What PEEP level should I use in my patient? This is a question we ask ourselves everyday in clinical practice when we are dealing with mechanical ventilation in the intensive care unit (ICU).
Nowadays this question does not have a unique answer, which causes an important variability in clinical practice. Recent studies in patients with ARDS (Acute respiratory distress syndrome) present this variability as well as non-compliance of clinical practice guidelines recommendations. For example, in LUNG-SAFE1 study only 53% of ARDS patients were ventilated with tidal volume less than 7ml/kg while PEEP level used in these patients, with fraction of inspired oxygen (FiO2) greater than 70%, was even lower than the recommendations of low PEEP level group in ALVEOLI study.2 This argument regarding PEEP level is not exclusive for patients with ARDS but this parameter is controversial in other situations such as surgical patients or patients with exacerbations of chronic obstructive pulmonary disease (COPD), both to set FiO2 and to improve patient–ventilator interaction.3
It is demonstrated that the use of lung protective ventilation strategies improve the outcome of patients with ARDS and there is broad consensus in using a limited tidal volume and a limited lung pressure, as well as using PEEP levels higher than those used (and still being used) in routine clinical practice.4,5 However, there is no consensus regarding the appropriate strategy to select a PEEP level in each patient, so that different strategies are used with different purposes, what, at the moment, could be the two basic strategies: optimal PEEP determination depending on pulmonary mechanics of each patient or PEEP level adjustment depending on the quotient PaO2/FiO2.6
Recently, we have learned that in order to set tidal volume and PEEP level we have to keep in mind hemodynamics and pulmonary mechanics monitorization in patients with ARDS, so what is important to set ventilatory parameters is knowing lung recruitment capacity (to avoid atelectrauma) and keep the balance with an adequate tidal volume (to avoid overstretching phenomena). Undoubtedly, adequate balance between lung recruitment and overstretching improves not only lung function but also cardiac function in patients with ARDS.
In a meta-analysis conducted by our group analyzing the effect of high PEEP versus conventional PEEP in ARDS,7 we have already described that the use of high PEEP was not associated to increase mortality. However, if we consider only those studies in which high PEEP level is selected depending on the pulmonary mechanics characteristics, obtained by performing pressure-volume curves, the use of a high PEEP level was associated with a significant reduction in mortality (RR 0.59, 95% CI 0.43–0.82) and the incidence of barotrauma (RR 0.24, 95% CI 0.09–0.70).
Recently Amaro et al.8 have published the Driving Pressure Concept. Driving pressure could be the most important force in mechanical ventilation, it is the ratio as an index indicating the “functional” size of the lung an would provide a better predictor of outcomes in patients with ARDS than volume tidal alone. This ratio (ΔP=Volume tidal/static compliance), can be routinely calculated for patients who are not making inspiratory efforts as the plateau pressure minus PEEP.8
Pintado et al.9 published a randomized study comparing a lung protective ventilation strategy with two types of setting PEEP level in 70 patients with ARDS: based on PaO2/FiO2 or based on pulmonary mechanics, searching for the best pulmonary compliance point. Main results have shown the group selected based on compliance had more organ dysfunction-free days (median 6 vs. 20.5 days; P=0.02), more days without respiratory failure (7.5 vs. 14.5 days; P=0.03), and more days without hemodynamic failure (16 vs. 22 days; P=0.04). There was nonsignificant reduction in mortality at 28 days (39% vs. 21%).
This issue of “Medicina Intensiva” journal,10 presents a post hoc analysis of this study which included patients with severe ARDS according to Berlin consensus conference criteria, reporting that in severe ARDS patients, they found more organ dysfunction-free days at 28 days (12.83±10.70 vs. 3.09±7.23, p=0.04) and a trend toward lower 28-days mortality when PEEP was applied according to best static compliance (33.3% vs. 72.7%, p=0.16). In patients with moderate ARDS, they did not find those effects. An important limitation of this study is the sample size, this could explain some of the negatives results.
A very interesting finding of this study is that patients randomized to compliance-guided PEEP adjustment group had a strong trend to lower driving pressure mainly at the beginning of the evolution of the disease. This finding was very similar regardless of severity of ARDS. Recently, Amato et al.8 performed a multilevel mediation analysis with nine previous randomized trials on patients with ARDS to examine if the driving pressure (VT/respiratory-system compliance) was an independent variable associated with survival.
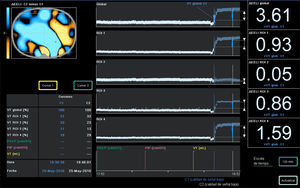
The authors of the study declare its limitations, originated mainly its post hoc analysis of a randomized trial with a low number of patients, however it is useful to create a hypothesis of great interest to justify conducting a multicenter clinical trial to resolve definitely the important question “What PEEP level should I use in my patient?”, meanwhile, from the respiration physiology perspective, PEEP optimization based on clinical situation and lung mechanics in each individual case might be the best option, perhaps making use of bedside imaging techniques as ultrasound or electrical impedance tomography (Figure 1), although it is not the easiest one at the routine clinical practice.
As we have seen in PROSEVA study11 regarding the use of prone-position during ventilation, the biggest benefit of this physiologic approach might lie in its specific use in high risk patients who need an individualized treatment.12
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.