.2 ± 1.3 | 4.9 ± 4.8 | 6.9 ± 3.2 | <.001 |
| Hemoglobin, g/dL | 12.6 ± 2.1 | 12.9 ± 1.8 | 12.3 ± 2.3 | 0.516 |
| Platelets, 109 × 10 g/L | 258 ± 148 | 220 ± 73 | 272 ± 147 | 0.364 |
| Creatinine, mg/dL | 1.51 ± 1.6 | 1.51 ± 1.1 | 2.15 ± 1.4 | <.001 |
| Serum urea nitrogen, mg/dL | 36 ± 32 | 46 ± 40 | 74 ± 67 | <.001 |
| Troponin T peak, μg/L | 1.3 ± 2.1 | 2.0 ± 3.2 | 1.3 ± 1.5 | 0.125 |
| NT-pro-BNP peak, pg/mL | 8.4 ± 15.0 | 5.3 ± 6.0 | 15.1 ± 18.0 | 0.001 |
| Vasoactive and inotropic drugs | ||||
| Vasoactive inotropic score | 12 ± 7 | 36 ± 9 | 115 ± 52 | <.001 |
| Norepinephrine equivalent score | 0.10 ± 0.08 | 0.32 ± 0.09 | 1.22 ± 1.30 | <.001 |
| Norepinephrine | 42 (59) | 68 (99) | 68 (100) | <.001 |
| Max dose, μg/kg/min | 0.16 ± 0.04 | 0.32 ± 0.09 | 1.05 ± 0.51 | <.001 |
| Epinephrine | 0 | 2 (3) | 5 (7) | 0.038 |
| Max dose, μg/kg/min | – | 0.05 | 0.19 | 0.036 |
| Vasopressin | 0 | 0 | 1 (1) | |
| Dopamine | 7 (10) | 5 (7) | 7 (10) | 0.936 |
| Max dose, μg/kg/min | 5 ± 3 | 2 ± 4 | 7 ± 2 | 0.159 |
| Dobutamine | 30 (42) | 47 (68) | 50 (74) | <.001 |
| Max dose, μg/kg/min | 4 ± 2 | 6 ± 4 | 9 ± 5 | <.001 |
| Milrinone | 1 (1) | 2 (3) | 1 (1) | 0.972 |
| Phenylephrine | 2 (3) | 3 (4) | 7 (10) | 0.070 |
| Max dose, μg/kg/min | 0.3 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.066 |
| Levosimendan | 2 (3) | 5 (7) | 3 (4) | 0.651 |
| Max dose, μg/kg/min | 0.05 | 0.09 ± 0.1 | 0.10 | 0.606 |
| Therapies and procedures | ||||
| Mechanical ventilation | <.001 | |||
| None | 43 (61) | 27 (39) | 11 (16) | |
| Non-invasive mechanical ventilation | 10 (14) | 8 (12) | 7 (10) | |
| Invasive mechanical ventilation | 18 (25) | 34 (49) | 50 (74) | |
| Oro-tracheal intubation, days | 3.7 ± 4 | 5.4 ± 5 | 10.0 ± 14 | <.001 |
| Renal replacement therapy | 1 (1) | 3 (4) | 14 (21) | 0.001 |
| Mechanical circulatory support | 0.001 | |||
| None | 69 (97) | 57 (83) | 52 (76) | |
| Intra-aortic balloon pump | 2 (3) | 11 (16) | 10 (15) | |
| Intravascular microaxial blood pump | 0 | 1 (1) | 2 (3) | |
| ECMO | 0 | 0 | 8 (12) |
LVEF: left ventricular ejection fraction; VIS: Vasoactive Inotropic Score. ECMO: extracorporeal membrane oxygenation; NT-pro-BNP: N-terminal pro-B type natriuretic peptide; VIS: Vasoactive Inotropic Score.
Table 1 also presents vital signs upon admission, illness severity scores, laboratory data, as well as the utilization of vasoactive and inotropic drugs, and therapeutic interventions. Mean blood pressure (62 ± 10 mmHg) and heart rate (101 ± 23 beats per minute) did not vary significantly across the three groups. The Society for Cardiovascular Angiography and Interventions (SCAI) shock stage was D or E in 4%, 32% and 79% of T1, T2 and T3 patients, respectively (p < 0.001). With increasing VIS, a progressive decline in mean pH was observed (7.35 ± 0.11 in T1, 7.32 ± 0.10 in T2, and 7.26 ± 0.16 in T3; p < 0.001), coupled with an elevation in peak lactate levels (2.5 ± 1.0 mmol/L in T1, 3.3 ± 1.7 mmol/L in T2, and 4.8 ± 2.5 mmol/L in T3; p < 0.001). The predominant vasoactive and inotropic agents administered were noradrenaline (86%), dobutamine (61%), and dopamine (9%), with doses of 0.6 ± 0.5 mg/kg/min, 7 ± 5 μg/kg/min, and 5 ± 4 μg/kg/min, respectively. Invasive mechanical ventilation was utilized in 25%, 49% and 74% of patients in T1, T2, and T3 (p < 0.001), with durations of 4 ± 4 days, 5 ± 5 days, and 10 ± 14 days (p < 0.001), respectively. Renal replacement therapy was required by 21% of T3 patients, whereas it was used by only 1% and 4% of T1 and T2 patients, respectively (p < 0.001). Mechanical circulatory support was employed in 3%, 17%, and 24% of patients in T1, T2, and T3, respectively (p = 0.001). Only 7 patients (3.4%) developed LVOTO or worsening of it directly related to the use of inotropic or vasoactive treatment, requiring the reduction or discontinuation of these drugs.
The analysis of complications during the hospital course, stratified by the VIS tertile, is summarized in Table 2. The duration of hospital stay was similar across the three groups (17 ± 18 days). Notably, while the incidence of ventricular arrhythmias, severe bradycardia, atrial fibrillation, pulmonary embolism, stroke, systemic embolism, and infections showed comparable distribution among the three groups, acute kidney injury and major bleeding occurred with progressively higher frequency in T1, T2, and T3. In-hospital mortality exhibited a progressive increase across the tertiles, with rates of 4%, 14%, and 47% in T1, T2, and T3, respectively (p < 0.001).
In-hospital course, complications and outcomes.
| VIS 1−23 (n = 71) | VIS 24−57 (n = 69) | VIS > 57 (n = 68) | p | |
|---|---|---|---|---|
| In-hospital stay, days | 14 ± 15 | 16 ± 17 | 20 ± 22 | 0.077 |
| Ventricular arrhythmias | 9 (13) | 11 (16) | 9 (13) | 0.918 |
| Asystole/atrioventricular block | 1 (2) | 5 (7) | 5 (7) | 0.126 |
| Atrial fibrillation | 12 (17) | 13 (19) | 17 (25) | 0.238 |
| Pulmonary embolism | 0 | 2 (3) | 1 (1) | 0.469 |
| Stroke | 2 (3) | 4 (6) | 4 (6) | 0.399 |
| Major bleeding | 3 (4) | 6 (9) | 11 (16) | 0.021 |
| Infection | 23 (32) | 30 (43) | 28 (41) | 0.284 |
| Acute kidney failure | 22 (31) | 28 (41) | 48 (71) | <.001 |
| In-hospital death | 3 (4) | 10 (14) | 32 (47) | <.001 |
| Cardiovascular | 1 (33) | 6 (60) | 17 (53) | <.001 |
| Non-cardiovascular | 2 (66) | 4 (40) | 15 (47) | 0.001 |
| 30-day mortality | 2 (3) | 6 (9) | 25 (37) | <.001 |
VIS: Vasoactive Inotropic Score.
The Kaplan–Meier 30-day and 1-year mortality curves of the three VIS groups are shown in Fig. 2. Fig. 3 shows the survival curves based on the type of vasoactive support used. It is noteworthy that those patients with combined support had a worse prognosis, while those with only inotropic support had the best survival
In the multivariable-adjusted analysis, it was found that 30-day mortality was significantly higher in T3 patients compared to T1, while no difference emerged between T1 and T2, after adjusting for potential confounders (Table 3). Specifically, when compared to the low VIS tertile (T1, referent group), the HR for 30-day mortality was 2.36 (95% CI 0.46−11.95; p = 0.301) for T2, and 8.80 for T3 (1.96−39.48; p = 0.005). The remaining factors were: age (HR 1.04 per-year increase; 95% CI 1.01−1.07; p = 0.016), acute kidney injury (HR 2.27; 95% CI 1.01−5.13; p = 0.048), and invasive mechanical ventilation (HR 2.15; 95% CI 0.89−5.20; p = 0.089), as well as the VIS tertile.
Univariable and multivariable Cox proportional hazard regression analysis for factors associated with 30-day and 1-year mortality.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| 30-Day Mortality | ||||
| Age (per 1-year increase) | 1.03 (1.00−1.06) | 0.055 | 1.04 (1.01−1.07) | 0.016 |
| Male sex | 1.42 (0.64−3.19) | 0.389 | … | … |
| Diabetes | 2.37 (1.14−4.95) | 0.021 | ||
| Active neoplasm | 3.10 (0.94−10.20) | 0.062 | … | … |
| LVEF (per 1-point increase) | 0.99 (0.96−1.02) | 0.513 | … | … |
| Right ventricular failure | 0.82 (0.20−3.46) | 0.792 | … | … |
| Left ventricular outflow tract obstruction | 1.27 (0.50−2.51) | 0.782 | … | … |
| Severe mitral regurgitation | 0.77 (0.18−3.21) | 0.716 | … | … |
| Acute kidney failure | 3.66 (1. 36−8.19) | 0.002 | 2.27 (1.01−5.13) | 0.048 |
| Invasive mechanical ventilation | 3.85 (1.66−8.94) | 0.002 | 2.15 (0.89−5.20) | 0.089 |
| VIS | ||||
| 1−23 | 1.00 (reference) | … | 1.00 | … |
| 24−57 | 3.26 (0.66−16.15) | 0.148 | 2.36 (0.46−11.95) | 0.301 |
| >57 | 14.54 (3.42−61.71) | <.001 | 8.80 (1.96−39.48) | 0.005 |
| 1-Year Mortality | ||||
| Age (per 1-year increase) | 1.04 (1.01−1.06) | 0.006 | 1.08 (1.02−1.14) | 0.012 |
| Male sex | 1.57 (0.82−2.97) | 0.389 | … | … |
| Diabetes | 2.04 (1.11−3.78) | 0.022 | … | |
| Active neoplasm | 4.09 (1.61−10.36) | 0.003 | … | |
| LVEF (per 1-point increase) | 0.99 (0.96−1.01) | 0.365 | … | … |
| Right ventricular failure | 0.78 (0.24−2.51) | 0.674 | … | … |
| Left ventricular outflow tract obstruction | 1.24 (0.66−2.35) | 0.504 | … | … |
| Severe mitral regurgitation | 2.03 (0.91−4.53) | 0.084 | … | … |
| Acute kidney failure | 2.83 (1.53−5.24) | 0.001 | … | |
| Invasive mechanical ventilation | 3.16 (1.67−6.00) | <.001 | 2.18 (0.67−7.10) | 0.193 |
| VIS | ||||
| 1−23 | 1.00 | … | 1.00 | … |
| 24−57 | 2.24 (0.77−6. 65) | 0.140 | 0.89 (0.17−4.56) | 0.890 |
| >57 | 9.11 (3.54−23.43) | <.001 | 4.55 (1.11−18.63) | 0.035 |
HR: hazard ratio; LVEF: left ventricular ejection fraction; VIS: Vasoactive Inotropic Score.
Similarly, the second part of Table 3 presents the findings from both univariable and multivariable analysis for the secondary outcome 1-year mortality. In the multivariable analysis, compared to patients in the low VIS tertile (T1, referent group), T2 patients had similar 1-year mortality (HR 0.89; 95% CI 0.17−4.56; p = 0.890), while T3 patients had a significantly higher risk (HR 4.55; 95% CI 1.11−18.63; p = 0.035), even after adjusting for the potential confounders age (HR 1.08 per 1-year increase; 95% CI 1.02−1.14; p = 0.012) and use of invasive mechanical ventilation (HR 2.18; 95% CI 0.67−7.10; p = 0.193).
DiscussionThe main findings of this study are as follows: 1) half of the patients with CS-TTS received vasopressors or inotropes, with noradrenaline and dobutamine being the most commonly used agents; 2) the degree of drug support did not significantly differ based on the type of TTS trigger; 3) a high level of pharmacological hemodynamical support, as quantified by VIS, was associated with worse short- and long-term outcomes.
The observed in-hospital mortality rates (4%–47%) in our study differ from the 10–15% previously reported for CS-TTS. This discrepancy may result from differences in patient selection, as our cohort specifically included individuals requiring vasoactive or inotropic support, representing more severe cases. Additionally, stratification by VIS revealed a wide mortality range, with lower-risk patients showing rates consistent with prior studies, while those with high VIS experienced significantly worse outcomes. Variability in comorbidities and management strategies across centers could also have contributed to these findings.30
The prognostic value of VIS has been consistently reported in several studies involving post-cardiothoracic surgery patients25,26 and, more recently, in patients with non-surgical causes of CS.27,28 In contrast to previous cohorts of CS of different etiologies, where the use of vasoactive and inotropic drugs has been reported in more than 90% of patients,31 such drugs were employed in only half of our CS-TTS cohort. This disparity may reflect not only the historically reported milder clinical course of CS-TTS compared to other causes of CS,8 but also a potential hesitancy in administering vasopressors and inotropes in CS-TTS due to concerns about potential side effects in this complex clinical scenario.
In a cohort of 493 with CS of multiple causes requiring inotropes or vasopressors, Na et al.27 reported a 30-day mortality as low as 5% in patients with VIS 1–10, which progressively increased to 10%, 20%, and 26% with VIS of 11–20, 21–38, and 39–85, respectively, reaching 61% in patients with VIS greater than 85. After multivariable analysis, the fourth and fifth VIS quintiles were associated with higher in-hospital mortality, while no significant differences were found between the first versus second or third VIS quintiles. In another cohort of 836 patients with CS secondary to acute myocardial infarction, Choi et al.28 found worse 6-month mortality rates in the third and fourth VIS quartiles (VIS ranges of 30–90, and >90, respectively). These findings, consistent across studies, reinforce the association between higher VIS and worse outcomes, even though direct comparisons between studies are challenging due to the variability in VIS ranges used for stratification. Moreover, these CS cohorts included patients with a more profound shock stage then patients from our cohort, as speculated from their higher serum lactate levels and extended use of mechanical circulatory support, with ECMO being employed in one-third of patients from both cohorts. In our study, patients in the high VIS tertile had a worse 30-day and 1-year mortality, while no differences were found between the low and middle VIS tertiles. This aligns with previous literature: overall, a high VIS is associated with worse outcome of CS, whereas the same cannot be said for low to intermediate VIS. In our cohort, we observed an increasing incidence of major bleeding across the VIS tertiles, reaching nearly 10% in the highest tertile. Although the statistical power is limited due to the small group sizes, this finding may be clinically relevant, particularly when considering therapeutic strategies in patients with severe CS-TTS. It is reasonable to hypothesize that the increased use of mechanical support in the highest tertile, combined with greater organ dysfunction, could have contributed to a higher predisposition to bleeding, possibly exacerbated by shock-induced coagulopathy. These data suggest that major bleeding may represent a significant complication in patients with high VIS, highlighting the need to carefully balance the use of mechanical and pharmacological support in such cases.
Interestingly, vasopressors carry a weight in VIS calculation that is about 5- to 10-fold higher than inotropes. For instance, in a patient receiving dobutamine 5 mg/kg/min and norepinephrine 0.15 mcg/kg/min, VIS would be 20, the same value as a patient only receiving norepinephrine at 0.2 mcg/kg/min. While it seems reasonable to assign a higher specific weight to vasopressors in patients with primary distributive shock, such as that typically observed after cardiothoracic surgery (for which VIS was originally developed), this may not be universally applicable to other types of shock.16,17 In this regard, mixed shock phenotypes represent a significant challenge, as they often combine elements of both distributive and CS, with distinct temporal dynamics that may affect VIS interpretation.32 In cases of CS-TTS triggered by physical insults such as infections, early stages are characterized by distributive shock complicated by myocardial dysfunction or by a mixed shock state in which a systemic insult simultaneously induces myocardial dysfunction and vasodilation; these presentations typically dominate during the first 24 h, the period in which VIS is calculated. In contrast, CS complicated by inappropriate vasodilation often emerges as a later phase in the clinical course, which may not be adequately captured by VIS at the 24-h mark. This temporal variation highlights the potential limitations of VIS as a static measure when applied to dynamic and evolving shock phenotypes.
Furthermore, outcome data stratified by the type of hemodynamic support suggest that patients treated with inotropes alone tend to have better outcomes than those receiving vasopressors alone, while those requiring combined inotropic and vasopressor support exhibit the worst outcomes (Fig. 3). This latter group likely reflects patients with more severe shock, as indicated by their higher VIS. These findings underscore the importance of considering the predominant shock phenotype and its temporal evolution when interpreting VIS. While inotropes may play a more central role in distributive or early mixed shock states, vasopressors appear to gain greater relevance in severe vasodilatory phases.33 Understanding these dynamics is essential for tailoring treatment strategies in complex conditions such as CS-TTS, where the interplay between myocardial dysfunction and vasodilation may evolve over time.
Recently, Vila-Sanjuán and collaborators found that inotropic or vasoactive treatments did not worsen ventricular function or cause LVOTO in TTS patients.34 This challenges the traditional recommendation of these drugs based on the adrenergic hypothesis of TTS. Preclinical studies indicate that norepinephrine, epinephrine, or isoprenaline can reproduce TTS's characteristic reversible apical ballooning and basal hypercontractility.35,36 Additionally, previous studies using various animal models have been able to trigger patterns similar to TTS, which worsened with higher doses of inotropic drugs.37 However, local catecholamine release at the myocardial level may occur independently of systemic increases through the hypothalamic-pituitary-adrenal axis. Neural impulses trigger norepinephrine release from sympathetic nerve terminals in the myocardium, which may explain why plasma catecholamine levels are not always elevated. Additionally, catecholamines directly released into the myocardium could be more toxic than those in the bloodstream.38 This highlights the complexity of TTS's pathophysiology beyond the adrenergic theory and suggests that inotropic and vasoactive treatments are unlikely to worsen the condition: the higher mortality in the high VIS tertile may reflect greater patient severity rather than a direct toxic effect of these drugs on the heart.
To determine whether VIS functions as an independent prognostic score beyond the severity of shock, it is crucial to consider global severity classifications, such as the SCAI staging, which has previously demonstrated predictive value in CS-TTS.39 Notably, VIS and SCAI are intrinsically linked, as the use of inotropic and vasoactive drugs is a fundamental component of the SCAI shock stages. When VIS and SCAI were analyzed together in relation to in-hospital mortality (Supplemental Table 2), the predictive value of VIS diminished and ultimately lost statistical significance. This finding suggests that a comprehensive clinical score reflecting overall shock severity, such as SCAI, holds greater prognostic relevance than one based solely on pharmacological variables. These results reinforce the idea that VIS primarily serves as a marker of illness severity rather than as an independent and modifiable prognostic factor.
Altogether, our study found that high VIS was associated with worse 30-day and 1-year mortality, while low and intermediate VIS had similar mortality rates.
Study limitationsOur study has inherent limitations owing to its retrospective nature, which warrant consideration when interpreting the results. These include potential missing data and selection bias, which may restrict the generalizability of findings. The absence of available invasive hemodynamic data could have hindered the diagnosis of CS, potentially resulting in the misclassification of other types of non-cardiogenic shock as CS. Because the diagnosis of CS did not require evidence of impaired tissue perfusion, the incidence of CS‐TTS may have been overestimated, and its severity might have been underestimated. Additionally, patients with a VIS of 0 were excluded from the analysis, which could be considered a limitation as they were not used as a reference group. Nonetheless, previous studies evaluating VIS have similarly excluded VIS 0 as a comparison group, acknowledging the heterogeneity of this population. This group may include both stable patients and potentially unstable individuals in whom vasoactive drug use was avoided due to concerns about adverse effects in the complex hemodynamic scenario of CS-TTS, as well as patients who were not candidates for advanced measures due to age and comorbidities. Given the observational design, unmeasured confounding variables may have influenced the outcomes. To mitigate this limitation, we performed a multivariable analysis adjusting for numerous variables identified as potential confounders. However, it is worth noting that this is the first multicenter study specifically focused on assessing the prognostic value of VIS in patients with CS-TTS.
ConclusionInotropes are employed in half of the patients with CS-TTS. In CS-TTS, a higher VIS in the first 24 h is associated with worse outcomes, while no differences emerged between low and intermediate VIS. Further prospective studies are needed to confirm these findings.
CRediT authorship contribution statementMT: data collection, statistical analysis, drafting and reviewing the manuscript.
SVS: data collection and reviewing the manuscript.
RV: data collection and reviewing the manuscript.
JS: data collection and reviewing the manuscript.
MMS: data collection and reviewing the manuscript.
JRR: data collection and reviewing the manuscript.
AM: data collection and reviewing the manuscript.
EBP: data collection and reviewing the manuscript.
MA: data collection and reviewing the manuscript.
MCP: data collection and reviewing the manuscript.
AU: data collection, statistical analysis, drafting and reviewing the manuscript.
ING: data collection and reviewing the manuscript.
Ethics approval number11/349-E.
Declaration of Generative AI and AI-assisted technologies in the writing processNon-use of some form of AI.
FundingThe Retako webpage is maintained thanks to the support by a non-conditional Fundación interhospitalaria para la investigación cardiovascular (FIC) scholarship.
DisclosuresNo relevant relationships with industry.
To all the researchers of the RETAKO team.