During the months of March and June 2020, many Spanish hospitals became monographic centers dedicated to patients infected with the SARS-CoV-2 coronavirus that produces COVID-19 disease. Any patient with respiratory failure became a suspected COVID-19 case, particularly in the presence of plasma D-dimer elevation. However, these same manifestations can be seen in pulmonary thromboembolism (PTE).1 The fact that COVID-19 disease is associated to PTE only adds to the state of confusion.2 On the other hand, physical immobility is one of the main risk factors for the development of PTE. In this regard, it can be assumed that during lockdown following the nationwide alert declared by the Spanish Government on 14 March 2020, citizens in general spent more time at rest than usual.
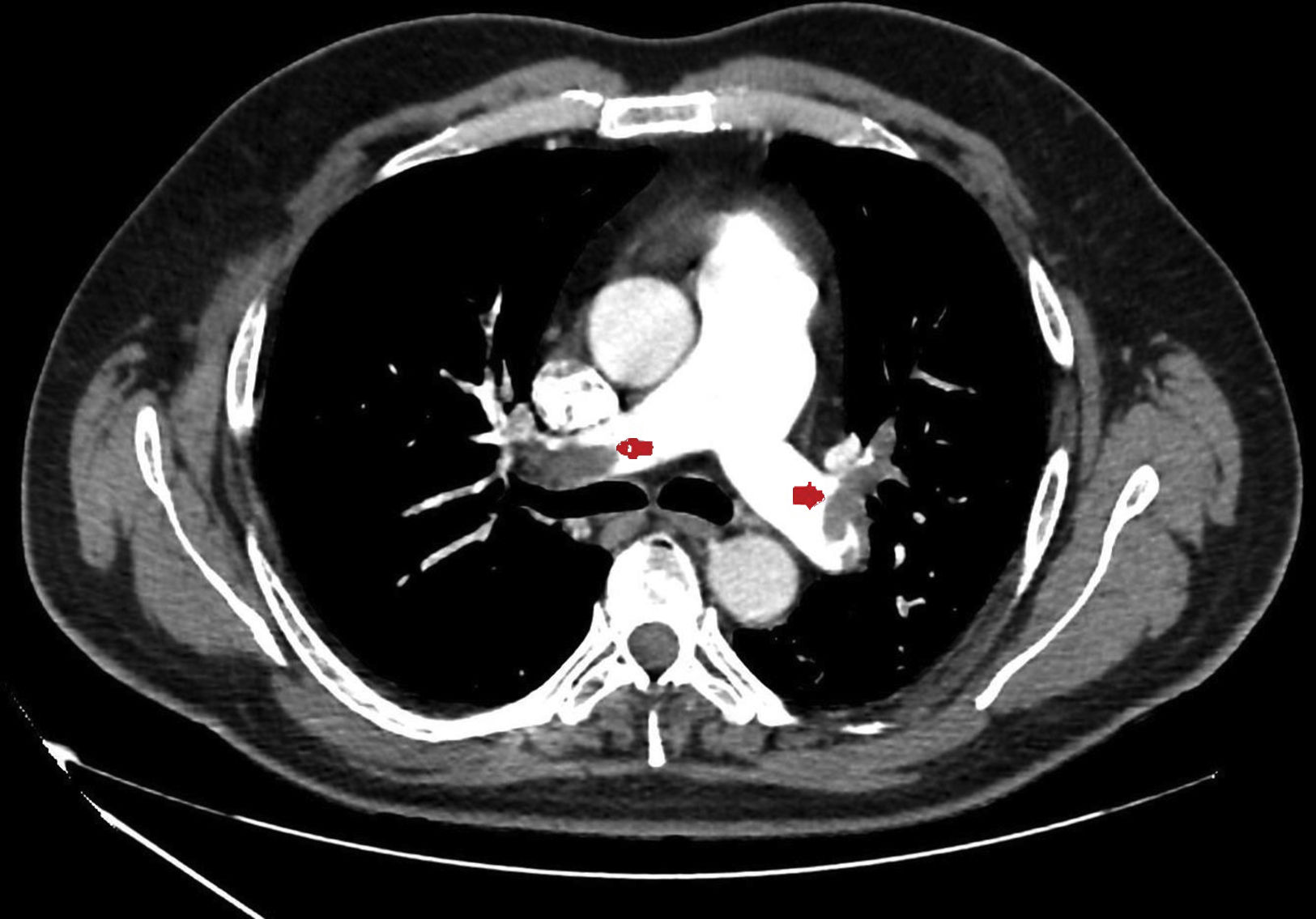
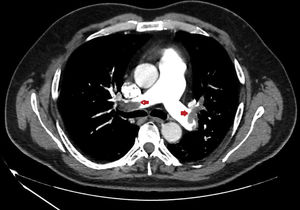
We present a series of four young patients (under 50 years of age) admitted to an Intensive Care Unit (ICU) due to severe PTE (Fig. 1) during a period of 10 days (22–30 April) after one month of lockdown because of the pandemic (starting on 15 March and extending until 11 May). The patients were treated with low molecular weight heparin, and their course proved favorable. Seeing so many cases in such a short period of time quickly drew our attention. In effect, over the last three years, our ICU admitted an average of 7 cases of PTE a year, with no particular increase in incidence being noted during the months of April.
At that time, vascular involvement in COVID-19 patients had already been postulated,3 and we initially suspected that the mentioned cases of PTE could be secondary to COVID-19 disease. However, the four PCR tests made (Seegene®), one per patient, proved negative. We then suspected that the patients might have passed the disease without symptoms, but that the vascular involvement had progressed towards PTE. Nevertheless, IgM and IgG antibody testing (electrochemoluminescence immunoassay, Roche®) also proved negative. Although false-negative results cannot be ruled out, the fact is that both PCR and serological testing were negative in all four cases.
Having discarded the possibility of COVID-19, the fact that we were seeing severe PTE in young individuals that simply had to spend a considerable amount of time at home during lockdown, continued to draw our attention.
We know that no causal factor is identified in up to one-half of all venous thromboembolic events (comprising deep venous thrombosis and pulmonary embolism). The remaining 50% of the cases are attributable to transient or persistent clinical or environmental factors that cumulatively induce venous stasis, hypercoagulability and endothelial damage. The main risk factors include surgery, physical immobility, and cancer. Other associated factors are obesity, a history of venous thromboembolic events, inflammatory diseases and genetic factors.4
The association between immobility and PTE is well known. We are all aware of the risk of PTE after surgery, though this risk has been reduced thanks to the use of preventive anticoagulation measures. Only one of our four patients had undergone minor surgery two months ago. However, there are other forms of immobility that we tend to be less aware of.
A study published in the early 21st century reported an increased incidence of PTE in individuals on transcontinental flights.5 As a consequence of the subject of long air flights and the growing recreational and occupational use of computers, a number of studies have explored the association between physical immobility at home or at work and the risk of venous thromboembolic disease. The term “e-thrombosis” has been coined to describe this phenomenon.6 Immobility has arbitrarily been defined as remaining seated at least 8hours a day without standing up from the chair for at least three consecutive hours in that time, during the last four weeks. Prolonged immobility favors venous stasis of the lower extremities by reducing muscle contraction-induced compression of the veins. There is also evidence of increased coagulability and endothelial dysfunction.7 The resulting thrombi in turn may displace and become lodged within the pulmonary venous system, causing PTE. It has been suggested that between 15–30% of all cases of venous thromboembolic disease may be due to recreational or occupational immobility.8–10
We thus considered the possibility that physical immobility secondary to lockdown during the COVID-19 pandemic could explain the observed PTE peak in our ICU. In surveys made a posteriori, three of the four patients admitted having spent an average of over 8hours a day seated over the last few weeks – though none had remained seated for more than two consecutive hours without standing up (Table 1). These three patients were overweight, and one had a history of deep venous thrombosis and recent minor surgery. The fourth patient claimed to have spent an average of 6hours a day seated, and was the only subject in the series presenting coagulation anomalies with lupus antibody positivity as the cause of hypercoagulability.
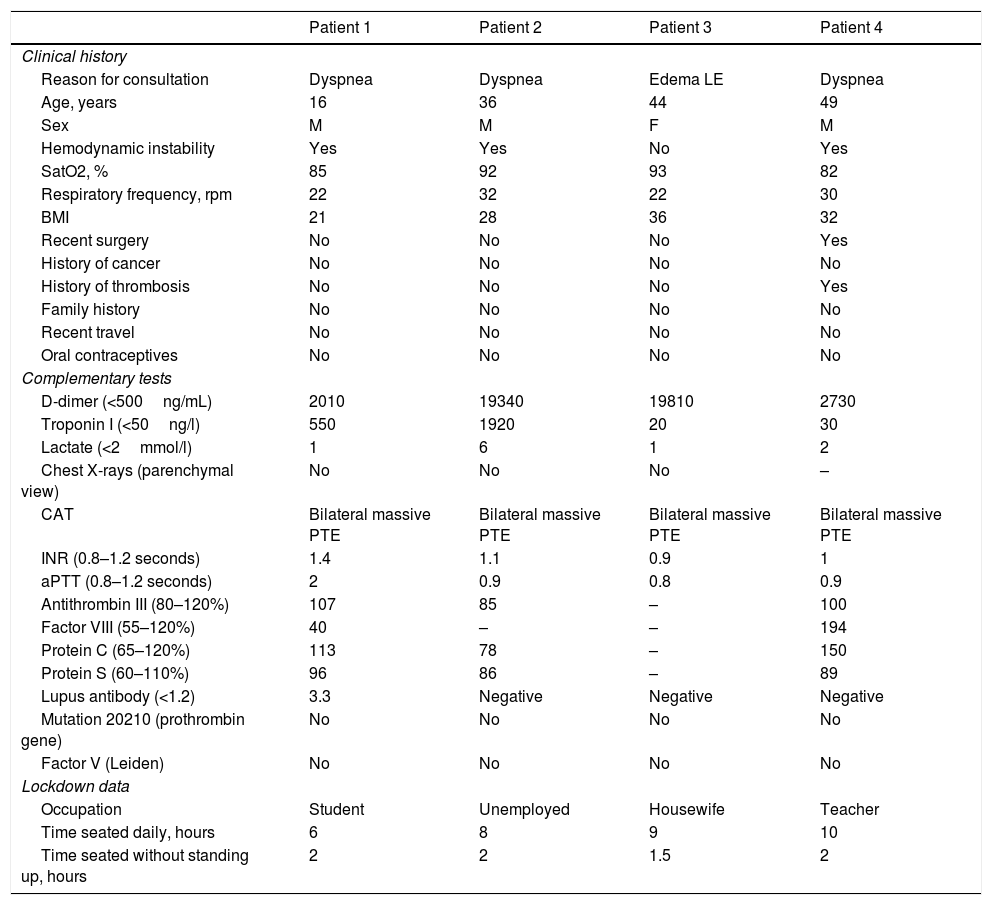
Principal characteristics of the patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Clinical history | ||||
| Reason for consultation | Dyspnea | Dyspnea | Edema LE | Dyspnea |
| Age, years | 16 | 36 | 44 | 49 |
| Sex | M | M | F | M |
| Hemodynamic instability | Yes | Yes | No | Yes |
| SatO2, % | 85 | 92 | 93 | 82 |
| Respiratory frequency, rpm | 22 | 32 | 22 | 30 |
| BMI | 21 | 28 | 36 | 32 |
| Recent surgery | No | No | No | Yes |
| History of cancer | No | No | No | No |
| History of thrombosis | No | No | No | Yes |
| Family history | No | No | No | No |
| Recent travel | No | No | No | No |
| Oral contraceptives | No | No | No | No |
| Complementary tests | ||||
| D-dimer (<500ng/mL) | 2010 | 19340 | 19810 | 2730 |
| Troponin I (<50ng/l) | 550 | 1920 | 20 | 30 |
| Lactate (<2mmol/l) | 1 | 6 | 1 | 2 |
| Chest X-rays (parenchymal view) | No | No | No | – |
| CAT | Bilateral massive PTE | Bilateral massive PTE | Bilateral massive PTE | Bilateral massive PTE |
| INR (0.8–1.2 seconds) | 1.4 | 1.1 | 0.9 | 1 |
| aPTT (0.8–1.2 seconds) | 2 | 0.9 | 0.8 | 0.9 |
| Antithrombin III (80–120%) | 107 | 85 | – | 100 |
| Factor VIII (55–120%) | 40 | – | – | 194 |
| Protein C (65–120%) | 113 | 78 | – | 150 |
| Protein S (60–110%) | 96 | 86 | – | 89 |
| Lupus antibody (<1.2) | 3.3 | Negative | Negative | Negative |
| Mutation 20210 (prothrombin gene) | No | No | No | No |
| Factor V (Leiden) | No | No | No | No |
| Lockdown data | ||||
| Occupation | Student | Unemployed | Housewife | Teacher |
| Time seated daily, hours | 6 | 8 | 9 | 10 |
| Time seated without standing up, hours | 2 | 2 | 1.5 | 2 |
Abbreviations: LE: Lower extremities, rpm: respirations per minute, BMI: body mass index (overweight >27; obesity >30), CAT: Computed axial tomography, PTE: Pulmonary thromboembolism, INR: International Normalized Ratio, aPTT: Activated partial thromboplastin time.
Lastly, genetic factors are studied despite the fact that their clinical relevance is not clear and that less than 15% of all patients with venous thromboembolic disease present genetic alterations.4 None of the three patients in which genetic testing proved possible in our series presented any of the most frequent alterations.
These data suggest that prolonged immobility at home, together with other risk factors (in our case patient overweight, a history of venous thromboembolic disease and thrombophilia), may be associated to an increased incidence of PTE. Although our study is based on simple clinical observation, we believe the association (without speaking of causality as such) to be plausible.
In conclusion, in young individuals, immobility at home during lockdown due to the COVID-19 pandemic could have been associated to an increased risk of PTE. In the event of a new outbreak of the pandemic, we must bear this possibility in mind, and the public health services should recommend physical exercise at home. In future, particularly if working from home (telecommuting) becomes consolidated as an occupational option, we must take this serious disease condition and its prevention into account.
Financial supportThe present study has received no financial support.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Franch-Llasat D, Mayor-Vázquez E, Pedregosa-Díaz J, Herrero-Redondo M, Ortin-Font X, Roche-Campo F. e-Thrombosis en época COVID-19. Efectos colaterales del confinamiento. Med Intensiva. 2021;45:122–124.