Echocardiography enables the intensivist to assess the patient with circulatory failure. It allows the clinician to identify rapidly the type and the cause of shock in order to develop an effective management strategy. Important characteristics in the setting of shock are that it is non-invasive and can be rapidly applied. Early and repeated echocardiography is a valuable tool for the management of shock in the intensive care unit. Competency in basic critical care echocardiography is now regarded as a mandatory part of critical care training with clear guidelines available. The majority of pathologies found in shocked patients are readily identified using basic level 2D and M-mode echocardiography.
The four core types of shock (cardiogenic, hypovolemic, obstructive, and septic) can readily be identified by echocardiography. Echocardiography can differentiate the different pathologies that may be the cause of each type of shock. More importantly, as a result of more complex and elderly patients, the shock may be multifactorial, such as a combination of cardiogenic and septic shock, which emphasises on the added value of transthoracic echocardiography (TTE) in such population of patients.
In this review we aimed to provide to clinicians a bedside strategy of the use of TTE parameters to manage patients with shock. In the first part of this overview, we detailed the different TTE parameters and how to use them to identify the type of shock. And in the second part, we focused on the use of these parameters to evaluate the effect of treatments, in different types of shock.
La ecocardiografía permite al intensivista valorar al paciente con fallo circulatorio agudo. Esta técnica ayuda a identificar, rápidamente y de una manera no invasiva, el tipo y la causa del shock para instaurar una estrategia terapéutica. La realización de exámenes ecocardiográficos precoces y repetidos es una valiosa herramienta para el manejo del shock en la unidad de cuidados intensivos. La mayoría de patologías responsables del shock pueden ser identificadas con un nivel básico de ecocardiografía en 2D y modo M. En la actualidad, las competencias en ecocardiografía básica se consideran mandatorias en la formación de los profesionales de Medicina Intensiva.
Los cuatro tipos básicos de shock (cardiogénico, hipovolémico, obstructivo y séptico) pueden ser adecuadamente identificados con la ecocardiografía. Además, la ecografía puede diferenciar las diferentes patologías que pueden ser la causa de cada uno de los tipos de shock. Es importante señalar que, dada la complejidad y la edad avanzada de muchos pacientes críticos, el shock puede ser multifactorial (p.ej.: combinación de shock séptico y cardiogénico), lo que enfatiza el valor añadido de la ecocardiografía transtorácica (ETT) en esta población de pacientes.
En esta revisión, queremos proporcionar a los clínicos una estrategia, a pie de cama, del uso de los parámetros obtenidos con la ETT para manejo de los pacientes en shock. En la primera parte de este artículo, se detallan los diferentes parámetros ecocardiográficos y cómo pueden utilizarse para identificar los tipos de shock. En la segunda parte, se expone el uso de estos parámetros para evaluar el efecto de los tratamientos en los diferentes tipos de shock.
Shock is best defined as a life-threatening, generalized form of acute circulatory failure associated with inadequate oxygen utilization by the cells. It is a state in which the circulation is unable to deliver sufficient oxygen to meet the demands of the tissues, resulting in cellular dysfunction. The result is cellular dysoxia, i.e. the loss of the physiological independence between oxygen delivery and oxygen consumption, associated with increased lactate levels.1
Transthoracic echocardiography (TTE) is a key exam in the diagnosis of shock and therapy guidance.
The main challenge during shock management is to quickly restore hemodynamics and to identify rapidly the type and the cause of shock in order to optimize therapeutic interventions.
Echocardiography is now proposed as the first-line evaluation modality1,2 to allow rapid characterization of the type of shock and to guide the management of patients in specific clinical settings for whom the situation may evolve over time. Furthermore, repeated echocardiography may be necessary to evaluate the response to therapeutics.
How to diagnose the mechanism of shock ?Cardiogenic shockCardiogenic shock (CS) is a critical syndrome of life-threatening peripheral hypoperfusion and organ dysfunction due to primary cardiac dysfunction and inadequate cardiac output (CO). Several etiologies may be responsible of the initial cardiac insult. Indeed, during several years, the main causes of CS were dominated by acute myocardial infarction (AMI). Thanks to early treatments of AMI, the prevalence of ischemic cardiogenic shock is decreasing. And so, other aetiologies of CS are increasing, like myocarditis, Takotsubo syndrome, post-partum cardiomyopathy, valvular pathologies or end-stage cardiomyopathies.3
Cardiac functionA common cause of shock is severe ventricular dysfunction. To rule it out, we can perform a basic echocardiography in which, with a quick view of the heart, we determine its contractile capacity.
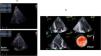
Left ventricular ejection fraction (LVEF) (Fig. 1A)Depending on the origin of CS and initial state of the cardiac function, TTE can be used for tracking LVEF evolution. Visual assessment by a simple eyeballing of LVEF is considered to be reliable in Cardiovascular Intensive Care Unit, when used by trained practitioners2 (Videos 1, 2).
Additionally, LVEF can be measured by the Simpson’s Biplane Formula,4 requiring area tracing of left ventricle (LV) cavity and contouring the endocardial border in both the apical four-chamber and two-chamber views in end-diastole and end-systole. LV is considered to have the shape of a cone. Area tracings of the LV cavity divide it into a number of discs (usually 20) and the total of volume of these discs is equal to LV volume. The difference between diastolic and systolic disc volumes divided by the diastolic volume gives LVEF value. In other words:
Other methods for LVEF measurement such as 3D echocardiography is more accurate but not usable in routine.
Other parameters of cardiac function- •
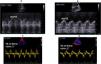
Mitral annular plane systolic excursion (MAPSE) (Fig. 2A and B):
It is measured by the use of M-mode echocardiography from four sites of the atrioventricular plane corresponding to the septal, lateral, anterior, and posterior walls using the apical four- and two-chamber views by M-mode echocardiography. The M-mode cursor should always be aligned parallel to the LV walls. The systolic excursion of mitral annulus should be measured from the lowest point at end-diastole to aortic valve closure (end of the T-wave on the electrocardiogram). MAPSE represents the amount of displacement of the mitral annular plane towards the apex and thus assesses the global change in size of the LV cavity (in the long-axis direction). The average normal value of MAPSE derived from previous studies for the four annular regions (septal, anterior, lateral, and posterior) ranged between 12 and 15 mm5,6 and a value of 8 mm was associated with a depressed LVEF (<50%) with a specificity of 82% and a sensitivity of 98%.5 In addition, a mean value for MAPSE of 7 mm could be used to detect an EF < 30% with a sensitivity of 92% and a specificity of 67% in dilated cardiomyopathy patients with severe congestive heart failure.6 It is of note that the association between MAPSE and EF is only valid in case of normal or dilated left ventricles7,8 while the correlation is rather poor in patients with LV hypertrophy.9
- •
Tissue Doppler peak systolic wave at the mitral lateral annulus (S′) (Fig. 2C and D):
Tissue Doppler imaging enables measurements of atrioventricular annular and regional myocardial velocities, and may be more sensitive than conventional echocardiography in detecting abnormalities of LV systolic and diastolic function.10 Two previous studies showed that there was a close correlation between systolic annular displacement directly measured by M-mode and that indirectly estimated by temporal integration of velocities measured by either pulsed tissue Doppler or colour Doppler in healthy subjects.11,12 Similar results were reported, showing a significant correlation between S′. and MAPSE both at rest and during exercise in heart failure patients with preserved LVEF13 S′ value >10 cm/s is correlated to preserved LVEF, 6–8 cm/s corresponds to altered LVEF between 30 and 45%, and S′ value <6 cm/s is associated with LVEF < 30%.14
Regional wall motionEven if systolic ventricular function estimated by LVEF is one of the strongest predictors of total and cardiovascular mortality, assessment of regional wall motion is part of visual echocardiographic examination. Wall motion is assessed in each 17 segments of the LV and LV segments can be akinetic, hypokinetic or dyskinetic, that may be due to a chronic or acute coronary disease.
More precise methods have been developed during the past decades for a better quantification of global and regional myocardial function, as the Strain, Strain Rate and Speckle Tracking (Fig. 1B). These methods can track the motion and the deformation of the myocardium during systole and diastole and point out regional wall motion abnormalities (RWMA) that are not visible on visual echocardiography. LV Global Longitudinal Strain alteration precedes the LVEF one and was demonstrated to be strongly correlated to mortality.4
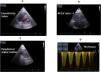
Cardiac output (CO)Measurement of CO remains a corner- stone in the hemodynamic assessment of critically ill patients and in particular in CS patients as decreased CO is often observed in such population. Several methods for determining CO have been described using both two-dimensional and Doppler echocardiography.15,16 Of these methods, the one using the left ventricular outflow tract (LVOT) and aortic valve as the conduit, is probably the most reliable and most commonly used as there is an excellent agreement with the reference CO measured by thermodilution in most situations 15 (Fig. 3B and C). The measurement of stroke volume (SV) is usually made at the LVOT. When using the TTE approach, the operator measures the LVOT diameter from the parasternal long-axis view immediately below the hinge point of the aortic valve leaflets (Fig. 3A). The LVOT area (cm2) is calculated from this diameter measurement using the formula:
Next, the operator places the pulsed wave Doppler (PWD) sample volume in the LVOT to measure the velocity time integral (VTI) of blood flow in the LVOT, using the five-chamber apical view. The SV is calculated as follows:
Obstructive shockThere are two main causes of obstructive shock:
Pericardial tamponadeLeads to right ventricle collapse and decrease of RV output and by consequence LV output. From a subcostal view, we can assess the presence of pericardial effusion, which compromises the functionality of the heart. In a basic analysis of shock, the existence of severe effusion (>2 cm), collapse of the cavities in their respective diastoles, dilation of the Inferior Vena Cava with absence of respiratory variations and in some situations, visualisation on the two-dimensional TTE of “Swinging heart” which is associated to a large pericardial effusion testifies often cardiac tamponade.17
Doppler assessment provides unique information regarding haemodynamic of pericardial tamponade. the following Doppler features are observed during inspiration: in the left heart, there will be a reduction in effective filling gradient (EFG) of the LV (pressure difference between pulmonary capillaries and left ventricle) due to a reduction in pulmonary capillary pressure while left atrium (LA) and LV diastolic pressures are relatively maintained due to reduced transmission of intrathoracic pressure into the heart. Therefore, LV filling will be reduced18 and consequently, the transmittal Doppler early diastolic (E)-wave and in turn LV outflow will be reduced. In the right heart, the opposite is observed; RV filling is increased with increased RV volume as the septum moves to the left (ventricular interdependence), increased tricuspid E-wave and increased RV outflow velocity.
In critically ill patients, however, mechanical ventilation, bronchospasm, significant pleural effusion, respiratory distress, and arrhythmias make the Doppler findings difficult to interpret.
Pulmonary embolismTTE can help to establish a prompt diagnosis of acute pulmonary embolism (PE) and to identify patients with high-risk features. Additionally, when the patient is hemodynamically unstable, TTE may be the only immediately available and appropriate imaging investigation. 19
Using a basic approach, the cause of the shock towards PE when we observe the evidence of hyperechogenic images in the right cavities, in this context, has a high specificity of PE. Additionally, signs of the consequences of acutely increased pulmonary artery/right heart pressures can be observed including dilatation of right heart chambers and more precisely the evolution of an initial abnormal ratio of RV diameter or area to LV diameter or area (Fig. 4, Video 3).
Indices of right ventricle dilation and/or pulmonary hypertension: parasternal short-axis view at the mid-ventricular level illustrating measurements of left ventricular diameters for calculation of the eccentricity index (A), and assessment of septal motion (C), right and left ventricle basal diameter ratio in apical 4-chamber view (B), peak velocity of tricuspid regurgitation (TR Vmax) obtained in apical 4-chamber view with continuous wave Doppler through tricuspid valve (D).
Tricuspid regurgitation is frequent in patients with intermediate-to-high-risk pulmonary embolism. It allows the estimation of RV systolic pressure and thus of pulmonary arterial systolic pressure (PAsP) in the absence of pulmonary valve stenosis. PAsP can be estimated from the peak velocity of the tricuspid regurgitation (TR) jet (V) according to the simplified Bernoulli equation but may underestimate it when tricuspid regurgitation is very severe (Fig. 4D).
In the absence of perceptible TR or inadequate alignment of PWD, Pulmonary Regurgitation (PR) from the parasternal short-axis view is usable to estimate pulmonary artery diastolic and mean pressures (PAdP and PAmP). The measurement of RVOT VTI can estimate RV output which is associated with increased pulmonary embolism-related mortality when it is low.19 Moreover, a decreased Pulmonary artery acceleration time and the presence of a “notch” on the RVOT VTI are valid signs of pulmonary hypertension (60/60 sign: right ventricular ejection acceleration time <60 ms with peak systolic gradient of tricuspid regurgitation <60 mmHg; a mid-systolic notch).20,21
Septic shockSepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection.22 The recognition of sepsis can be challenging. It requires an accurate history taking, physical examination and interpretation of laboratory data. Three pathophysiological mechanisms can be involved in septic shock: hypovolemia, vasoplegia, and cardiac dysfunction.
VasoplegiaA major pathological contribution to shock in sepsis is peripheral vasoplegia and although this is not measurable with echo, the cardiac findings can be taken into account when estimating it.
For example, in shock a hyperdynamic, after optimal fluid resuscitation, usually a clue to the presence of marked peripheral vasodilatation.
Furthermore, the absence of elevation in LV filling pressure has been reported as a specific characteristic of this hemodynamic profile, not only when evaluated by the E/e’ but also when measured in the past using a pulmonary artery catheter.23,24 It was suggested to be related to an increase in LV compliance due to sepsis.25
HypovolemiaIt is constant during sepsis and no need for a diagnostic tool at the early phase. Nevertheless, after the initial phase, optimization of fluid therapy using dynamic parameters is mandatory as suggested by the most recent recommendations.22 This will be detailed below.
Sepsis-related cardiomyopathySevere sepsis is frequently associated with cardiopulmonary dysfunction driven by a cascade of cellular and molecular processes.26 Myocardial dysfunction occurs frequently, early and involves both ventricles.26,27 Parker et al. were the first to describe LV hypokinesis in septic shock.23
They reported that survivors manifested severely depressed LVEF but that adequate LV stroke output was maintained as a result of acute LV dilation.28 LVEF might not be a reliable index of LV systolic function in patients with early septic shock, as this is a state characterized by low systemic vascular resistance that unloads the LV.24 Therefore, normal or supra normal EF in early sepsis might lead clinicians to make the wrong inference about cardiac reserve.
Speckle tracking echocardiography (STE) is a relatively novel and sensitive method for assessing ventricular function and may unmask myocardial dysfunction not appreciated with conventional echocardiography.29 STE may unmask systolic dysfunction not seen with conventional echocardiography. RV dysfunction unmasked by STE, especially when severe, was associated with high mortality in patients with severe sepsis or septic shock.
Hypovolemic shockUsing a basic ultrasound, we can suspect the presence of severe hypovolemia.
Some of the parameters assessed during basic echocardiography although not very sensitive, together with the medical history and clinical examination may raise suspicion of hypovolemia as a main cause of shock. The most frequently assessed by intensivists are: kissing walls of the LV which is a collapse of the walls of the LV during systole and the reduced left ventricular end-diastolic area (Video 4).
Although the administration of fluid is the first treatment this therapeutic option needs to be optimized later during the course of shock as it may pose two essential problems: the increase in cardiac output induced by a bolus of fluid after the initial phase is inconstant,30 and the deleterious effects of fluid overload are now clearly demonstrated.31 This is why many tests and indices have been developed to detect preload dependence and predict fluid responsiveness. This part will be developed later in the management part.
How to manage shock patients?Cardiogenic shockBy directly visualizing cardiac cavities and structures, TTE is an easy and reliable tool for the evaluation and the follow up of cardiac function, adjusting fluid balance, optimizing vasopressors and indicating monitoring or weaning of mechanical cardiac support (MCS) therapeutics.
Cardiac functionLVEF improvement after revascularization for acute myocardial infarction or inotropic support introduction for cardiogenic shock for instance should be followed up. However, this need to be interpreted within other hemodynamic parameters in case of shock for an overall assessment of tissue perfusion. Other parameters can be also reassessed after revascularisation as the MAPSE and the S′, in order to evaluate the efficacy of the revascularisation.
Additionally, and in the case of acute coronary disease, it is important to follow up regional wall motion abnormalities (RWMA) evolution after coronary revascularization. The quantification of global and regional myocardial function, as the Strain, Strain Rate and Speckle Tracking can be used also to follow the RWMA after revascularization.
Cardiac output (CO)Assessment of CO is important not only to identify the type of shock in particular cardiogenic shock together with other parameters, but also to evaluate the response to medical and surgical interventions, such as administration of inotropic agents for the treatment of right and left heart failure. Indeed, CO is SV multiplied by heart rate. The measurement of SV is usually made at the LVOT.
The VTI, SV or CO can be serially measured noninvasively before and after medical therapies in order to evaluate their effects, all the three variables are interchangeable each can be used as a sole parameter.
In addition, it is to be noticed that Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) is now taking part in cardiogenic shock resuscitation and treatment, its management may be guided by TTE.32
First, adequate venous canula position in right atrium can be assessed by TTE. Secondly, CO is often diminished after VA-ECMO implantation because of competitive cardiac and assistance flows this may be easily detected at the bedside by TTE. Furthermore, a direct visualisation by two dimensional TTE of the aortic valve may show that it remains closed indicating an urgent LV unloading. The indication of the maintenance of VA-ECMO, the need of further concomitant mechanical circulatory support devices or the detection of complications such as pericardial effusion or intracavitary/valves thrombosis need to be daily reassessed by repeating TTE.
Finally, TTE is now a corner stone of the VA-ECMO weaning protocol, by assessing LVEF, LVOT VTI, S′ and RV function under VA-ECMO and during weaning protocol.33
The main limitations of echocardiographic measurements of SV, CO, and VTI in the LVOT are that all of them, require accurate alignment with the LVOT, and consistent sampling that should occur just beneath the aortic valve. The use of an LVOT diameter adds a second potentially more significant error measurement. It is now recommended to use the stroke distance (i.e., LVOT and RVOT VTI) alone for serial measurements after therapeutic interventions, with the assumption that LVOT and RVOT diameters remain constant.
In clinical practice, only LVOT VTI is measured, considering LVOT to be constant and heart rate to be in stable range. The increase in LVOT VTI reflects CO improvement and myocardial contractile reserve.
In the absence of intracardiac shunt, LV output is equal to RV output. The latter can be estimated by measuring RVOT diameter from the parasternal short-axis view and RVOT VTI. Like LVOT VTI, RVOT VTI is obtained by placing PWD with a correct alignment in the RVOT. Inadequacy between LV output and RV output can be the sign of an atrial septal defect or a ventricular septal defect. In these cases, and without major pulmonary hypertension, left to right shunts lead to an increased RV output and a decreased LV output and by consequence haemodynamic instability.
It has to be noticed that RV output can be measured through the modified subcostal window, enhancing monitoring, especially in mechanically ventilated patients.34
LV filling pressuresAlthough invasive methods are considered as the “gold standard” for measuring intracardiac filling pressures, echocardiography is routinely used as a non-invasive alternative.35 This has been achieved using an algorithm based on LVEF status (altered or preserved), Doppler-derived parameters from mitral inflow velocities (E- and A-peak wave velocities, E/A ratio, E velocity DT) and tissue Doppler-derived mitral annular (e’-peak wave and E/e’ ratio).
It has to be underlined that LV filling pressures should not be considered a part of the clinical context, ventilation mode and other echocardiographic data such as LVEF.
Frequently, during cardiogenic shock, filling pressures are high, the improvement of cardiac function is often associated to their decrease.
Obstructive shockThere are two main causes of obstructive shock:
Pericardial tamponadeLeads to right ventricle collapse and decrease of RV output and by consequence LV output. Acute cardiac tamponade with hemodynamic compromise requires urgent pericardiocentesis or surgical removal of pericardial fluid.36
As pericardial tamponade treatment is mostly procedural rather than medical, echocardiography identifies the optimal site for pericardiocentesis by visualizing the location and distribution of pericardial effusion. The para-apical site is the most common entry site for pericardiocentesis and procedural rate is around 95%.17 Some may also recommend injecting agitated saline solution through the pericardiocentesis needle in the pericardial effusion to avoid the puncture of the ventricular cavity.37,38
Pulmonary embolismTTE can help to establish a prompt diagnosis of acute pulmonary embolism and to identify patients with high-risk features. Additionally, when the patient is hemodynamically unstable, TTE may be the only immediately available and appropriate imaging investigation.19,39
Indeed, echocardiography plays a determinant role in making therapeutic decisions in shock patients as it may help to rule out the diagnosis of severe pulmonary embolism in the absence of acute core pumonale. The main findings in acute pulmonary embolism are the consequences of acutely increased pulmonary artery/right heart pressures, these parameters simply assessed are used also to evaluate the efficacy of the treatment in particular thrombolysis.
Rapid decrease in PAsP reflects adequate dissolution of the thrombus. Finally, the improvement of the RV output and the RV function can be helpful to monitor the evolution of the thrombus.
Septic shockThree pathophysiological mechanisms can be involved in septic shock: vasoplegia, hypovolemia, and cardiac dysfunction. Obviously, optimal management of septic shock needs to be readjusted in function of the predominant dysfunction, this may be guided by echocardiography and fluid responsiveness indicators.
Preload responsivenessAlthough during septic shock, patients frequently present with hypovolemia, beyond the very initial phase, an increase in CO after fluid administration is observed in only 50% of the patients.40 Moreover, fluid overload is now widely admitted to be an independent predictor of mortality.41 In this regard, recent guidelines recommend the use of dynamic parameters for the assessment of fluid responsiveness rather than static parameters.22 Preload responsiveness can be assessed by measuring the response of VTI LVOT through dynamic tests as passive leg raising or to a combination of end-expiratory and end-inspiratory occlusions in patients under mechanical ventilation.42
Adequate TTE haemodynamic evaluation should be made regarding ventilation status (spontaneous breathing or mechanical ventilation) and cardiac rhythm (sinusal or not).
Increase in LVOT VTI of >12.5% during passive leg raising predicted the increases in SV in response to intravenous fluids with spontaneously breathing activity.43 In mechanically ventilated patients, recruitment manoeuvres can change cardiac loading conditions and decrease cardiac preload. For instance, the decrease in stroke volume during a recruitment manoeuvre predicted fluid bolus responsiveness in surgical patients during anaesthesia.44,45 Consecutive end-inspiratory occlusion and end-expiratory occlusion change VTI ≥ 13% in total predicted fluid responsiveness more accurately with less inter-observer variability.42
Among various indices, the assessment of respiratory variation of the diameter of the inferior vena cava (IVC min and IVC max) has received growing interest since it can be easily using two dimensional echocardiography in most critically-ill patients.46 However, there are some main concerns for the use of this parameter to predict preload responsiveness in critically ill patients.
Firstly, it has been demonstrated that neither the IVC diameter nor IVC variability accurately predict fluid responsiveness in spontaneously breathing in critically ill patients.47 Secondly, even in mechanically ventilated patients (one of the largest published series of ventilated patients) assessed using advanced critical care echocardiography for any type of acute circulatory failure. IVC variability had a low diagnostic accuracy to predict preload responsiveness with AUC of 0.608.48
Septic cardiomyopathyInitial assessment of MAPSE, speckle tracking and global longitudinal strain of LV and LVEF in 2D-mode may play a role in septic cardiomyopathy prognostication.29 Feng and al. reported in their analysis of MIMIC-III database that early use of TTE in septic shock had a significant benefit in terms of 28-day mortality, with more fluids, administered during the first day, greater use of dobutamine and a trend to be more quickly weaned from vasopressors.49
Furthermore, and as mentioned above during cardiogenic shock: LVOT VTI and LVEF may be used to evaluate the effectiveness of inotropes in case of cardiac dysfunction.
Hypovolemic shockHypovolemic shock is characterized by a reduction of intravascular volume and subsequent reduction in preload, so tracking the goals of resuscitation of a hypovolemic shock is likely the same as septic shock, about assessing fluid responsiveness with LVOT VTI and passive leg raising for instance and considering dynamic parameters instead of static ones.
ConclusionTTE provides the intensivists with valuable tools for assessment of circulatory failure particularly where the aetiology is undifferentiated or multifactorial. In one hand, it allows the diagnosis of the type of shock and its exact cause, in the other hand, it permits to track the effect of the initiated treatments.
It is a non‐invasive tool, easy to initiate and it can be applied at the bedside anytime during the day or night. An initial basic study can lead to the initiation of treatment, with a more advanced study subsequently providing incremental and vital additional information.
FundingNone.
Conflict of interestNone.