To analyze the use and impact of the intra-aortic balloon pump (IABP) upon the 30-day mortality rate and short-term clinical outcome of non-selected patients with ST-elevation acute myocardial infarction (acute STEMI) complicated by cardiogenic shock (CS).
DesignA single-center retrospective case–control study was carried out.
SettingCoronary Care Unit.
PatientsData were collected from 825 consecutive patients with acute STEMI admitted to a Coronary Care Unit from January 2009 to August 2015. Seventy-three patients with CS upon admission subjected to emergency percutaneous coronary intervention (PCI) were finally included in the analysis and were stratified according to IABP use (44 patients receiving IABP).
VariablesCardiovascular history, hemodynamic situation upon admission, angiographic and procedural characteristics, and variables derived from admission to the Coronary Care Unit.
ResultsCumulative 30-day mortality was similar in the patients subjected to IABP and in those who received conventional medical therapy only (29.5% and 27.6%, respectively; HR with IABP 1.10, 95% CI 0.38–3.11; p=0.85). Similarly, no significant differences were found in terms of the short-term clinical outcome between the groups: time on mechanical ventilation, days to hemodynamic stabilization, vasoactive drug requirements and stay in the Coronary Care Unit. Poorer renal function (HR 3.9, 95% CI 1.4–10.6; p=0.008), known peripheral artery disease (HR 3.3, 95% CI 1.2–9.1; p=0.019) and a history of diabetes mellitus (HR 3.2, 95% CI 1.2–8.1; p=0.018) were the only variables independently associated to increased 30-day mortality.
ConclusionIn our “real life” experience, IABP does not modify 30-day mortality or the short-term clinical outcome in patients presenting STEMI complicated with CS and subjected to emergency percutaneous coronary revascularization.
Analizar el uso e impacto del balón de contrapulsación intraaórtico (BCIA) en la mortalidad a 30 días y en los desenlaces clínicos a corto plazo de pacientes con infarto agudo de miocardio con elevación del segmento ST complicado con shock cardiogénico.
DiseñoEstudio de casos y controles unicéntrico y retrospectivo.
ÁmbitoUnidad Coronaria.
PacientesLos datos fueron obtenidos de 825 pacientes consecutivos admitidos en una unidad coronaria con diagnóstico de infarto agudo de miocardio con elevación del segmento ST desde enero de 2009 hasta agosto de 2015. Un total de 73 pacientes en situación de shock cardiogénico al ingreso derivados a una revascularización coronaria percutánea urgente fueron incluidos para el análisis y estratificados en función de la utilización del BCIA (44 pacientes recibieron BCIA).
VariablesAntecedentes cardiológicos, situación hemodinámica al ingreso, características angiográficas y periprocedimiento, y variables derivadas de la estancia en la Unidad Coronaria.
ResultadosLa mortalidad a 30 días fue similar entre los tratados con BCIA y aquellos con tratamiento convencional (29,5 y 27,6%, respectivamente; HR con BCIA 1,10, IC 95% 0,38-3,11; p=0,85). Así mismo, no encontramos diferencias significativas con respecto a los desenlaces clínicos a corto plazo: días en ventilación mecánica, tiempo hasta la estabilidad hemodinámica, requerimiento de fármacos vasoactivos y días de estancia en la Unidad Coronaria. En el análisis multivariante, las únicas variables asociadas de forma independiente con una mayor mortalidad a 30 días fueron peor función renal al ingreso (HR 3,9, IC 95% 1,4-10,6; p=0,008), antecedentes de enfermedad arterial periférica (HR 3,3, IC 95% 1,2-9,1; p=0,019) y diabetes mellitus (HR 3,2, IC 95% 1,2-8,1; p=0,018).
ConclusiónEn nuestra experiencia de la «vida real», la utilización del BCIA no modifica la mortalidad a 30 días ni los desenlaces clínicos a corto plazo en pacientes con infarto agudo de miocardio con elevación del segmento ST complicado con shock cardiogénico que son derivados a una estrategia de revascularización coronaria percutánea urgente.
Despite advances in primary angioplasty programs, coronary revascularization techniques and medical treatment, cardiogenic shock (CS) complicating acute myocardial infarction still occurs in the range from 5 to 15% and remains the leading cause of hospital mortality associated with ST-segment elevation myocardial infarction (STEMI).1–4 In this clinical setting, since 19685 intra-aortic balloon counterpulsation (IABP) has been the most widely used method for temporary mechanical circulatory support with implantation rates from 2007 to 2011 of 50,000 per year based on a national survey in the USA.6 Nevertheless, evidence supporting the benefit was based on registries with conflicting results.7
The most recent large randomized trial on the use of IABP in patients with myocardial infarction complicated with CS undergoing early revascularization (IABP-SHOCK II trial),8 showed neither benefit on 30-day mortality nor on any of the secondary endpoints. These results, in addition to the previous limited IABP evidence, led to downgrade the recommendation supporting routine use of IABP in CS in the setting of STEMI from Class IIb B from 2012 ESC guidelines9 to III A in 2014 myocardial revascularization guidelines.10
The aim of our study was to assess the impact of IABP in unselected patients presenting with STEMI complicated with CS undergoing percutaneous coronary intervention (PCI). We aimed to determine if IABP implantation could influence 30-day survival and short-term clinical outcomes during coronary care unit admission.
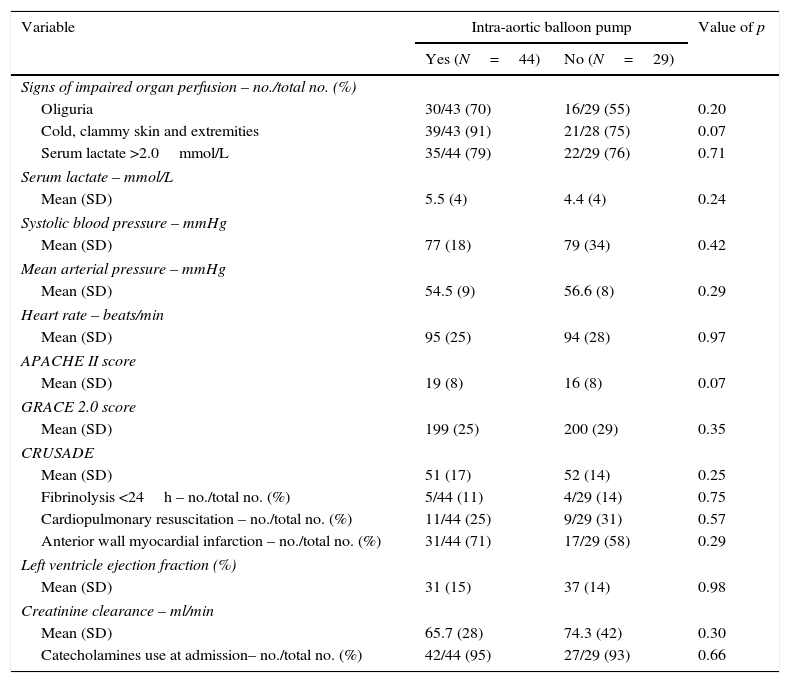
Patients and methodsPatient populationAll patients admitted to our tertiary referral hospital for an urgent PCI with diagnosis of STEMI complicated with CS from January 2009 to August 2015 were included. CS was defined as a persistent state of hypotension (systolic blood pressure <90mmHg for more than 30min or needed catecholamines to maintain a systolic blood pressure above 90mmHg) and impaired organ perfusion (lactate levels >2.0mmol/L, abnormal mental status, cold clammy skin, oliguria defined as urine output of less than 30ml/h).
Baseline characteristics including cardiovascular risk factors, cardiovascular history, hemodynamic situation and prognostic scores (APACHE II,11 GRACE12 and CRUSADE13) at admission, angiographic and procedural characteristics and variables derived from the coronary care unit admission were recorded from patient medical history.
Patients were divided into two groups based on treatment with IABP or not. IABP was inserted on the catheterization laboratory in all cases and the decision of insertion was left to the discretion of the operator. Due to the retrospective nature of the study, a multiple regression analysis was performed with IABP placement as dependent variable. As covariates, we included clinical and angiographic variables that could potentially influence operator decision as follows: requirement of more than one inotropic agent, time to reperfusion, infarct related artery, anterior wall location, left ventricle ejection fraction less than 35% (measured by Simpson's biplane method), APACHE II score >20.
All patients underwent early revascularization either by primary PCI or rescue PCI in addition to the best available medical treatment.
All patients were verbally informed (if their level of consciousness was suitable) and their consent was asked before the initiation of interventionist procedure. A signed written informed consent that included authorization to perform all the techniques required during the interventionist procedure was obtained from patient relatives because of the critical situation.
End pointsThe primary study endpoint was 30-day all-cause mortality. Secondary endpoints included hospital reinfarction, hospital stent thrombosis, major bleeding requiring transfusion, stroke in hospital and variables derived from coronary care admission: serum lactate levels and renal function at baseline and during the first 48h of follow-up (creatinine clearance was calculated using the Cockcroft–Gault-formula), time to hemodynamic stabilization (defined as mean arterial pressure ≥65mmHg without vasopressor drugs, serum lactate levels <2mmol/L and urine output >0.5ml/kg/h), length of time on mechanical ventilation and length of stay in the coronary care unit.
Statistical analysisDiscrete variables are presented as percentages and were compared using the X2 or Fisher's exact test as appropriate. Continuous variables are presented as mean±standard deviations and were compared using the Student's t test or Mann–Whitney U test as appropriate.
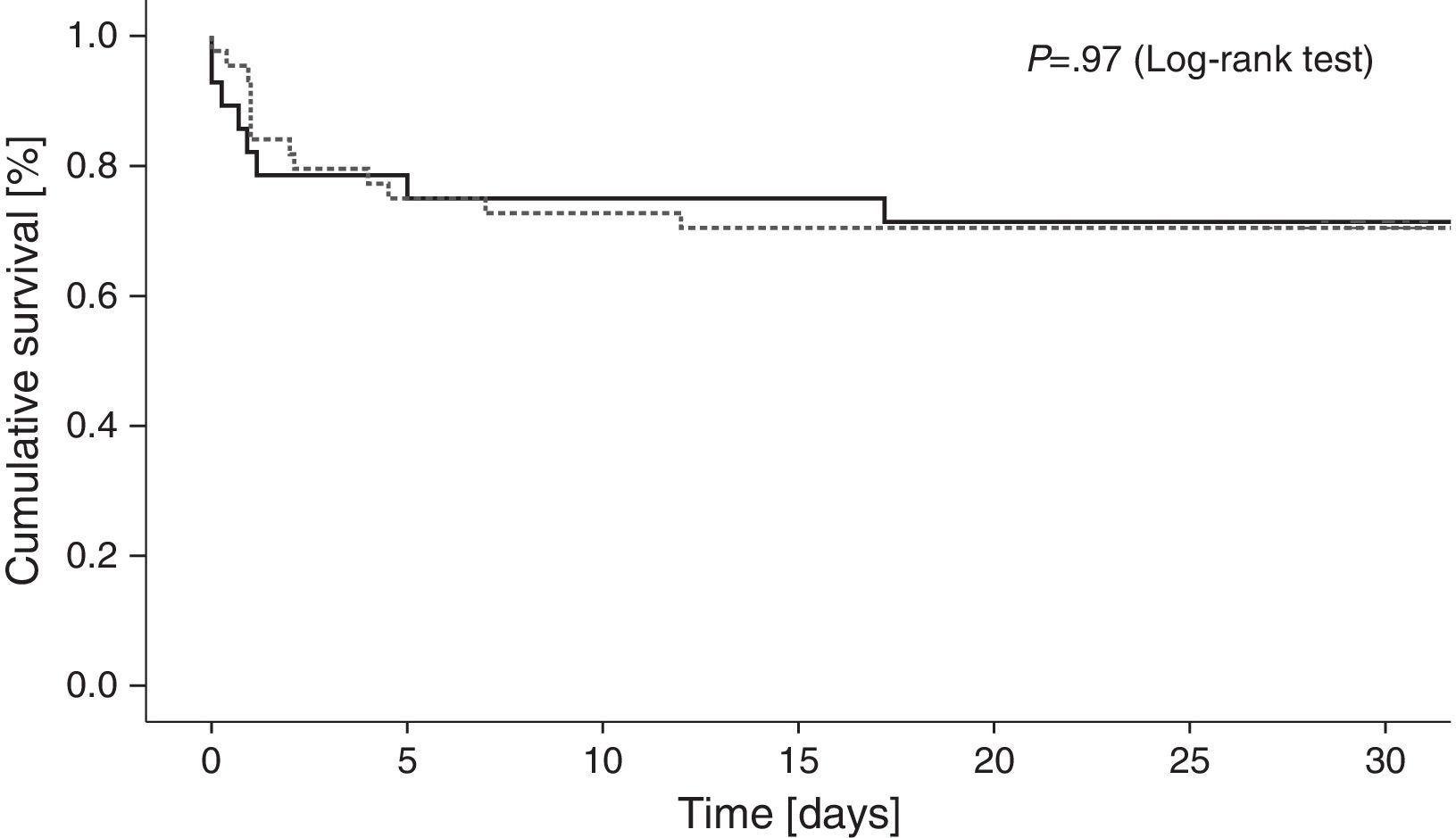
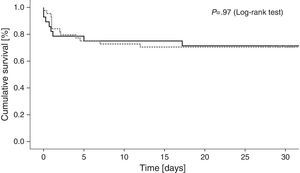
For the primary endpoint, the X2 test was used to compare mortality between the two groups. 30-day cumulative mortality was characterized with Kaplan–Meier curves. Log-rank test was used for the comparison between groups.
To evaluate the association between IABP use and 30-day mortality, Cox regression analysis was performed, with 30-day mortality as dependent variable. As covariates, we included IABP and variables that showed a p value <0.1 in the univariate analysis, as well as baseline and hospital presentation factors known to be associated with death.
All tests were 2-tailed, and a p value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc., Chicago, Illinois).
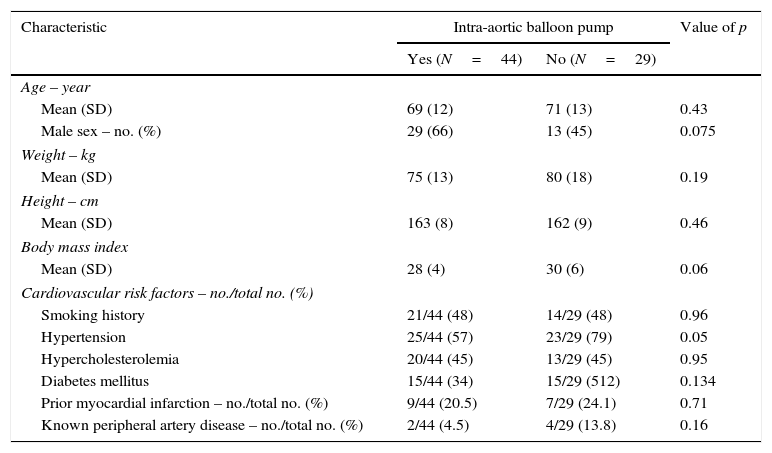
ResultsBetween January 2009 and August 2015, 825 patients diagnosed with STEMI were admitted. Of those, 73 (8.8%) were on CS and included for the analysis. 44 (60%) were treated with IABP in addition to medical therapy (IABP group) and 29 (40%) with optimal medical therapy only (non-IABP group). Table 1 shows baseline characteristics of the patients based on the insertion of an IABP or not. Both groups were comparable on cardiovascular risk factors, prognostic scores at admission (APACHE II, GRACE, CRUSADE), hemodynamic situation, cardiopulmonary resuscitation requirement or need of inotropic agents (Table 2).
Baseline characteristics of patients.
| Characteristic | Intra-aortic balloon pump | Value of p | |
|---|---|---|---|
| Yes (N=44) | No (N=29) | ||
| Age – year | |||
| Mean (SD) | 69 (12) | 71 (13) | 0.43 |
| Male sex – no. (%) | 29 (66) | 13 (45) | 0.075 |
| Weight – kg | |||
| Mean (SD) | 75 (13) | 80 (18) | 0.19 |
| Height – cm | |||
| Mean (SD) | 163 (8) | 162 (9) | 0.46 |
| Body mass index | |||
| Mean (SD) | 28 (4) | 30 (6) | 0.06 |
| Cardiovascular risk factors – no./total no. (%) | |||
| Smoking history | 21/44 (48) | 14/29 (48) | 0.96 |
| Hypertension | 25/44 (57) | 23/29 (79) | 0.05 |
| Hypercholesterolemia | 20/44 (45) | 13/29 (45) | 0.95 |
| Diabetes mellitus | 15/44 (34) | 15/29 (512) | 0.134 |
| Prior myocardial infarction – no./total no. (%) | 9/44 (20.5) | 7/29 (24.1) | 0.71 |
| Known peripheral artery disease – no./total no. (%) | 2/44 (4.5) | 4/29 (13.8) | 0.16 |
Clinical course at admission.
| Variable | Intra-aortic balloon pump | Value of p | |
|---|---|---|---|
| Yes (N=44) | No (N=29) | ||
| Signs of impaired organ perfusion – no./total no. (%) | |||
| Oliguria | 30/43 (70) | 16/29 (55) | 0.20 |
| Cold, clammy skin and extremities | 39/43 (91) | 21/28 (75) | 0.07 |
| Serum lactate >2.0mmol/L | 35/44 (79) | 22/29 (76) | 0.71 |
| Serum lactate – mmol/L | |||
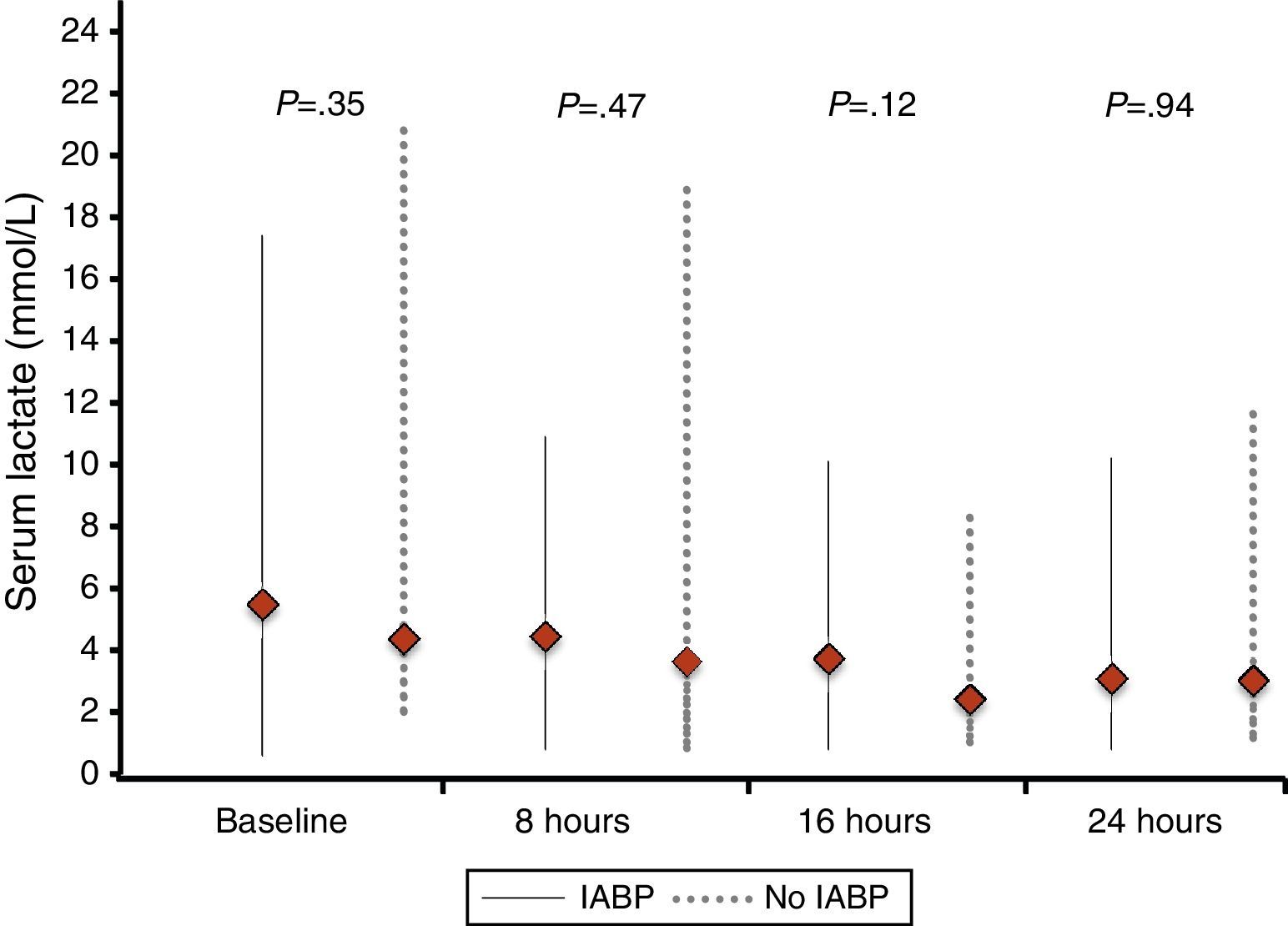
| Mean (SD) | 5.5 (4) | 4.4 (4) | 0.24 |
| Systolic blood pressure – mmHg | |||
| Mean (SD) | 77 (18) | 79 (34) | 0.42 |
| Mean arterial pressure – mmHg | |||
| Mean (SD) | 54.5 (9) | 56.6 (8) | 0.29 |
| Heart rate – beats/min | |||
| Mean (SD) | 95 (25) | 94 (28) | 0.97 |
| APACHE II score | |||
| Mean (SD) | 19 (8) | 16 (8) | 0.07 |
| GRACE 2.0 score | |||
| Mean (SD) | 199 (25) | 200 (29) | 0.35 |
| CRUSADE | |||
| Mean (SD) | 51 (17) | 52 (14) | 0.25 |
| Fibrinolysis <24h – no./total no. (%) | 5/44 (11) | 4/29 (14) | 0.75 |
| Cardiopulmonary resuscitation – no./total no. (%) | 11/44 (25) | 9/29 (31) | 0.57 |
| Anterior wall myocardial infarction – no./total no. (%) | 31/44 (71) | 17/29 (58) | 0.29 |
| Left ventricle ejection fraction (%) | |||
| Mean (SD) | 31 (15) | 37 (14) | 0.98 |
| Creatinine clearance – ml/min | |||
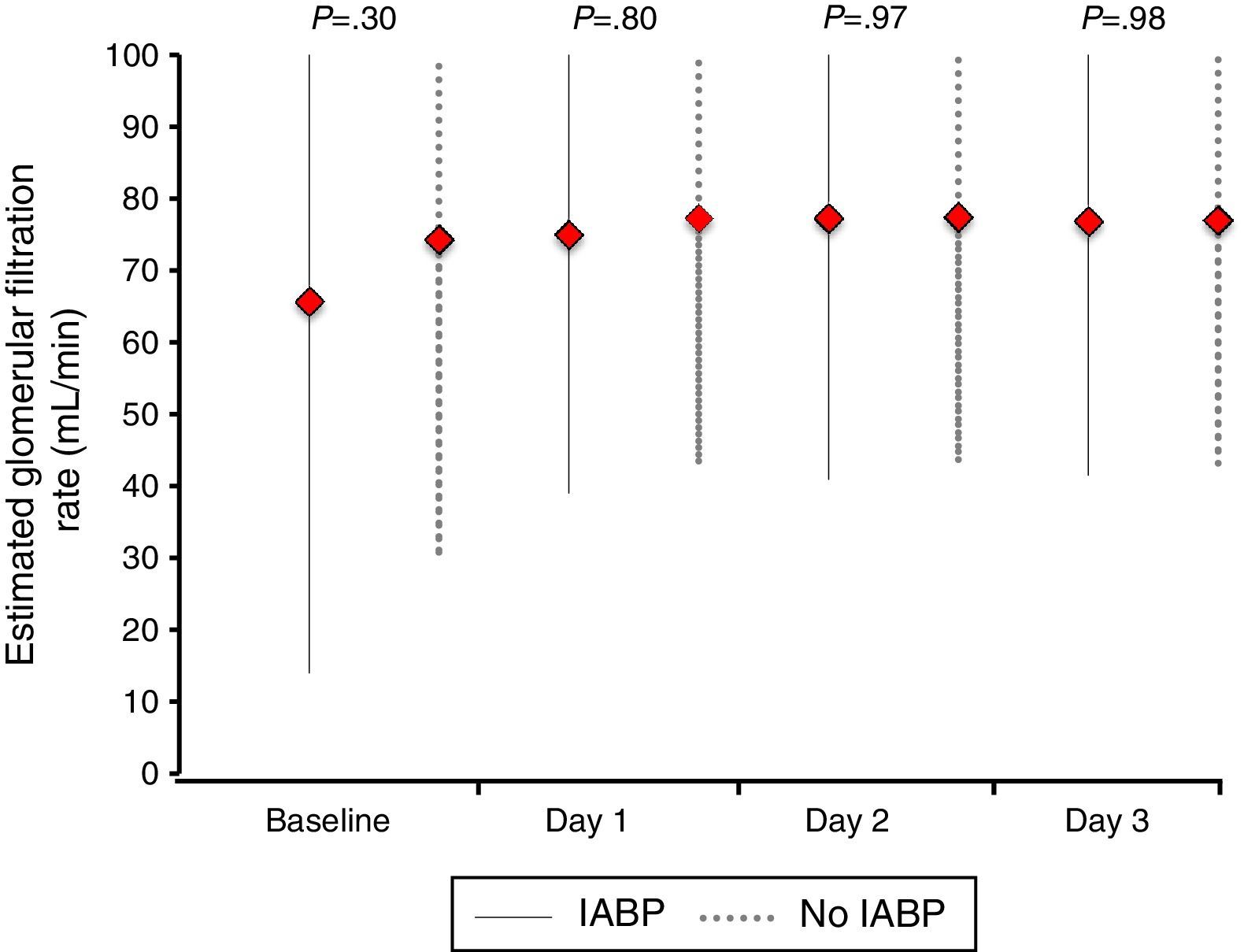
| Mean (SD) | 65.7 (28) | 74.3 (42) | 0.30 |
| Catecholamines use at admission– no./total no. (%) | 42/44 (95) | 27/29 (93) | 0.66 |
APACHE II score: Acute Physiology and Chronic Health Evaluation score, GRACE 2.0: Global Registry of Acute Coronary Events score. Creatinine clearance was calculated using the Cockcroft–Gault-formula.
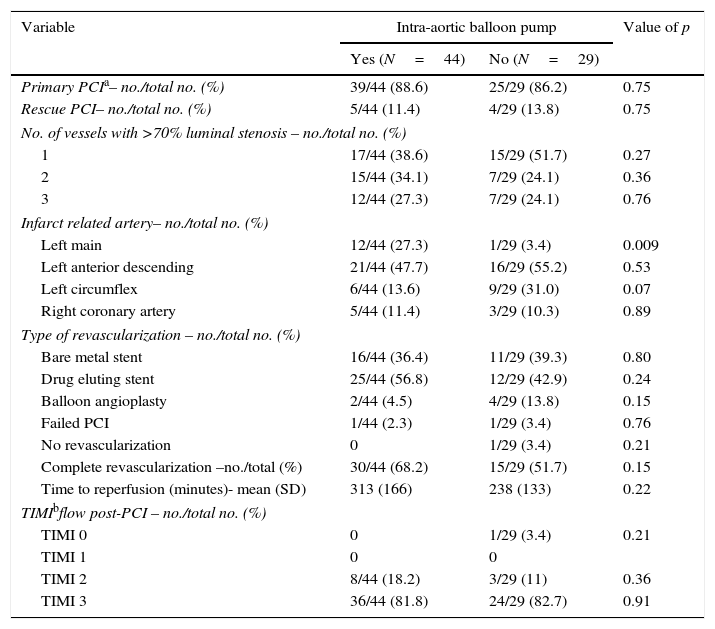
Primary PCI was the most frequent revascularization strategy (88.6% in IABP group vs. 86.2% in non-IABP group, p=0.75) with left anterior descending artery as the culprit lesion in most cases (47.7% IABP group vs. 55.2% non-IABP group, p=0.53). Of notice, 13 patients (18%) with left main disease as infarct related artery, 12 of them were treated with IABP (p=0.009). The rate of stent implantation and stent type (drug-eluting stent or bare-metal stent) was also comparable between the two groups (Table 3).
Invasive treatment details.
| Variable | Intra-aortic balloon pump | Value of p | |
|---|---|---|---|
| Yes (N=44) | No (N=29) | ||
| Primary PCIa– no./total no. (%) | 39/44 (88.6) | 25/29 (86.2) | 0.75 |
| Rescue PCI– no./total no. (%) | 5/44 (11.4) | 4/29 (13.8) | 0.75 |
| No. of vessels with >70% luminal stenosis – no./total no. (%) | |||
| 1 | 17/44 (38.6) | 15/29 (51.7) | 0.27 |
| 2 | 15/44 (34.1) | 7/29 (24.1) | 0.36 |
| 3 | 12/44 (27.3) | 7/29 (24.1) | 0.76 |
| Infarct related artery– no./total no. (%) | |||
| Left main | 12/44 (27.3) | 1/29 (3.4) | 0.009 |
| Left anterior descending | 21/44 (47.7) | 16/29 (55.2) | 0.53 |
| Left circumflex | 6/44 (13.6) | 9/29 (31.0) | 0.07 |
| Right coronary artery | 5/44 (11.4) | 3/29 (10.3) | 0.89 |
| Type of revascularization – no./total no. (%) | |||
| Bare metal stent | 16/44 (36.4) | 11/29 (39.3) | 0.80 |
| Drug eluting stent | 25/44 (56.8) | 12/29 (42.9) | 0.24 |
| Balloon angioplasty | 2/44 (4.5) | 4/29 (13.8) | 0.15 |
| Failed PCI | 1/44 (2.3) | 1/29 (3.4) | 0.76 |
| No revascularization | 0 | 1/29 (3.4) | 0.21 |
| Complete revascularization –no./total (%) | 30/44 (68.2) | 15/29 (51.7) | 0.15 |
| Time to reperfusion (minutes)- mean (SD) | 313 (166) | 238 (133) | 0.22 |
| TIMIbflow post-PCI – no./total no. (%) | |||
| TIMI 0 | 0 | 1/29 (3.4) | 0.21 |
| TIMI 1 | 0 | 0 | |
| TIMI 2 | 8/44 (18.2) | 3/29 (11) | 0.36 |
| TIMI 3 | 36/44 (81.8) | 24/29 (82.7) | 0.91 |
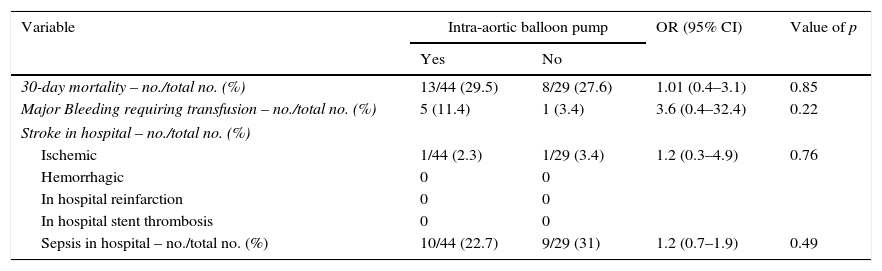
Cumulative 30-day mortality was similar among patients treated with IABP and those treated with conventional medical therapy only (29.5% and 27.6%, respectively; odds ratio [OR] with IABP 1.10, 95% confidence interval [CI], 0.38–3.11; p=0.85) [Table 4]. Fig. 1 shows Kaplan–Meier survival curves according to IABP use. Table 4 shows clinical outcomes of patients with and without IABP: no reinfarction or acute stent thrombosis were detected during 30-day follow-up and no differences between groups were found regarding the rate of in-hospital stroke or sepsis. There was a trend of higher bleeding events in the IABP group (11.4% and 3.4%, respectively; OR with IABP 3.6, 95% CI 0.4–32.4; p=0.22), however without reach statistical significance.
Clinical outcomes of patients with and without intra-aortic balloon pump.
| Variable | Intra-aortic balloon pump | OR (95% CI) | Value of p | |
|---|---|---|---|---|
| Yes | No | |||
| 30-day mortality – no./total no. (%) | 13/44 (29.5) | 8/29 (27.6) | 1.01 (0.4–3.1) | 0.85 |
| Major Bleeding requiring transfusion – no./total no. (%) | 5 (11.4) | 1 (3.4) | 3.6 (0.4–32.4) | 0.22 |
| Stroke in hospital – no./total no. (%) | ||||
| Ischemic | 1/44 (2.3) | 1/29 (3.4) | 1.2 (0.3–4.9) | 0.76 |
| Hemorrhagic | 0 | 0 | ||
| In hospital reinfarction | 0 | 0 | ||
| In hospital stent thrombosis | 0 | 0 | ||
| Sepsis in hospital – no./total no. (%) | 10/44 (22.7) | 9/29 (31) | 1.2 (0.7–1.9) | 0.49 |
OR: odds ratio; CI: confidence interval.
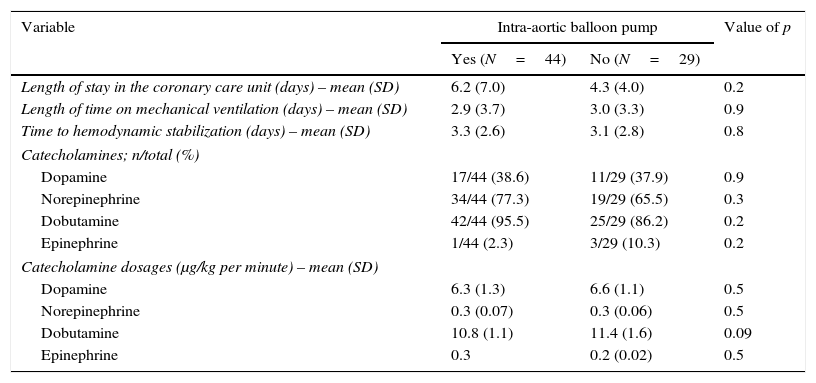
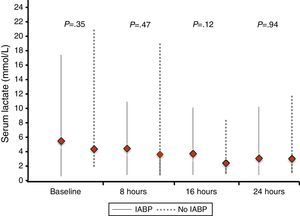
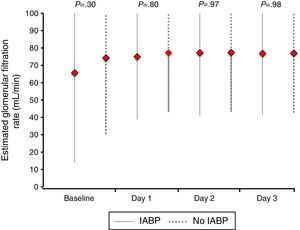
Similarly, no differences were found regarding data related to intensive care stay: length of time on mechanical ventilation (53% of patients received invasive mechanical ventilation), days to hemodynamic stabilization, vasoactive drug requirement and length of stay in the coronary care unit were similar in both groups (Table 5), as well as serum lactate levels (Fig. 2) and renal function at baseline and during 72h follow-up (Fig. 3).
Coronary care clinical outcomes.
| Variable | Intra-aortic balloon pump | Value of p | |
|---|---|---|---|
| Yes (N=44) | No (N=29) | ||
| Length of stay in the coronary care unit (days) – mean (SD) | 6.2 (7.0) | 4.3 (4.0) | 0.2 |
| Length of time on mechanical ventilation (days) – mean (SD) | 2.9 (3.7) | 3.0 (3.3) | 0.9 |
| Time to hemodynamic stabilization (days) – mean (SD) | 3.3 (2.6) | 3.1 (2.8) | 0.8 |
| Catecholamines; n/total (%) | |||
| Dopamine | 17/44 (38.6) | 11/29 (37.9) | 0.9 |
| Norepinephrine | 34/44 (77.3) | 19/29 (65.5) | 0.3 |
| Dobutamine | 42/44 (95.5) | 25/29 (86.2) | 0.2 |
| Epinephrine | 1/44 (2.3) | 3/29 (10.3) | 0.2 |
| Catecholamine dosages (μg/kg per minute) – mean (SD) | |||
| Dopamine | 6.3 (1.3) | 6.6 (1.1) | 0.5 |
| Norepinephrine | 0.3 (0.07) | 0.3 (0.06) | 0.5 |
| Dobutamine | 10.8 (1.1) | 11.4 (1.6) | 0.09 |
| Epinephrine | 0.3 | 0.2 (0.02) | 0.5 |
IABP indication was left to the discretion of the operator as mentioned on methods. Results from multiple regression analysis showed that left main disease as infarct related artery was the only variable independently associated with IABP placement (OR 11.07 95% CI 1.3–94.1; p=0.02).
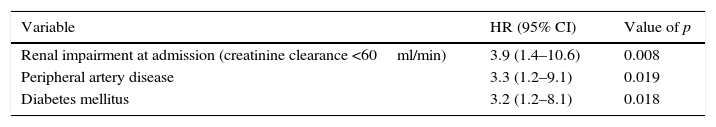
To assess the association between 30-day mortality and IABP, Cox regression analysis was performed. To construct the model we included as covariates the following variables: IABP use, age, diabetes mellitus, hypertension, known peripheral artery disease, serum lactate levels >2mmol/L, significant renal insufficiency (creatinine clearance <60ml/min) at admission, time to reperfusion, anterior wall STEMI, left ventricle ejection fraction below 35%, cardiopulmonary resuscitation and left main disease as culprit lesion. Results from the multivariate regression analyses are shown in Table 6. Worse renal function (creatinine clearance <60ml/min at admission) [HR 3.9, 95% CI 1.4–10.6; p=0.008), known peripheral artery disease (HR 3.3 95% CI 1.2–9.1; p=0.019) and previous history diabetes mellitus (Hazard ratio [HR] 3.2, 95% CI 1.2–8.1; p=0.018), were independently associated with higher 30-day mortality. IABP was not an independent predictor of 30-day mortality.
Multivariate regression analysis for 30-day mortality.
| Variable | HR (95% CI) | Value of p |
|---|---|---|
| Renal impairment at admission (creatinine clearance <60ml/min) | 3.9 (1.4–10.6) | 0.008 |
| Peripheral artery disease | 3.3 (1.2–9.1) | 0.019 |
| Diabetes mellitus | 3.2 (1.2–8.1) | 0.018 |
HR: hazard ratio; CI: confidence interval.
Creatinine clearance was calculated using the Cockcroft–Gault-formula.
The main finding of our real life study is that among patients with STEMI complicated with CS that received an early coronary revascularization technique (primary/rescue PCI), the use of IABP had no impact on short-term survival. Similarly, no benefit was found on short-term clinical outcomes regarding IABP use or not. Our results are consistent with the current evidence that do not recommend the routine use of IABP therapy in CS patients.
In our registry, IABP was used in up to 60% of patients with STEMI complicated with CS at admission, which implies an implantation rate significantly higher than previously reported. Recent evidence shows a significant decrease in the use of IABP in Europe. Thereby, results from the Euro Heart Survey on PCI that included 33 European countries reported an IABP use of 25%.14 Similar data were found in ALKK-PCI registry with an overall use of 25.5%.15 This significant reduction is probably a consequence of the results of previous registries16 and the meta-analysis of Sjauw et al.7 that showed no mortality benefit of IABP in patients treated with primary PCI. The largest randomized trial to date evaluating IABP use on STEMI patients complicated with CS,8 reaffirms these findings.
Our results are consistent to those previously reported.7,8,17–19 Thrombolysis within 24h of admission was performed in 12.3% of our population and when these patients are transferred to rescue PCI, the impact of IABP on 30-day mortality was similar to those who received primary PCI. In this respect, a previous meta-analysis showed benefits in terms of mortality in the subgroup of patients that received thrombolysis as revascularization strategy and treated with IABP.7 This beneficial effect on patients with CS and a previous pharmacological revascularization strategy is probably related to the diastolic augmentation with the subsequent improvement in coronary perfusion, issue of critical importance for a thrombolytic agent to effectively dissolve an occlusive coronary thrombus.20 This fact probably explains why in rescue PCI patients (revascularization modality less dependent on coronary perfusion pressure that thrombolytic therapy) the synergistic effect is lost.
Several multivariable prognostic models have been developed to predict clinical outcomes in CS patients. Classical factors associated with higher mortality from the major CS trials (SHOCK, TRIUMPH, IABP-SHOCK II)8,21,22 included older age, lower left-ventricle ejection fraction, worse renal function, signs of impaired organ perfusion, need for vasopressor support, cardiac arrest, anterior infarction during presentation among others. Similarly to previously reported, our results from multivariable modeling showed that worse renal function and known peripheral artery disease were independently associated with 30-day all cause mortality.
Previous history of diabetes mellitus was independently associated with 30-day mortality. This finding is in contrast to SHOCK trial registry23 in which after adjustment, diabetics have an in-hospital survival rate that is marginally lower than that of non-diabetics (odds ratio for death, 1.36; 95% CI 1.00–1.84; p=0.051). However, in a more recent study24 evaluating the importance of preexisting diabetes on the incidence and prognosis of CS, no difference on 30-day and 5-year mortality were found (diabetics: 30-day 63%, 5-year 91%; non diabetics: 30-day 62%, 5-year 86%; p>0.05).
Anterior wall STEMI is the most common cause of impaired LVEF in the setting of an STEMI.25 However, in the subgroup analysis from IABP-SHOCK II trial,8 patients with an anterior STEMI had similar 30-day mortality to those with non-anterior STEMI type (RR 0.81, 95% CI 0.58–1.13 vs. 1.16, 95% CI 0.85–1.57, respectively; p value for interaction 0.14). We included anterior STEMI type as a covariate in the 30-day mortality multivariate regression analysis and no differences were found. In this regard, a recent study evaluated the impact of IABP on in-hospital mortality in patients presenting with acute anterior-STEMI without cardiogenic shock26 and found similar in-hospital rate of cardiac death between the two cohorts (5.6% vs. 0%; p=0.12).
IABP improves myocardial perfusion, reduces myocardial oxygen consumption and furthermore decreases afterload and improves cardiac index,27 which are the principal variables that most be corrected in cardiogenic shock patients.25 We evaluated the impact of IABP use on the clinical course during intensive care stay and no clinical benefit in terms of hypoperfusion biomarkers (serum lactate levels), length of time on mechanical ventilation, days to hemodynamic stabilization, and length of stay in the coronary care unit were found. Our results are similar to those reported on the IABP-SHOCK II trial in which no significant differences between groups regarding process-of-care outcomes were reported.8
Our study has important limitations. First, although patients were consecutively selected, the information and analysis is retrospective and as such has many limitations including nonrandom allocation of IABP. In this setting, we remark IABP insertion in 12 of 13 patients with left main disease as culprit lesion and a trend of its placement in sickest population estimated by APACHE II score. Second, the exact moment of IABP insertion (before, during, or after PCI) was not registered so we were unable to analyze the impact of IABP timing on the clinical course of our study population, although all IABP were implanted on the catheterization laboratory. Third, the sample size is reduce and limited to a single center and as consequence our conclusions may not apply to other patient population.
In our “real life” experience, IABP use does not modify 30-day mortality neither short-term clinical outcome in STEMI patients complicated with CS undergoing an urgent percutaneous coronary revascularization. Worse renal function, known peripheral artery disease and previous history of diabetes mellitus were independent predictors of 30-day mortality.
Conflict of interestThe authors declare no conflicts of interest.