Secondary injury due to oxidation may occur during ischemic stroke, possibly leading to oxidative damage to deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Higher blood concentrations of 8-hydroxy-2′-deoxyguanosine (8-OHdG) (through the oxidation of guanosine from DNA) have been found in ischemic stroke patients than in healthy subjects, and in patients with versus without post-ischemic stroke depression. The present study was carried out to explore the possible association between serum DNA and RNA oxidative damage and mortality in patients with cerebral infarction.
MethodsA prospective, multicenter observational study was carried out in the Intensive Care Units of 6 Spanish hospitals. We included patients with severe malignant middle cerebral artery infarction (MMCAI) defined as ischemic changes evidenced by computed tomography in more than 50% of the middle cerebral artery territory and a Glasgow Coma Score (GCS)<9. Serum concentrations of the three oxidized guanine species (OGS) (8-hydroxyguanine from DNA or RNA, 8-hydroxyguanosine from RNA, and 8-OHdG from DNA) on the day of MMCAI diagnosis were determined. The study endpoint was 30-day mortality.
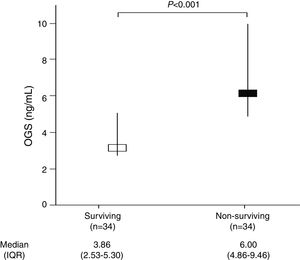
ResultsWe found higher serum OGS levels (p<0.001) in non-surviving (n=34) than in surviving patients (n=34). Logistic regression analyses showed serum OGS levels to be associated to 30-day mortality controlling for lactic acid, GCS and platelet count (OR=1.568; 95%CI=1.131–2.174; p=0.01).
ConclusionsThe novel observation in this study is the association between global serum OGS concentration and mortality in ischemic stroke patients.
En el infarto cerebral puede aparecer una lesión cerebral secundaria debido a la oxidación del ácido desoxirribonucleico (ADN) y del ácido ribonucleico (ARN). Se han encontrado concentraciones sanguíneas de 8-hidroxi-2’-desoxiguanosina (8-OHdG) (por la oxidación de la guanosina del ADN) más altas en pacientes con infarto cerebral que en individuos sanos, y en pacientes con depresión tras un infarto cerebral. El objetivo de nuestro estudio fue determinar si existe una asociación entre el daño oxidativo del ADN y del ARN, y la mortalidad de los pacientes con infarto cerebral.
MétodosEstudio prospectivo, observacional y multicéntrico realizado en unidades de cuidados intensivos de 6 hospitales españoles. Se incluyeron pacientes con un infarto maligno grave de la arteria cerebral media (MMCAI), definido como la presencia de cambios isquémicos en la tomografía en más del 50% del territorio de la arteria cerebral media y menos de 9 puntos en la escala Glasgow Coma Scale (GCS). Se determinaron los niveles séricos de las 3 especies oxidadas de la nucleobase guanina (OGS) (8-hidroxiguanina del ADN o ARN, 8-hidroxiguanosina del ARN y 8-OHdG del ADN) en el día del diagnóstico del MMCAI. La variable principal fue la mortalidad a 30 días.
ResultadosEncontramos concentraciones séricas de OGS (p<0,001) más altas en los pacientes fallecidos (n=34) que en los supervivientes (n=34). La regresión logística mostró que los niveles séricos de OGS se asociaban con la mortalidad a los 30 días controlando por ácido láctico, GCS y recuento plaquetario (odds ratio=1,568; IC 95%=1,131-2,174; p=0,01).
ConclusionesEl nuevo hallazgo de nuestro estudio fue la asociación entre los niveles séricos de OGS globales y la mortalidad de los pacientes con infarto cerebral.
Ischemic stroke causes high consumption resources, disabilities and deaths.1 Apart from the primary injury due to brain vasculature obstruction that causes a reduction of oxygenated blood and substrates to neurons, a secondary injury due to oxidation could appear during ischemic stroke.2–5 The hyperoxidative state of the ischemic stroke could produces oxidative damage on the ribonucleic acid (RNA),6,7 deoxyribonucleic acid (DNA),8–11 lipids, and proteins.
Four types of nucleobases compound DNA and RNA. The nucleobases guanine, cytosine and adenine are present in RNA and DNA, uracil in RNA, and thymine in DNA. Guanine, with the lowest redox potential, is the most prone nucleobase to oxidation.8–11 There is three oxidized guanine species (OGS) that are 8-oxo-guanine (8-oxo-Gua) or 8-hydroxyguanine (8-OHGua) from DNA or RNA oxidation, 8-oxo-guanosine (8-oxo-G) or 8-hydroxyguanosine (8-OHG) from RNA oxidation, and 8-oxo-deoxyguanosine (8-oxo-dG) or 8-hydroxy-2′-deoxyguanosine (8-OHdG) from DNA oxidation.
Higher levels of 8-OHG12–15 and 8-OHGua16,17 have been found in patients with different diseases than in healthy subjects. However, 8-OHdG is the most studied OGS, and higher 8-OHdG levels in patients with cardiovascular disease, heart failure and periodontal disease than in healthy subjects have been found in some meta-analyses.18–20
There is scarce data about nucleic acid oxidative damage in ischemic stroke patients.21–23 In a study higher 8-OHdG plasma concentrations were found in ischemic stroke patients than in healthy subjects.21 High concentrations of 8-OHdG in serum22 and urine23 were associated with post-ischemic stroke depression. The objective of our study was to determine whether an association between serum DNA and RNA oxidative damage and mortality in patients with cerebral infarction exists.
MethodsDesign and subjectsThis was an observational and prospective study. This multicentre study was carried out after the approval of the Institutional Review Board of all participating hospitals and with the written informed consent from the next to kin of each patient. This study was performed in the Intensive Care Units of 6 Spanish hospitals: H. Universitario Dr. Negrín (Las Palmas de Gran Canaria), H. Clínico Universitario de Valencia, H. General de La Palma, H. Insular de Las Palmas de Gran Canaria, H. Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife), and H. Universitario de Canarias (San Cristóbal de La Laguna).
We included patients with severe malignant middle cerebral artery infarction (MMCAI), defined as ischemic changes in computer tomography in more than 50% of the middle cerebral artery territory and a Glasgow Coma Scale (GCS)24 lower than 9. Patients with inflammatory or malignant disease, pregnancy, age less than 18 years, intracerebral hemorrhage or subarachnoid hemorrhage were excluded of the study. The patients were recruited between 2009 and 2012.
Variables recordedWe recorded the following variables at the moment of MMCAI diagnosis: age, sex, chronic obstructive pulmonary disease (COPD), chronic renal failure, diabetes mellitus, arterial hypertension, heart failure, temperature, GCS, sodium, lactic acid, glycemia, bilirubin, creatinine, pressure of arterial oxygen (PaO2), fraction inspired oxygen (FI02), platelets, leukocytes, leukocytes, leukocytes, leukocytes, hemoglobin, international normalized ratio (INR), fibrinogen, activated partial thromboplastin time (aPTT), Acute Physiology and Chronic Health Evaluation II (APACHE II) score,25 infarction volume, hemorrhagic transformation, midline shift, and decompressive craniectomy. The end-point study was 30-day mortality.
Determination of serum concentrations of OGSSerum blood samples on the day of MMCAI diagnosis (within the first 4h after diagnosis) were collected and frozen at −80°C until serum concentration determinations. Serum concentration of the three OGS were determined with the DNA/RNA Oxidative Damage ELISA Kit® (Cayman Chemical Corporation, Ann Arbor, USA), which has an assay detection limit of 10pg/mL, intra-assay coefficient of variation (CV) of 4.7–11.6%, and inter-assay CV of 4.5–10.7%. To check the adequate dilution of the samples we follow the recommendations of the kit. A first test was performed with 9 samples diluted at 1/25, 1/50 and 1/100 with ELISA Buffer preparation that is included in the kit. After verifying that the best dilution was 1/50, the rest of the samples were diluted to 1/50. Those determinations were carried out in the Laboratory Department of Hospital Universitario de Canarias (Tenerife, Spain).
Statistical methodsWe used medians and interquartile ranges to report continuous variables, and frequencies and percentages to report categorical variables. We used Wilcoxon–Mann–Whitney test for the comparison of continuous variables between patient groups, and chi-square test for the comparison of categorical variables. We carried out a multiple logistic regression analysis to determine the association between serum OGS levels and other variables with 30 day-mortality. We performed a receiver operating characteristic (ROC) curve to explore the prediction capacity of 30-day mortality by serum OGS levels. We constructed Kaplan–Meier 30-day mortality curves with patients showing higher and lower serum OGS levels than 4.82ng/mL (cut-off value selected by Youden J index). Jouden Index was used to select the cut-off of serum OGS levels since it shows the maximun prognostic capability with the best ratio between sensibility and specificity.26 We considered as statistically significant all p-values<0.05. We used NCSS 2000 (Kaysville, Utah), LogXact 4.1 (Cytel Co., Cambridge, MA), and SPSS 17.0 (SPSS Inc., Chicago, IL, USA) to perform the statistical analyses.
ResultsTable 1 shows that non-surviving (n=34) compared to surviving (n=34) patients showed higher serum levels of OGS (p<0.001) (Fig. 1) and lactic acid (p=0.049), and lower GCS (p=0.01) and platelet count (p=0.02). We did not find statistically significant differences between non-surviving and surviving patients in age, sex, chronic renal failure, COPD, diabetes mellitus, arterial hypertension, body temperature, sodium, creatinine, glycemia, bilirubin, PaO2, PaO2/FI02 ratio, leukocytes, aPTT, INR, fibrinogen, hemoglobin, APACHE-II score, infarction volume, midline shift, hemorrhagic transformation, and descompressive craniectomy.
Clinical and biochemical characteristics of surviving and non-surviving patients.
| Surviving(n=34) | Non-surviving(n=34) | p-Value | |
|---|---|---|---|
| Age (years) – median (IQR) | 59 (47–68) | 63 (53–70) | 0.36 |
| Gender female – n (%) | 14 (41.2) | 13 (38.2) | 0.99 |
| Diabetes mellitus – n (%) | 4 (11.8) | 9 (26.5) | 0.22 |
| Arterial hypertension – n (%) | 19 (55.9) | 16 (47.1) | 0.63 |
| COPD – n (%) | 1 (2.9) | 1 (2.9) | 0.99 |
| Chronic renal failure – n (%) | 2 (5.9) | 2 (5.9) | 0.99 |
| Heart failure – n (%) | 1 (2.9) | 1 (2.9) | 0.99 |
| APACHE-II score – median (IQR) | 20 (16–25) | 22 (19–27) | 0.06 |
| GCS score – median (IQR) | 7 (6–8) | 6 (3–7) | 0.01 |
| Temperature (°C) – median (IQR) | 36.4 (36.0–37.0) | 36.9 (36.0–37.3) | 0.15 |
| Sodium (mEq/L) – median (IQR) | 139 (136–145) | 140 (139–145) | 0.38 |
| Glycemia (g/dL) – median (IQR) | 127 (100–170) | 136 (118–162) | 0.40 |
| Creatinine (mg/dl) – median (IQR) | 0.80 (0.60–1.13) | 1.00 (0.70–1.25) | 0.19 |
| Bilirubin (mg/dl) – median (IQR) | 0.60 (0.40–0.83) | 0.60 (0.33–1.10) | 0.95 |
| Lactic acid (mmol/L) – median (IQR) | 1.20 (0.90–1.70) | 1.55 (1.00–2.70) | 0.049 |
| PaO2 (mmHg) – median (IQR) | 156 (105–293) | 115 (94–267) | 0.26 |
| PaO2/FI02 ratio – median (IQR) | 300 (198–369) | 254 (192–325) | 0.24 |
| Leukocytes – median×103/mm3 (IQR) | 12.4 (9.6–16.9) | 13.9 (9.7–20.1) | 0.32 |
| Hemoglobin (g/dL) – median (IQR) | 12.1 (11.4–14.0) | 12.5 (11.0–14.8) | 0.81 |
| Platelets – median×103/mm3 (IQR) | 202 (171–265) | 175 (136–216) | 0.02 |
| INR – median (IQR) | 1.06 (1.00–1.20) | 1.20 (1.01–1.31) | 0.07 |
| aPTT (seconds) – median (IQR) | 28 (25–30) | 27 (26–32) | 0.91 |
| Fibrinogen (mg/dl) – median (IQR) | 443 (416–489) | 419 (337–631) | 0.90 |
| Infarction volume (ml) – median (IQR) | 173 (100–231) | 180 (60–277) | 0.64 |
| Midline shift (mm) – median (IQR) | 6.0 (2.5–11.5) | 9.0 (3.5–15.0) | 0.43 |
| Thrombolysis – n (%) | 11 (32.4) | 10 (29.4) | 0.99 |
| Hemorrhagic transformation – n (%) | 7 (20.6) | 6 (17.6) | 0.99 |
| Decompressive craniectomy – n (%) | 9 (26.5) | 7 (20.6) | 0.78 |
| OGS (ng/mL) – median (IQR) | 3.86 (2.53–5.30) | 6.00 (4.86–9.46) | <0.001 |
IQR=interquartile range; COPD=Chronic Obstructive Pulmonary Disease; APACHE II=Acute Physiology and Chronic Health Evaluation; GCS=Glasgow Coma Scale; PaO2=pressure of arterial oxygen/fraction inspired oxygen; FIO2=pressure of arterial oxygen/fraction inspired oxygen; INR=international normalized ratio; aPTT=activated partial thromboplastin time; OGS=oxidized guanine species.
Logistic regression analysis showed that serum OGS levels were associated with 30-day mortality after control for lactic acid, GCS, and platelet count (Odds Ratio=1.568; 95% CI=1.131–2.174; p=0.01) (Table 2).
Multiple binomial logistic regression analysis to predict 30-day mortality.
| Variable | Odds ratio | 95% confidence interval | P |
|---|---|---|---|
| Serum OGS (ng/mL) | 1.568 | 1.131–2.174 | 0.01 |
| Lactic acid (mmol/L) | 1.269 | 0.702–2.292 | 0.43 |
| GCS (points) | 0.705 | 0.507–0.980 | 0.04 |
| Platelet count (each 1000/mm3) | 0.996 | 0.987–1.005 | 0.34 |
OGS=oxidized guanine species; GCS=Glasgow Coma Scale.
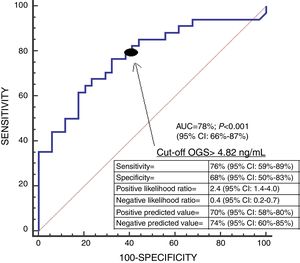
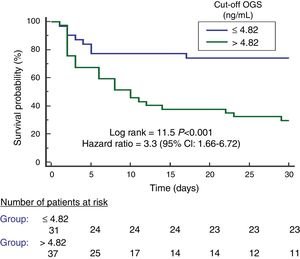
We found an area under the curve to predict 30-day mortality for serum OGS levels of 78% (95% CI=66–87%; p<0.001) (Fig. 2). We found in Kaplan–Meier analysis that patients with serum OGS levels>4.82ng/mL showed a higher 30-day mortality (Hazard ratio=3.3; 95% CI=1.66–6.72; p<0.001) (Fig. 3).
Previously, higher 8-OHdG plasma concentrations in ischemic stroke patients than in healthy subjects,21 and higher concentrations of 8-OHdG in serum22 and urine23 in patients with post-ischemic stroke depression were found. We determined in our study serum levels of global OGS, which included 8-OhdG and also of the other two OGS (8-oxo-guanosine or 8-hydroxyguanosine from RNA, and 8-oxo-guanine or 8-hydroxyguanine from DNA or RNA). In our study, we found for the first time, higher serum OGS levels in non-surviving than in surviving MMCAI patients, and the association between serum OGS concentrations and mortality of MMCAI patients. Those findings of our study are in accordance with the findings of other studies that found higher concentrations in patients with worst evolution of ischemic stroke defined as the presence of post-ischemic stroke depression22,23; although the end-point of our study was more severe (30-day mortality).
In addition, we found differences between non-surviving and surviving patients in other variables that previously were associated with mortality in those patients, such as lower GCS, lower platelet count, and higher lactic acid29–31; however, we only found that GCS was associated with 30-day mortality in the logistic regression analysis.
The mortality rate in our series (50%) was similar to the rate reported in other studies (38–61%),27,28 the mortality rate in our patients with decompressive craniectomy (44%) and without it (52%) was also similar to the rate reported in other studies (36–43% with decompressive craniectomy and 40–76% without it),27,28 and the rate of decompressive craniectomy in our series (24%) was similar to the rate reported in other studies (18–21%).27,28
In our study, the mean age was 58±14 years and 40% of patients were women. The incidence of ischemic stroke is higher in men, but in middle-age the rate begins to increase in women concomitant with the onset of menopause (and female sex hormone loss), and the rate is even higher in elderly females (>85 years) than in elderly males.32–35
We must recognize some limitations of our study. First, we only determined serum OGS levels at the time of MMCAI diagnosis. Second, we have not determined serum OGS levels in healthy subjects. Third, higher circulating 8-OHdG levels have been found in patients with cardiovascular diseases and we have not excluded those patients in our study; however, we have not found significant differences between surviving and non-surviving patient groups. Fourth, we did not report data about number and causes of exclusion. However, we think that the strength of our study is that we reported serum levels of the three OGS and not only of 8-OhdG levels.
We believe that the new findings of our study with cerebral infarction patients and the findings in animal models of cerebral infarction about the benefits of administration of antioxidant agents reducing DNA oxidative damage in brain samples, infarction volume and neurological deficit36–48 could motivate the interest to research about the use of serum DNA and RNA oxidative damage levels as biomarkers of mortality prediction and to research about the use of antioxidants agents in cerebral infarction.
ConclusionsThe association between serum concentrations of global OGS and mortality of ischemic stroke patients was the novel finding of our study.
Author contributionsLLo conceived, designed and coordinated the study, participated in acquisition and interpretation of data, and drafted the manuscript.
MMM, PAG, LR, MA, JJC, JSV, AAC and VGM participated in acquisition of data.
AGC and APC carried out the analyses of DNA and RNA oxidative damage.
AJ participated in the interpretation of data.
All authors revised the manuscript critically for important intellectual content and made the final approval of the version to be published.
FundingsThis study was supported by a grant (OA18/011) from Fundación DISA a la Investigación Médica 2017 (Santa Cruz de Tenerife. Spain). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestsThe authors declare that they have no competing interests.