To explore the behavior of C-reactive protein (CRP) after orthotopic liver transplantation (OLT) during the first postoperative days, and its usefulness as a marker of severe early allograft dysfunction (EAD).
DesignA prospective, single-center cohort study was carried out.
SettingThe Intensive Care Unit (ICU) of a regional hospital with a liver transplant program since 1997.
PatientsThe study comprised a total of 183 patients admitted to our ICU immediately after liver transplantation between 2009 and 2015.
Variables of interestC-reactive protein levels upon ICU admission and after 24 and 48h, severe EAD and hospital mortality.
ResultsThe CRP levels after OLT were: upon ICU admission 57.5 (51.6–63.3)mg/L, after 24h 80.1 (72.9–87.3)mg/L and after 48h 69.9 (62.5–77.4)mg/L. Severe EAD patients (14.2%) had higher mortality (23.1 vs 2.5; OR 11.48: 2.98–44.19) and lower CRP upon ICU admission (39.3 [29.8–48.7]mg/L) than the patients without EAD (0.5 [53.9–67.0]; p<0.05] – the best cut-off point being 68mg/L (sensitivity 92.3%; specificity 40.1%; Youden index 0.33).
Lower CRP upon ICU admission was correlated to higher mortality (24.5 [9.2–39.7] vs 59.4 [53.4–65.4]; p<0.01, AUC 0.79 [0.65–0.92]).
ConclusionLiver transplant is a strong inflammatory stimulus accompanied by high levels of C-reactive protein. A blunted rise in CRP on the first postoperative day after OLT may be a marker of poor allograft function and is related to hospital mortality.
Explorar el comportamiento de la proteína C reactiva (PCR) en el postoperatorio inmediato de trasplante hepático y su utilidad como marcador de disfunción grave del injerto hepático.
DiseñoEstudio de cohortes prospectivo, unicéntrico.
ÁmbitoUnidad de cuidados intensivos (UCI) de un hospital regional.
PacientesCiento ochenta y tres pacientes ingresados en nuestra UCI inmediatamente después del trasplante hepático entre 2009-2015.
Variables de interésNiveles de PCR al ingreso en UCI, 24 y 48h, disfunción grave del injerto hepático, mortalidad intrahospitalaria.
ResultadosLos niveles de PCR en el postoperatorio inmediato de trasplante fueron: al ingreso en UCI 57,5 (51,6-63,3)mg/L, a las 24h 80,1 (72,9-87,3)mg/L y a las 48h 69,9 (62,5-77,4)mg/L. Los pacientes con disfunción grave del injerto (14,2%) tuvieron una mayor mortalidad (23,1 vs. 2,5; OR 11,48: 2,98-44,19) y PCR más baja al ingreso en UCI (39,3 [29,8-48,7]mg/L) que los pacientes sin disfunción grave (0,5 [53,9-67]; p<0,05), siendo el mejor punto de corte para la PCR de 68mg/L (sensibilidad 92,3%; especificidad 40,1%; índice de Youden 0,33).
La PCR baja al ingreso tuvo correlación directa con la mortalidad (24,5 [9,2-39,7] vs. 59,4 [53,4-65,4]; p<0,01, AUC 0,79 [0,65-0,92]).
ConclusiónEl trasplante hepático es un estímulo inflamatorio intenso que se acompaña de niveles elevados de PCR. Un ascenso truncado de la PCR, en el primer día del postoperatorio de trasplante hepático, puede ser un marcador de funcionamiento inadecuado del injerto hepático y está relacionado con la mortalidad intrahospitalaria.
Orthotopic liver transplantation (OLT) is the main treatment for patients with a severe liver disease, but this is a complex surgical procedure and subject to a high number of complications.1 An important issue in the management of these patients is the evaluation of the function of the implanted organ but, considering the wide spectrum of functions performed by the liver, its assessment is not easy to undertake.
Markers for cytolysis, biliary production, ammonia, lactate and coagulation factors synthesized by the liver are widely used for the assessment of graft function, but there is no clear agreement about how and when to use them.2,3 In this scenario, the identification of other useful parameters for the evaluation of graft function could potentially hasten the detection of severe early allograft dysfunction (EAD) and help to initiate measures to diminish its intensity or even halt its development.4
Many tests have been proposed to increment the array of tools at our disposal such as the rate of elimination of molecules cleared by the liver (i.e. indocyanine green or C-methacetin),4–6 or a change in serum levels of different inflammatory markers.
C-reactive protein (CRP) is an acute phase reactant7 synthesized mainly by the liver, and its synthesis initiates about 6h after an inflammatory insult, with exponential increments every 8h, until its maximal peak 50h after the insult. Surgical procedures such as OLT are known to induce inflammation, therefore, an increase of CRP should be expected. Nonetheless, bearing in mind the role of the liver in its synthesis, disturbances in hepatic function should result in a dampened increase in CRP serum level.8 Accordingly, CRP production will depend on one hand on the impact of the inflammatory stimulus of the surgery and on the other hand on the functional capability of the allograft.8 For this reason, we hypothesize that when liver allograft function is altered, CRP production is blunted, and recognizing this will help the early detection of severe allograft dysfunction.9
Patients and methodsStudy designWe conducted a prospective, single center, cohort study, registering a series of consecutive OLT recipient patients admitted to our intensive care unit (ICU) from February 2009 to February 2015. For the design of the study and the preparation of the manuscript, we adhered to the recommendations of the STROBE initiative (Strengthening the Reporting of Observational Studies in Epidemiology).10
SettingThe ICU of the Regional University Hospital in Malaga, Spain. During the period covered by this study (2009–2015) all patients were managed according to an institutional hospital protocol covering all phases of the transplant process (preoperative, operative and postoperative stages) that was maintained without substantial changes.
Our protocol includes the use of the piggy-back technique and end to end anastomosis of the common bile duct in most cases, immediate postoperative care in our ICU for all patients, and clear indications for the immunosuppressive strategy: calcineurin inhibitors plus steroids (the most frequently used), mammalian target of rapamycin inhibitors plus steroids or interleukin 2 receptor antibodies plus steroids according to patient's characteristics and past medical history.
Ethics approval and consent to participateThis study was compliant with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines and approved by the Committee for Ethics in Research of the Regional University Hospital of Malaga.
Informed consent was obtained on admission to the ICU by either the patient or next of kin. Confidentiality was assured by registering variables in a disaggregated database.
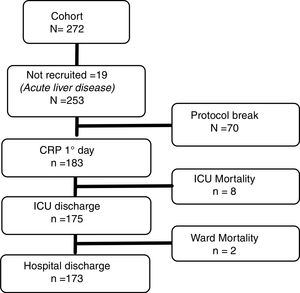
Patient information and data collectionRecruitment was conducted between February 2009 and February 2015. A flow-chart of study recruitment is presented in Fig. 1. Patients admitted to the ICU for postoperative care after OLT that complied with the study protocol (samples of CRP could be taken at ICU admission and at 24 and 48h and did not present exclusion criteria) were enrolled in the study.
Exclusion criteria: age less than 18 years, emergent OLT after acute liver failure, clinical and microbiological evidence of active infection, and refusal from the patient or his/her representative to participate in the study.
As per institutional hospital protocol, serum lactate, transaminases, INR, bilirubin, and creatinine are measured on admission to the ICU after the liver transplant and every 12h until discharge from the unit. Plasma CRP was additionally measured at ICU admission, 24 and 48h. A quantitative method based on polystyrene coated monoclonal antibodies against CRP was used (Dimension Vista 1500 System®). This test detects CRP concentrations in a range of 2.9–190mg/L.
Prior definitionsSevere dysfunction of liver allograft was defined using the MEAF (Model for Early Allograft Function Scoring) score11 with a cut-off over 8 points. This score is calculated by an equation comprising of three markers usually measured to evaluate liver function: ALT, INR, and total bilirubin.
The MEAF score was developed when recruitment was closing but due to the fact that all the required variables were already registered prospectively in our database, the statistical analysis was not due to be performed until the end of the recruiting period and in our population we found that this score performs better,2 we opted for its inclusion in the study. The primary outcome was severe graft dysfunction by MEAF score and the secondary outcome was in-hospital mortality.
Statistical analysisAs a first step, normality was probed with the Kolmogorov–Smirnov test, detecting that variables following a normal distribution were: MELD (Model for End-Stage Liver Disease), MEAF and CRP in days 1 and 3. Despite this fact and for the sake of readability of results, the mean and confidence interval of the mean were selected for the description of those continuous variables in which this parameter was more informative than the median. For the rest of variables, the median and interquartile range are the statistics shown. Categorical variables are presented as percentages. Chi-square, U-Mann Whitney, and Kruskal–Wallis tests were applied with a p-value <0.05. Corresponding Odds Ratio (OR) and 95% confidence interval of OR are shown when applicable.
Being a prospective cohort and not having information about the standard deviation of CRP in these populations we were not able to calculate beforehand the sample size needed so we calculated the power of our results.
To determine optimal cut-off level of CRP for detection of severe allograft dysfunction, a receiver operating characteristics (ROC) curve was drawn, its correspondent area under the curve (AuC) (95% confidence interval) calculated, and the Youden Index used to define the best cut-off point. In order to show an upward ROC curve, to simplify visual understanding of the results, 1/CRP was employed to draw the curve, after performing all calculations with raw CRP values.
In order to test a relationship between CRP and outcome, a model of logistic regression was computed by the backward conditional stepwise method, including all variables with a statistical relationship below 0.15 (sex, age, chronic kidney disease, previous OLT, MELD previous to OLT, APACHE II, higher lactate, creatinine at admission, higher creatinine and CRP at admission) in the univariate analysis and severe EAD or in-hospital mortality as the dependent variable; these results are presented as OR (95% confidence interval).
For statistical analysis and creation of figures the statistical package R 3.1.2 for OsX,12 and Prism 6 for Mac Os X (GraphPad Software Inc®) were employed. In order to compute the power of our study we used the Statmate 2® for Windows software.
ResultsOf 272 patients admitted after OLT, none showed clinical or microbiological signs of infection and 19 had acute liver disease. 253 patients did not show exclusion criteria, among them, a valid determination of CRP was performed at ICU admission in 183 patients (180 at 24 and 155 at 48h respectively).
Mean age was 54.3 (52.8–55.7) years and 45 (24.6%) of our patients were female. Model for End-Stage Liver Disease (MELD) score before OLT was 16.5 (15.5–17.6) points and mean APACHE II at ICU admission was 14.4 (13.8–15.1). Arterial hypertension in 47 (25.7%), diabetes in 47 (25.7%) and chronic renal disease in 15 (8.2%) were registered as relevant comorbidities.
Main indications for OLT were: alcoholic liver disease in 87 (47.5%), chronic viral hepatitis in 51 (27.9%), biliary tree diseases in 16 (8.7%), cryptogenic 12 (6.6%), and other 9.3%. Thirteen patients (7.1%) had a previous OLT.
Median hospital stay before surgery was 1 (interquartile range 0–1) day, median ICU length of stay 3 (3–5) days, and total hospital length of stay 12 (9–20) days. In-hospital mortality was 10 cases (5.5%), 8 out of 183 (4.4%) in the early postoperative course during ICU stay and 2 out of 175 (1.1%) in the hospital ward after ICU discharge.
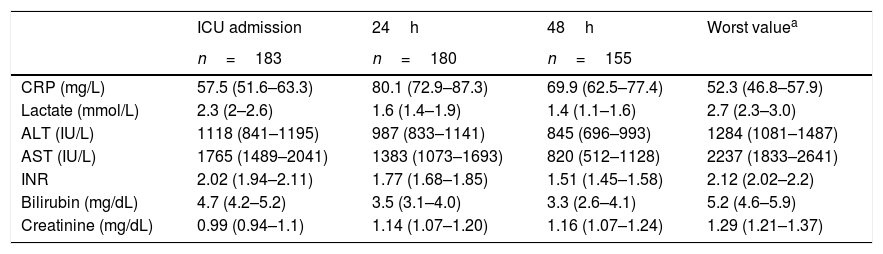
Mean CRP at ICU admission 57.5 (51.6–63.3)mg/L, at 24h 80.1 (72.9–87.3)mg/L and at 48h 69.9 (62.5–77.4)mg/L. Mean lower value for CRP 52.3 (46.8–57.9)mg/L. Concentration of CRP and classic markers of liver function are shown in Table 1.
Daily changes in C-reactive protein and other variables used to monitor liver function after liver transplant.
| ICU admission | 24h | 48h | Worst valuea | |
|---|---|---|---|---|
| n=183 | n=180 | n=155 | ||
| CRP (mg/L) | 57.5 (51.6–63.3) | 80.1 (72.9–87.3) | 69.9 (62.5–77.4) | 52.3 (46.8–57.9) |
| Lactate (mmol/L) | 2.3 (2–2.6) | 1.6 (1.4–1.9) | 1.4 (1.1–1.6) | 2.7 (2.3–3.0) |
| ALT (IU/L) | 1118 (841–1195) | 987 (833–1141) | 845 (696–993) | 1284 (1081–1487) |
| AST (IU/L) | 1765 (1489–2041) | 1383 (1073–1693) | 820 (512–1128) | 2237 (1833–2641) |
| INR | 2.02 (1.94–2.11) | 1.77 (1.68–1.85) | 1.51 (1.45–1.58) | 2.12 (2.02–2.2) |
| Bilirubin (mg/dL) | 4.7 (4.2–5.2) | 3.5 (3.1–4.0) | 3.3 (2.6–4.1) | 5.2 (4.6–5.9) |
| Creatinine (mg/dL) | 0.99 (0.94–1.1) | 1.14 (1.07–1.20) | 1.16 (1.07–1.24) | 1.29 (1.21–1.37) |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CRP: C-reactive protein; ICU: Intensive Care Unit; INR: International Normalized Ratio.
Data as mean (95% confidence interval for mean).
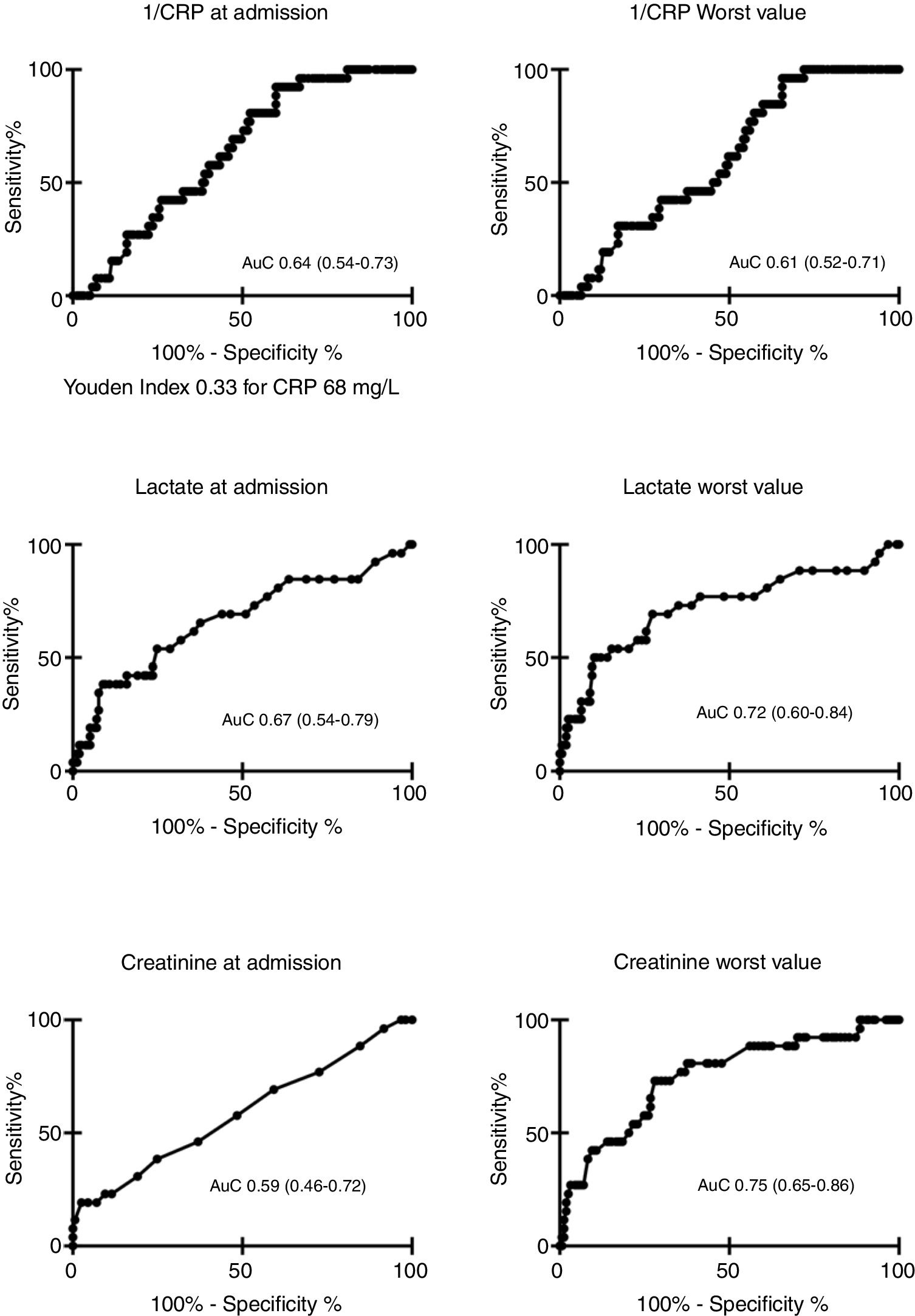
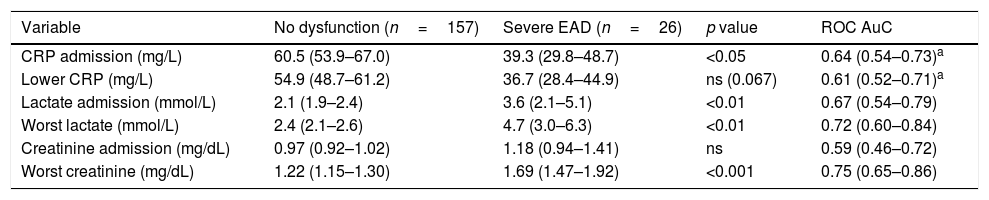
Twenty-six (14.2%) patients were diagnosed with severe EAD (MEAF score >8) and had higher mortality (23.1% vs 2.5%; OR 11.48 [95% CI 2.98–44.19]). CRP at ICU admission was 39.3 (29.8–48.7)mg/L, against 60.5 (53.9–67.0)mg/L in patients without severe EAD (p<0.05), and lowest CRP was 36.7 (28.4–44.9)mg/L in patients with severe EAD against 54.9 (48.7–61.2) in patients without it (p 0.067). Data for this and other related variables are shown in Table 2.
Changes in liver function markers in relation to severe EAD.
| Variable | No dysfunction (n=157) | Severe EAD (n=26) | p value | ROC AuC |
|---|---|---|---|---|
| CRP admission (mg/L) | 60.5 (53.9–67.0) | 39.3 (29.8–48.7) | <0.05 | 0.64 (0.54–0.73)a |
| Lower CRP (mg/L) | 54.9 (48.7–61.2) | 36.7 (28.4–44.9) | ns (0.067) | 0.61 (0.52–0.71)a |
| Lactate admission (mmol/L) | 2.1 (1.9–2.4) | 3.6 (2.1–5.1) | <0.01 | 0.67 (0.54–0.79) |
| Worst lactate (mmol/L) | 2.4 (2.1–2.6) | 4.7 (3.0–6.3) | <0.01 | 0.72 (0.60–0.84) |
| Creatinine admission (mg/dL) | 0.97 (0.92–1.02) | 1.18 (0.94–1.41) | ns | 0.59 (0.46–0.72) |
| Worst creatinine (mg/dL) | 1.22 (1.15–1.30) | 1.69 (1.47–1.92) | <0.001 | 0.75 (0.65–0.86) |
AuC: area under the curve; CRP: C-reactive protein; EAD: early allograft dysfunction; MEAF: Model for Early Allograft Function Scoring; ROC: receiver operating characteristics.
Data as the mean (95% confidence interval for the mean) and AuC (95% confidence interval for AuC). Severe EAD=MEAF score >8 points.
A regression model showed age (OR 0.95, 95%IC 0.90–0.99), higher lactate (1.27, 1.04–1.55), APACHE II (1.12, 1.01–1.20) and higher creatinine (3.51, 1.43–8.59) as the only variables with an independent relationship with graft dysfunction, with a Hosmer–Lemeshow goodness of fit of 0.58.
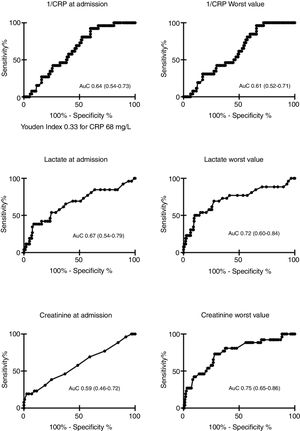
AuC data for CRP, lactate, and creatinine at ICU admission against MEAF score (above/below 8 points) is shown in Fig. 2. We compared only the behavior of CRP, lactate, and creatinine because the rest of parameters registered (transaminases, INR, bilirubin) were already included in the MEAF equation. The best cut-off for CRP to detect severe EAD (Fig. 2) at ICU admission was 68mg/L (Youden index 0.33), with a sensitivity of 92.3% and a specificity of 40.1%. We computed a power of 99% of our study to detect a statistically significant difference of 33.4mg/L in CRP levels between patients with and without severe EAD.
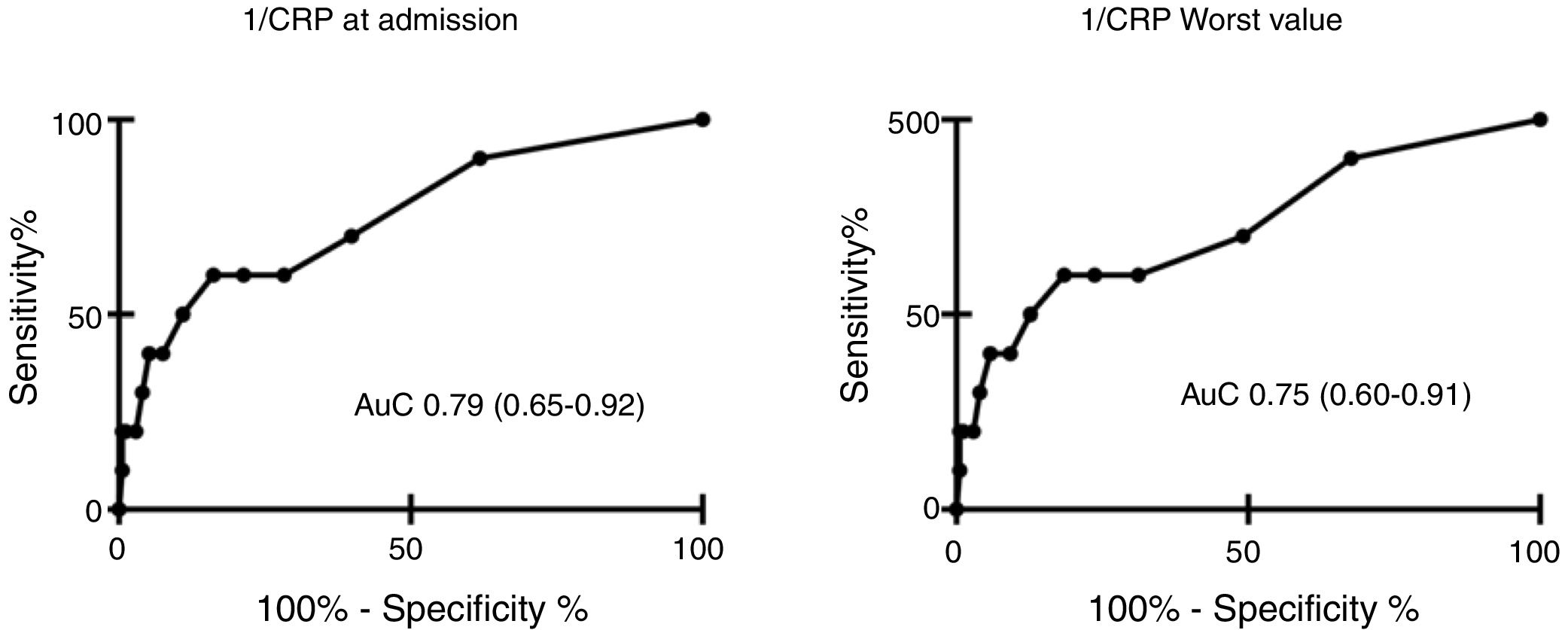
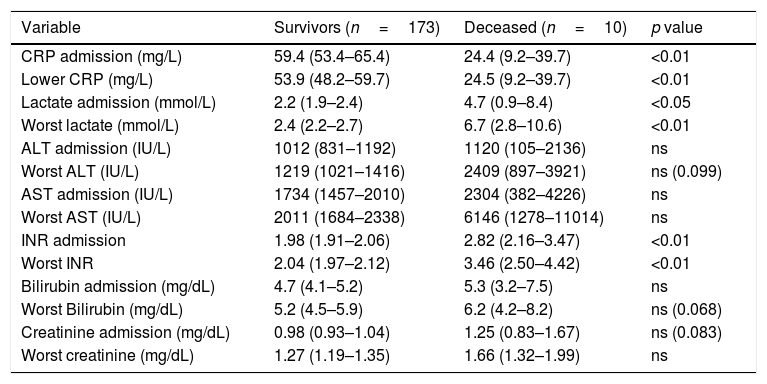
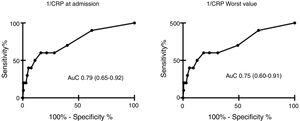
We found a negative relationship between CRP levels at ICU admission and in-hospital mortality, observing levels of 59.4 (53.4–65.4)mg/L in survivors vs 24.4 (9.2–39.7) in deceased patients, p<0.01. Lowest CRP levels were 53.9 (48.2–59.7)mg/L in survivors and 24.5 (9.2–39.7) in the deceased, p<0.01].
AuC was 0.79 (0.65–0.92) for CRP at ICU admission, and 0.75 (0.60–0.91) for lowest CRP. Results are shown in Table 3 and Fig. 3. A power of 99% was computed to detect a statistically significant difference of 32.24mg/L in CRP levels between patients that did or did not survive.
Changes in liver function markers in relation to in-hospital mortality.
| Variable | Survivors (n=173) | Deceased (n=10) | p value |
|---|---|---|---|
| CRP admission (mg/L) | 59.4 (53.4–65.4) | 24.4 (9.2–39.7) | <0.01 |
| Lower CRP (mg/L) | 53.9 (48.2–59.7) | 24.5 (9.2–39.7) | <0.01 |
| Lactate admission (mmol/L) | 2.2 (1.9–2.4) | 4.7 (0.9–8.4) | <0.05 |
| Worst lactate (mmol/L) | 2.4 (2.2–2.7) | 6.7 (2.8–10.6) | <0.01 |
| ALT admission (IU/L) | 1012 (831–1192) | 1120 (105–2136) | ns |
| Worst ALT (IU/L) | 1219 (1021–1416) | 2409 (897–3921) | ns (0.099) |
| AST admission (IU/L) | 1734 (1457–2010) | 2304 (382–4226) | ns |
| Worst AST (IU/L) | 2011 (1684–2338) | 6146 (1278–11014) | ns |
| INR admission | 1.98 (1.91–2.06) | 2.82 (2.16–3.47) | <0.01 |
| Worst INR | 2.04 (1.97–2.12) | 3.46 (2.50–4.42) | <0.01 |
| Bilirubin admission (mg/dL) | 4.7 (4.1–5.2) | 5.3 (3.2–7.5) | ns |
| Worst Bilirubin (mg/dL) | 5.2 (4.5–5.9) | 6.2 (4.2–8.2) | ns (0.068) |
| Creatinine admission (mg/dL) | 0.98 (0.93–1.04) | 1.25 (0.83–1.67) | ns (0.083) |
| Worst creatinine (mg/dL) | 1.27 (1.19–1.35) | 1.66 (1.32–1.99) | ns |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CRP: C-reactive protein; INR: International Normalized Ratio.
Data as the mean (95% confidence interval for the mean).
A regression model showed APACHE II (OR 1.34, 95%IC 1.08–1.66), higher lactate (1.42, 1.11–1.82), and CRP at ICU admission (0.95, 0.90–0.99) as the only variables with an independent relationship with mortality, with a Hosmer–Lemeshow goodness of fit of 0.40.
DiscussionChanges in serum CRP levels in relation to liver function and more specifically in relation to allograft function after liver transplant is an issue not yet resolved. Our results show that in fact, the liver transplant supposes a significant inflammatory stimulus accompanied by a rise in serum CRP during the postoperative period, but also show how those patients with a worse liver function had lower CRP serum levels and how a lower CRP postoperative increase is independently related to higher mortality. In our patients, a serum level below 68mg/dL on the first postoperative day is a sensible marker (over 92% sensibility) of severe EAD, but with a low specificity (40%), a fact that limits its usefulness.
As CRP is a non-specific marker of inflammation synthesized in the liver, its behavior in the setting of a liver transplant is uncertain. On one hand, we should expect CRP to rise because the transplant procedure is itself a strong pro-inflammatory stimulus but on the other hand, a damaged allograft or a retarded normalization of its function, could make it unable to react properly to inflammation, delaying or even aborting CRP production.
The role of CRP as a marker of poor graft function after OLT was first proposed by McCormick et al. These investigators, in a series of five liver transplant patients, detected that four of them showed an increase in serum CRP levels and the only patient who failed to show this increase died13 although this finding was challenged by Smith et al.,14 who argued that the CRP measurement method employed could explain this behavior.
Other reports as the study from Izumi et al.15 addressing CRP changes after OLT have shown contradictory results. This study demonstrated a significant increment in serum levels in acute (delta=58μ/ml) and chronic liver failure patients (delta=94μ/ml), with a maximum increment in the 4th day after transplant, not related to infection as previous reports had suggested, finally concluding that those changes could be at least in part related to the transplant procedure.
A study conducted by Their et al.,16 found an increment in serum CRP values in the postoperative course of liver and kidney transplant in 92% of their patients (median 43mg/L, range 6–130mg/L, maximum peak in the second day and return to normal levels by day 10). 67% of patients with rejection, increased CRP levels (median 52mg/L, range 9–157mg/L) 86% of them returning to normal levels after 5 days of rejection treatment. Also 88% of those patients with bacterial infection had elevated CRP levels (median 85mg/L, range 10–267mg/L). But, when vascular complications occurred, CRP barely rose above normal levels in few patients. Chung et al.17 study the intraoperative decline of CRP after OLT and found that CRP at day 1 after OLT below pre-transplant CRP had a 3-fold increased risk for detrimental outcome.
In any case, these results suggest that those patients with liver allograft dysfunction had a reduced peak of CRP compared to those patients complicated with infection, pointing to a diminished inflammatory response when EAD occurs, a hypothesis that seems confirmed by our findings. Kinetics of CRP in the first 3 postoperative days reported in this study coincide with several other studies,15,18,19 having the maximum peak in day two, but, unlike those studies our data shows that higher levels are attained the first postoperative day.
In other scenarios, like liver surgery20,21 or liver failure plus infection,8,22,23 low CRP levels have also been associated with liver dysfunction and outcome. Ananian et al.,20 demonstrated that after liver surgery, when acute dysfunction appears, CRP levels have an inverse correlation with hepatic function, a correlation that is maintained during the first month after surgery.
Rahman et al.,21 suggested that a blunted rise of CRP after hepatic resection can predict liver dysfunction and reported low CRP levels (28mg/L, 5–119mg/L) after ample liver resection compared to standard (41mg/L, 5–85mg/L) or minimal resections (51mg/L, 8–203mg/L) in the first day after surgery. These findings have been replicated in septic patients23 with liver failure where dampened rise of CRP were related to liver dysfunction rather than the inflammatory process.
We must acknowledge some problems with our study, in first place, that our relevant outcome variable, namely severe EAD, is based on clinical and laboratory parameters that in one way or other, mark a group of patients with a poorer outcomes, but is not defined by corresponding pathological findings, that could definitely prove that we are not mixing different problems (ischemia, rejection, allograft dysfunction).
Nonetheless, these diagnostic parameters are widely used and eventhough they can generate a selection bias, this is one present in all the studies addressing this topic and consequently do not rest external validity to our results beyond being a single-center study.
An additional problem when studying allograft dysfunction is the low incidence of this complication, making the development of larger multicenter studies necessary, to finally define the behavior of CRP in this scenario.
In our series, some groups of patients were excluded from the study (acute liver failure) and our decision was motivated because the acute liver failure patients CRP profile can be different from chronic liver disease patients due to different inflammatory stimuli and a deeper liver dysfunction.
One aspect that can be challenged in our protocol is the inclusion of a score that was published in the last stages of patients recruitment, namely the MEAF score.11 Even when this decision breaks the integrity of the “prospective” condition of the study, we opted for this strategy because based on reports published this seems a promising diagnostic tool. Even more, in an exploratory study we found this to perform the best in our specific population.2 Besides, all the variables included on this score were already collected prospectively in our study, the laboratory procedures are standardized and have not suffered significant variations during the period of study. Thus, we assumed that this approach did not compromise the validity of our conclusion and the evaluation of this new diagnostic tool adds usefulness to the study.
Finally, the number of losses in our population (due to unavailability to determine serum CRP at ICU admission) was high (27.7%) and this fact can suppose a bias in our results, but comparison between excluded and included patients did not show relevant differences (Table 1e shown in supplementary electronic material) and our data are consistent with those few studies previously published elsewhere. Also, the number of patients for the second and third day were diminishing because they died or were discharged to the surgery ward therefore in order to avoid a source of bias, we analyzed only data for CRP at admission and the first day.
ConclusionWe conclude that CRP serum levels are high in the early postoperative course after an OLT and a blunted rise in the first postoperative day, with a cut-off of 68mg/L, can be a marker of poor allograft function. Values below this threshold could be used as a screening marker for complications such as early allograft dysfunction. A dampened CRP rise seems to be related to in-hospital mortality in these patients.
Grants and financial supportThis work has not received external funding.
Authors’ contributionsAll authors participated in writing and revising the paper. H.G.M.E., S.P.G. and B.F.J.E. contributed on design, performance of research, data collection and analysis. Q.G.G., A.R.M.M. and D.R.M.J. contributed on performance of research and data collection.
Conflict of interestNone of the authors declare any kind of conflict with the contents of the present manuscript.
We thank to Katie Hurst MD, Juan Fernando Biguria-Rodriguez MD and Elisa López-Dolado PhD for reviewing this manuscript.