To assess the correlation between left ventricular outflow tract velocity time integral (LVOT VTI) and stroke volume index (SVI) calculated by thermodilution methods in ventilated critically ill patients.
DesignA prospective, descriptive, multicenter study was performed.
SettingFive intensive care units from university hospitals.
PatientsPatients older than 17 years needing mechanical ventilation and invasive hemodynamic monitoring were included.
InterventionsLVOT VTI was measured by pulsatile Doppler echocardiography. Calculations of SVI were performed through a floating pulmonary artery catheter (PAC) or a Pulse index Contour Cardiac Output (PiCCO®) thermodilution methods.
Main variablesThe relation between LVOT VTI and SVI was tested by linear regression analysis.
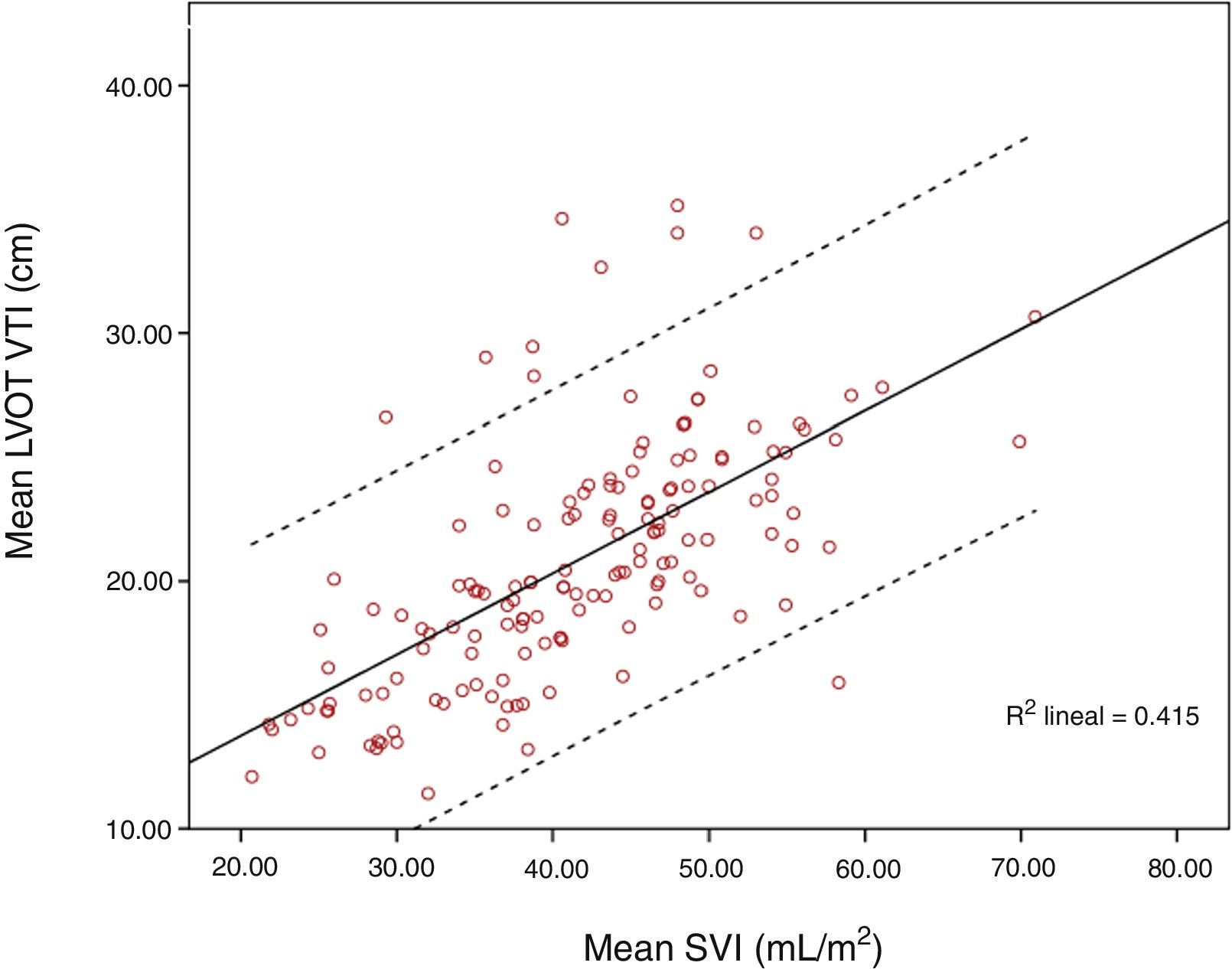
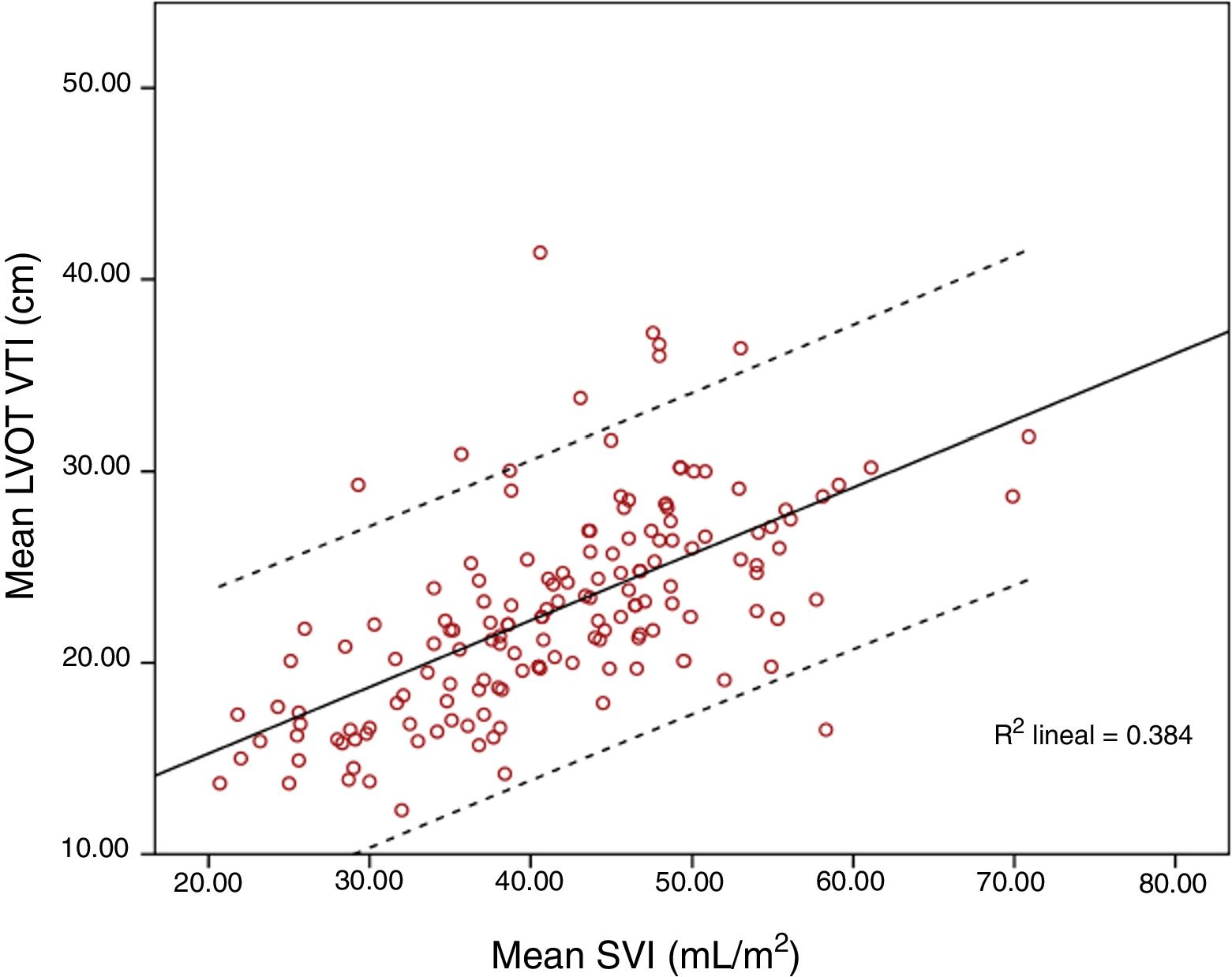
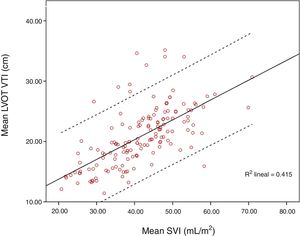
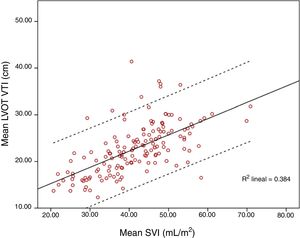
ResultsOne hundred and fifty-six paired measurements were compared. Mean LVOT VTI was 20.83±4.86cm and mean SVI was 41.55±9.55mL/m2. Pearson correlation index for these variables was r=0.644, p<0.001; ICC was 0.52 (CI 95% 0.4–0.63). When maximum LVOT VTI was correlated with SVI, Pearson correlation index was r=0.62, p<0.001. Correlation worsened for extreme values, especially for those with higher LVOT VTI.
ConclusionsLVOT VTI could be a complementary hemodynamic evaluation in selected patients, but does not eliminate the need for invasive monitoring at the present time. The weak correlation between LVOT VTI and invasive monitoring deserves additional assessment to identify the factors affecting this disagreement.
Evaluar la correlación entre la integral velocidad tiempo del tracto de salida del ventrículo izquierdo (IVT TSVI) y el índice volumen sistólico (IVS) calculado por métodos de termodilución en pacientes ventilados críticamente enfermos.
DiseñoSe realizó un estudio prospectivo, descriptivo y multicéntrico.
ÁmbitoCinco unidades de cuidados intensivos de hospitales universitarios.
PacientesSe incluyeron pacientes mayores de 17 años que necesitaron ventilación mecánica y monitorización hemodinámica invasiva.
IntervencionesLa IVT TSVI se midió mediante Doppler pulsátil. Los cálculos de SVI se realizaron a través de un catéter de arteria pulmonar (CAP) o un método de Pulse index Contour Cardiac Output (PiCCO®), con métodos de termodilución.
Variables principalesLa relación entre IVT TSVI e IVS se evaluó mediante análisis de regresión lineal.
ResultadosSe compararon 156 mediciones pareadas. La IVT TSVI media fue de 20,83±4,86cm y la media de IVS fue de 41,55±9,55ml/m2. El índice de correlación de Pearson para estas variables fue r=0,644, p<0,001; ICC fue 0,52 (IC 95%: 0,4-0,63). Cuando la IVT TSVI máxima se correlacionó con el IVS, el índice de correlación de Pearson fue r=0,62, p<0,001. La correlación empeoró para los valores extremos, especialmente para aquellos con mayor IVT TSVI.
ConclusionesLa IVT TSVI podría ser una evaluación hemodinámica complementaria en pacientes seleccionados, pero no elimina la necesidad de un control invasivo en la actualidad. La débil correlación entre la IVT TSVI y la monitorización invasiva requiere estudios adicionales para identificar los factores que afectan a este desacuerdo.
Since the introduction of ultrasound into the routine monitoring of critical patients the interest in achieving a reliable and accurate evaluation of cardiac function and hemodynamic values by echocardiography has been a subject of concern. Transthoracic cardiac ultrasound has the advantage of being available at bedside even outside of the intensive care unit (ICU), as a quick examination method avoiding discomfort to the patient and the complications associated with invasive monitoring.
Several echocardiographic methods and measurements are available for hemodynamic monitoring. In spite of the previously encouraging publications and recommendations, especially in the field of perioperative medicine, most of these measurements are experience dependent and difficult to obtain in mechanically ventilated patients.1–3
Data on the feasibility and accuracy of ultrasound application for hemodynamic monitoring in critically ill ventilated patients are limited. Echocardiography has several limitations primarily related to difficulties in obtaining reliable acoustic windows in the critical care settings.
Left ventricular outflow tract velocity time integral (LVOT VTI) can be measured in most critical care patients and has shown better reproducibility to assess left ventricular systolic function in mechanically ventilated and hemodynamically unstable patients, when compared with the Simpson method, the estimated “eyeball” ejection fraction, mean atrioventricular plane displacement and with the septal tissue velocity imaging methods.4 The lack of correlation between LVOT VTI and other invasive hemodynamic measurements would make obtained data inaccurate.
Left ventricular outflow tract (LVOT) could be considered as having fixed dimensions through the cardiac cycle and to be independent on preload state. Calculation of cardiac output requiring LVOT diameter measurement is strongly dependent on skills and observers.
Previous studies have demonstrated that LVOT mean velocity determined by pulsed wave Doppler allows cardiac index quantification through a simple formula.5 Hence, the requirement of calculate LVOT diameter to estimate cardiac output could be obviated. The aim of our study is to evaluate the correlation between LVOT VTI and stroke volume index (SVI), as a way to preclude the need for LVOT diameter size measurement.
Patients and methodsThe primary end-point of the study was to assess the correlation between LVOT VTI and SVI calculated by thermodilution methods. For this purpose, a prospective, descriptive study was performed. Five medical-surgical ICUs from different university hospitals participated in the study.
Selection of participantsFrom November 2015 to February 2016, consecutive patients older than 17 years admitted to the ICU needing mechanical ventilation and invasive hemodynamic monitoring were included in the study. Patients with aortic valve regurgitation, dynamic obstruction of the LVOT or with intra-aortic balloon pump insertion were excluded from the analysis.
Interventions and measurementsAnthropometric data were obtained from medical report or from relatives. In some cases, weight and size were measured in the ICU.
LVOT VTI was measured by pulsed wave Doppler transthoracic echocardiography from the apical 5 chamber view as usually defined and recommended.1 In every examination, performed by a single intensivist, five measurements of LVOT VTI were obtained and the mean and maximum values were recorded. The operator made a strong effort to align the probe with the LVOT flow. Operators were senior intensivists with experience in performing daily echocardiographic studies in critically ill patients.
Simultaneously, five calculations of SVI through a floating pulmonary artery catheter (PAC) or a Pulse index Contour Cardiac Output (PiCCO®, Pulsion Medical Systems, Germany) thermodilution methods (using cold saline) were performed by experienced staff nurses and the mean value was recorded. PAC or PiCCO® system were chosen according to the staff physician criteria, prior to patient inclusion in the study. Calculation of PAC and PiCCO® thermodilution values was performed using the software incorporated in the bedside monitors (IntelliVue MP60, Philips, Eindhoven, The Netherlands). Calibration of the system was carried out every eight hours.
Those thermodilution curves showing irregularities or lacking a clear early peak were considered inadequate and therefore rejected. If the difference between the lowest and highest values of the five measurements was >10%, additional cardiac output measurements were performed, and extreme values discarded. Physician performing the Doppler study was unaware of the results of the thermodilution study results.
Simultaneous results of LVOT VTI (mean and maximum) and SVI (mean) were paired for statistical analysis.
Measurements were recorded under stable hemodynamic and cardiac rhythm conditions, as often as needed by the responsible intensivist, but at least eight hours apart.
Statistical analysisQuantitative variables are expressed as mean±standard deviation (SD). Mean and maximum LVOT VTI were correlated with mean SVI obtained by PAC or by PiCCO®. Correlation was assessed by the Pearson correlation index and concordance by the intraclass correlation coefficient (ICC).
The relation between continuous variables was tested by linear regression analysis. The Student's t test was used to test the systematic differences among methods. A p value <0.05 was considered statistically significant.
IBM SPSS Statistics for Windows, Version 20.0. software (IBM Corp., Armonk, United States) provided statistical analysis.
The study protocol was approved by the institutional Investigational Committee from the principal investigator hospital. This committee ascertained that the study protocol was in accordance with the ethical standards collected in the Declaration of Helsinki, its amendments and that the national laws were observed.
Informed consent for the investigational use of the clinical data was obtained from patients when possible or, otherwise, from their relatives.
ResultsFifty patients were eligible for the study. One patient was excluded from the analysis due to the lack of a reliable acoustic window. Two additional patients were excluded because an intra-aortic balloon pump was inserted during the study period. Finally, 156 paired measurements were compared: 97 (62.2%) recorded with PiCCO® and 59 (37.8%) with PAC. In 19 evaluated patients PAC was inserted and in 28 the PiCCO® system was chosen for hemodynamic monitoring. As a mean, 3.3 paired measurements were registered in every patient (range 1–13). Thirty-nine paired measurements (25%) were recorded in atrial fibrillation.
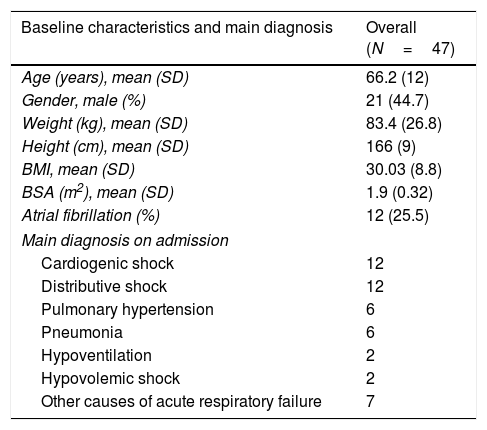
Table 1 shows the baseline characteristics of the remaining 47 patients.
Baseline characteristics of the overall population.
| Baseline characteristics and main diagnosis | Overall (N=47) |
|---|---|
| Age (years), mean (SD) | 66.2 (12) |
| Gender, male (%) | 21 (44.7) |
| Weight (kg), mean (SD) | 83.4 (26.8) |
| Height (cm), mean (SD) | 166 (9) |
| BMI, mean (SD) | 30.03 (8.8) |
| BSA (m2), mean (SD) | 1.9 (0.32) |
| Atrial fibrillation (%) | 12 (25.5) |
| Main diagnosis on admission | |
| Cardiogenic shock | 12 |
| Distributive shock | 12 |
| Pulmonary hypertension | 6 |
| Pneumonia | 6 |
| Hypoventilation | 2 |
| Hypovolemic shock | 2 |
| Other causes of acute respiratory failure | 7 |
Mean LVOT VTI was 20.83±4.86cm and mean SVI was 41.55±9.55mL/m2. Pearson correlation index for these variables was r=0.644, p<0.001; ICC 0.52 (CI 95% 0.4–0.63). When SVI and maximum LVOT VTI were compared, Pearson correlation index was r=0.62, p<0.001. Correlation worsened for extreme values, especially for those with higher LVOT VTI (Figs. 1 and 2).
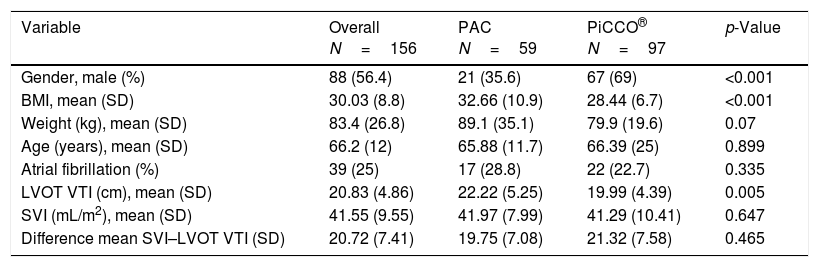
Results obtained by PAC and PiCCO® are shown in Table 2. LVOT VTI was different when both invasive monitoring systems were compared (LVOT VTI for PAC 22.22±5.25cm, for PiCCO® 19.99±4.39, p=0.005). Body mass index (BMI) was higher for patients in whom the PAC was chosen as the invasive monitoring method (BMI for PAC 32.66±10.92, BMI for PiCCO® 28.44±6.76, p<0.001). PiCCO® was preferably employed in men (male gender represented for PAC and PiCCO® 35.6% and 69% respectively, p<0.001).
Comparison of paired measurements performed with PAC and PiCCO®.
| Variable | Overall N=156 | PAC N=59 | PiCCO® N=97 | p-Value |
|---|---|---|---|---|
| Gender, male (%) | 88 (56.4) | 21 (35.6) | 67 (69) | <0.001 |
| BMI, mean (SD) | 30.03 (8.8) | 32.66 (10.9) | 28.44 (6.7) | <0.001 |
| Weight (kg), mean (SD) | 83.4 (26.8) | 89.1 (35.1) | 79.9 (19.6) | 0.07 |
| Age (years), mean (SD) | 66.2 (12) | 65.88 (11.7) | 66.39 (25) | 0.899 |
| Atrial fibrillation (%) | 39 (25) | 17 (28.8) | 22 (22.7) | 0.335 |
| LVOT VTI (cm), mean (SD) | 20.83 (4.86) | 22.22 (5.25) | 19.99 (4.39) | 0.005 |
| SVI (mL/m2), mean (SD) | 41.55 (9.55) | 41.97 (7.99) | 41.29 (10.41) | 0.647 |
| Difference mean SVI–LVOT VTI (SD) | 20.72 (7.41) | 19.75 (7.08) | 21.32 (7.58) | 0.465 |
N denotes the number of paired measurements; SD: standard deviation.
Non-invasive calculation of cardiac index by ultrasound uses to be performed by the conventional method that includes the calculation of aortic annular area. This method is exposed to mistakes related to operator experience and to the pitfalls and difficulties to secure a suitable acoustic window, especially in ventilated patients. This is the reason why it would be of great interest to develop a simple echocardiographic method, minimizing unnecessary calculations. Beside this, assessing the concordance between LVOT VTI and invasive hemodynamic parameters is of great importance for developing accurate methods of echocardiographic monitoring. These concerns set up the stage for the aim of our study.
Echocardiography has significant limitations in critically ill patients. Therefore, it is of great value to find out patient characteristics and conditions that could lead to inaccurate results. In the present study, we excluded patients with aortic regurgitation and LVOT abnormalities. Other cardiac abnormalities that can interfere with LVOT VTI measurements, such as dilated left ventricles, are common in fluid overloaded critical care patients. Atrial fibrillation, frequently present in seriously ill patients, could interfere with echocardiographic assessment. Nevertheless, we recorded five measurements of LVOT VTI in every patient, in accordance with current recommendation for patients on atrial fibrillation.
Our results show a modest agreement between LVOT VTI and SVI determined by thermodilution. The subsets of patients included have some characteristics than can lead to several pitfalls for accurately assessing cardiac function by ultrasound. These pitfalls are derived not exclusively from the quality of acoustic windows but also from physiological changes induced by mechanical ventilation or preload conditions.
Previously published studies pointed out to the fact that cardiac index monitoring in critically ill patients by cardiac ultrasound is feasible and accurate, including some ventilated patients. Evangelista et al. reported a close correlation (r=0.97) between left ventricular outflow tract mean velocity determined by pulsed wave Doppler and cardiac index determined by the thermodilution method, in the absence of left ventricular outflow abnormalities.5 In their study, cardiac index was calculated through a simple formula (CITD=172 MVpwD – 172, where CITD denotes cardiac index by thermodilution and MVpwD denotes mean velocity by pulsed wave Doppler). This study excluded patients with severe obesity and was not designed for patients on mechanical ventilation.
In the recent study published by Muñoz et al., cardiac output measured by thermodilution in patient after cardiac surgery was compared with cardiac output calculated (area×VTI) through transesophageal echocardiography (measuring VTI in LVOT and mitral annulus). Results from cardiac output measurements by transesophageal echocardiography did not correlate with those obtained by thermodilution.6 The authors did not discuss explanations for their findings.
LVOT obstruction can modify LVOT VTI values leading to uncertain results. This problem is not restricted to the dynamic obstruction observed in some hypertrophic cardiomyopathies, but can also be present in some low preload states (e.g. hypovolemic and distributive shock). In patients with left ventricular hypertrophy, obstruction can be precipitated by hypovolemia or by exogenous or endogenous catecholamines.7 These circumstances are common in the critical care setting and were present in most of the patients included in this study. The influence of these factors in our results has to be determined in future studies.
Although the aim of our study did not include to compare PAC and PiCCO® methods, correlation was closer to 1 for paired measurements with PiCCO® (r=0.766, p<0.001) than for those assessed with PAC (r=0.493, p<0.001). BMI was different for these two monitoring methods (32.66±10.92 and 28.44±6.76 for PAC and PiCCO® respectively; p<0.001). Physician responsible could have chosen the monitoring method taking into account obesity and its associated pathologies, such as pulmonary hypertension.
We did not have added anthropometric data to the calculation of LVOT VTI to improve correlation with SVI. Leye et al., have demonstrated that LVOT diameter is linearly correlated with body surface area (BSA). In their study they proposed BMI, height and gender specific equations to calculate LVOT diameter.8 Nevertheless, when their validated formula has been included to calculate LVOT dimensions, the correlation with Fick and thermodilution methods was poor, even for patients with preserved left ventricle function and normal ventricle size, as concluded by Maeder et al.9 These investigators did not find a good correlation between those gold standards and echocardiographic left ventricular function assessed by four methods (considering LVOT VTI, LVOT diameter as measured as well as estimated from body surface area and stroke volume indices assessed using the biplane and monoplane methods).
Pulmonary hypertension, a common issue in the critical care setting, can lead to right ventricle failure and tricuspid regurgitation, which are associated with underestimation of cardiac output measured by thermodilution. One study from Balik et al., concluded that high degree tricuspid regurgitation can cause inaccurate cardiac output calculated by thermodilution through a PAC.10 Additionally, there are some concerns about the accuracy of transthoracic Doppler echocardiography to evaluate pulmonary hypertension, which can be underestimated when compared with measurements determined by PAC.11,12 In our study, pulmonary pressures were measured only in the case of PAC insertion. Hence, we are lacking data about right ventricle function in most patients and we are unable to draw some conclusions about the impact that pulmonary hypertension could exerts on our results.
Although widely employed for critically ill patients monitoring, several concerns exist about the reliability of PiCCO® system in some settings, including patients receiving positive end-expiratory pressure, those suffering from pulmonary vascular occlusion, inhomogeneous lung injury or pleural effusions.13 All these circumstances are common among ICU patients and raise doubts about the accuracy of transpulmonary thermodilution as a reference when compared with other monitoring methods.
ConclusionsLVOT VTI could be a complementary hemodynamic evaluation tool in selected patients, but does not eliminate the need for invasive monitoring at the present time. LVOT VTI could introduce additional mistakes to those derived from the cross section of the aorta in the calculation of stroke volume in ventilated patients. The weak correlation between LVOT VTI and invasive measurement of SVI in extreme values deserves additional assessment in larger studies that could identify the factors affecting this disagreement.
Authors’ contributionsR. Blancas has full access to all the data in the study and he takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author assumes full responsibility for the integrity of the submission as a whole.
Dr. R. Blancas confirms that the study objectives and procedures are honestly disclosed. Moreover, he has reviewed study execution data and confirms that procedures were followed to an extent that convinces all authors that the results are valid and generalizable to a population similar to that enrolled in this study.
Dr. Óscar Martínez-González has performed statistical analysis.
Dr. Daniel Ballesteros, Dr. Antonio Núñez, Dr. Jimena Luján, Dr. Diego Serrano, Dr. Alberto Hernández, Dr. Cristina Martínez-Díaz, Dr. Carmen Martín Parra, Blanca López Matamala, Dr. María Angeles Alonso and Dr. Miriam Chana have performed echocardiographic studies and collected data.
Conflicts of interestThe authors declare that they have no conflict of interest.
The authors have to thank Dr. A. Evangelista, from the Hospital Vall d́Hebron (Barcelona, Spain), Dr. M. Ruiz, from the Regions Hospital (St. Paul, Minnesota, United States of America) and Dr. B. Oliva, from the Centro Nacional de Investigaciones Cardiovasculares Carlos III (Madrid, Spain) for their critical review of this manuscript.