Despite current initiatives aimed to improve sepsis awareness and early treatment, patient outcomes still depend upon the performance of accurate interventions which in turn rely on practical aspects surrounding the time of presentation of patients. Effective accomplishment of core interventions in sepsis demands a correct evaluation and requires a system that coordinates emergency services, general wards, surgical teams, intensive care department and pharmacy to provide optimal treatment.
Recognizing the need to improve early identification of sepsis, new definitions were published in 2016.1 Nonetheless, catastrophic consequences still exist as a consequence of the lack of sepsis awareness and systemic errors even within experienced health care institutions where presiding training programs promoting early sepsis diagnosis and management protocols are being encouraged at the same time.2 Less severe sepsis cases are those cases that frequently remain unrecognized, as they develop subtle clinical signs and appear less sick; comprising a subgroup of patients at risk of being inadequately treated, with associated mortality rates exceeding 25% in some studies.3
Noteworthy, training programs directed to improve awareness of sepsis are not sufficient to obtain palpable results.2 In recent years, high-quality evidence has demonstrated protocolized care for early resuscitation in sepsis to be the recommended approach not only aimed to reduce deaths, but also to prevent systemic errors and their overall individual, social and health care system consequences.4 Thus, many educational programs on protocolized care for early sepsis care and shock resuscitation have been derived from this recommendation.
As a step forward, the Surviving Sepsis Campaign (SSC) have produced and updated different bundles for the acute management of sepsis in order to provide a reliable tool containing evidence-based recommendations for the best care of patients. A bundle is a selected set of interventions or processes of care distilled from evidence-based practice guidelines that when implemented as a group provide a more robust picture of the quality of care provided. Individual hospitals can and should codify bundle elements into customized clinical protocols that function best in their institutions. However, to provide standard-of-care therapies to patients, no element of the bundle should be ignored. Despite several studies have shown that bundle care is associated to better outcomes, some elements of the initial bundles have not confirmed their efficacy or are no longer available, like activated protein C or quantitative resuscitation.5 A new SSC bundle update was recently published,6 in which authors combine both the 3- and 6-hour bundles and simplify them into the 1-hour bundle as a means of providing education and achieving improvements on sepsis management (Table 1).
Surviving Sepsis Campaign Bundles from 2012 to 2018.
| 2012 | 2016 | 2018 |
|---|---|---|
| 6-hour Resuscitation bundle: 1. Measure serum lactate. 2. Obtain blood cultures prior to antibiotic administration. 3. Administer broad-spectrum antibiotics within 3hours from time of presentation. 4. In the event of hypotension and/or lactate>4mmol/L: a. Deliver an initial minimum of 20ml/kg of crystalloid (or colloid equivalent). b. Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65mm Hg. 5. In the event of persistent hypotension despite fluid resuscitation and/or lactate>4mmol/L: a. Achieve central venous pressure (CVP) of ≥8mm Hg. b. Achieve central venous oxygen saturation (ScvO2) of ≥70%. 24-hour Management bundle: 1. Administer low-dose steroids for septic shock in accordance with a standardized ICU policy. 2. Administer drotrecogin alfa (activated) in accordance with a standardized ICU policy. 3. Maintain glucose control ≥ lower limit of normal, but <150mg/dl. 4. Maintain inspiratory plateau pressures <30cm H2O for mechanically ventilated patients. | 3-hour bundle: 1. Measure serum lactate. 2. Obtain blood cultures prior to antibiotic administration. 3. Administer broad-spectrum antibiotics within 3hours from time of presentation. 4. Administer 30ml/kg crystalloid for hypotension or lactate ≥4mmol/L 6-hour bundle: 1. Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65mm Hg. 2. In the event of persistent hypotension despite fluid resuscitation and/or lactate >4mmol/L: a. Measure central venous pressure (CVP). b. Measure central venous oxygen saturation (ScvO2) of ≥70%. 3. Remeasure lactate if initial lactate was elevated. | hour-1 bundle: 1. Measure lactate level. Re-measure if initial lactate is >2mmol/L (Weak recommendation, low quality of evidence). 2. Obtain blood cultures prior to administration of antibiotics (Best practice statement). 3. Administer broad-spectrum antibiotics (Strong recommendation, moderate quality of evidence). 4. Rapidly administer 30ml/kg crystalloid for hypotension or lactate ≥4mmol/L (Strong recommendation, low quality of evidence). 5. Apply vasopressors if patient is hypotensive during or after fluid resuscitation to maintain MAP ≥65mm Hg (Strong recommendation, moderate quality of evidence). |
Substantial agreement exists among international experts regarding establishment of earlier interventions to overcome barriers precluding definitive actions to be taken by clinicians when facing a suspected case of sepsis. Indeed, novel concepts have been recently introduced, such as the door-to-needle time for antibiotic administration, depicting global concerns about setting up a time window after the onset of symptoms.7 Nevertheless, in some situations, attaining an effective application of institutional protocols during the golden hour of sepsis may represent a real challenge to be achieved. First, significant delays from first medical contact to administration of appropriate therapy have been observed in the pre- and in-hospital setting. Of note, community-acquired sepsis cases transported by emergency medical systems have been associated with high adjusted in-hospital mortality rates associated with delays from first medical contact to antibiotic administration.8 Second, there are several aspects of sepsis management in which observational evidence has showed that current practices regarding some of its core interventions are highly variable. For instance, a large multinational study showed that a great proportion of hemodynamic management assessments were based on misunderstanding of clinical indications for fluid challenges and goals of fluid therapy.9 Third, a variable but non-negligible proportion of first medical contacts are provided by non-critical care professionals. Furthermore, existent variations in internal policies among different institutions determining immediate medication availability,7 general beliefs regarding sepsis prognosis, institutional policies, etc., which thwart any initiative aimed to encourage quality improvements. In addition, as pointed out by the IDSA, stipulating an aggressive, fixed-time period may lead to unintended consequences, namely an increased likelihood that broad-spectrum antibiotics will be given more frequently to patients with infection-like syndromes in the rush to meet the fixed timeframe stipulated for infected patients.
In some previous algorithm-based training programs for the management of other clinical situations, in which time has a major role (“time is life”), the performance of life support proceedings have led to significant improvements in acute emergency care.10 As patient outcomes are time-dependent and the first medical contact is a crucial element contributing to patient outcomes, all health care professionals facing a suspicious case of sepsis should be able to perform initial skilled life support interventions even while awaiting for a clearer diagnosis and treatment. In fact, less severe sepsis cases pose a great opportunity to apply the hour-1 bundle when chances to survive are higher.
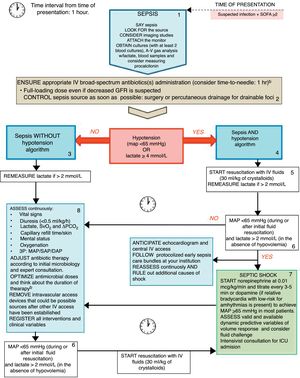
Aiming at improving the very early treatment window in sepsis we propose the “rational life support in sepsis” algorithm (Fig. 1) that may be useful to better clear-cut the current recommended skills to be acquired by first medical contacts regardless of their area of expertise. Indeed, we intended to provide a reliable tool at bedside which, rather than substituting institutional protocols for early management for sepsis care, is intended to facilitate training of health care professionals on early sepsis management and to create a global consciousness among medical and non-medical professionals.
The Rational Life Support in Sepsis (RALSS) algorithm. This algorithm is based on the Surviving Sepsis Campaign (SSC) hour-1 bundle of care, as well as adapted to the institutional protocol and available resources. At time zero, clinician on charge should suggest verbally and share with other staff members the diagnosis (say sepsis), look for the source (and consider imaging studies), attach the monitor to the patient and obtain at least 2 blood and other cultures according to clinical suspicion (preferably before administration of broad-spectrum antibiotics) (box 1). Afterwards, administration of appropriate IV antibiotic(s) and the best strategy for control of sepsis source should be ensured (if a drainable focus of infection is present) (box 2). Sepsis without hypotension(box 3)or Sepsis and hypotension(box 4) pathway should be followed according to patient status. If hypotension is present, start resuscitation with IV fluids (30ml/kg) and re-measure lactate if initial value was >2mmol/L or if clinical deterioration exists although the initial lactate was ≤2mmol/L (box 5). If hypotension persists and lactate is >2mmol/L start vasopressors only in those patients who have received an initial resuscitation with IV fluids and are not considered to be hypovolemic (box 6 and box 7). In septic shock patients assess for fluid responsiveness and ask for an intensivist consultation (box 7). Continuous assessment of clinical and laboratory variables and control of focus of infection reassessment may be carried out to enhance initial resuscitation interventions (box 8). Complementary assessments may be requested, and institutional protocol activated. During all interventions continuous reassessment and ruling out of other sources of shock should be conducted (box 9). ID denotes Infectious Diseases, MAP mean arterial pressure, SAP systolic arterial pressure, DAP diastolic arterial pressure, ScVO2 central venous oxygen saturation, PCO2 Partial pressure of carbon dioxide, ICU intensive care unit. a Do not significantly delay antimicrobial therapy while awaiting for cultures or blood samples. Out-of-hospital approach should attempt to store baseline blood samples for rapid analysis at hospital admission. b Broad-spectrum antibiotics considering the likely etiology of infection, specific drug properties and increased extra-renal drug elimination during sepsis/septic shock, history of multidrug-resistant microorganisms, presence of acute kidney injury (±renal replacement therapies) or liver failure and presence of obesity.
Sepsis is a time-dependent medical emergency requiring effective interventions focused on reducing the time interval between a suspected diagnosis and effective treatment. Advocating for general healthcare staff training on the acquisition of skilled life support interventions focused on the initial management recommendations of the hour-1 bundle and strengthen by the use of pragmatic tools should probably be assessed, in order to reduce delays from first medical contact to appropriate therapy; and to avoid systemic errors when facing a suspected case of sepsis. Such a strategy may significantly contribute to current initiatives on the development of educational programs for sepsis prevention and to promote the SSC targets, as it is focused on the endorsement of aspects unequivocally being associated with best practice standards.
FundingNone.
Conflicts of interestThe authors declare no conflicts of interest.