Acute respiratory distress syndrome (ARDS) is still related to high mortality and morbidity rates. Most patients with ARDS will require ventilatory support. This treatment has a direct impact upon patient outcome and is associated to major side effects. In this regard, ventilator-associated lung injury (VALI) is the main concern when this technique is used. The ultimate mechanisms of VALI and its management are under constant evolution. The present review describes the classical mechanisms of VALI and how they have evolved with recent findings from physiopathological and clinical studies, with the aim of analyzing the clinical implications derived from them. Lastly, a series of knowledge-based recommendations are proposed that can be helpful for the ventilator assisted management of ARDS at the patient bedside.
El síndrome de dificultad respiratoria aguda (SDRA) sigue asociándose a unas elevadas tasas de morbimortalidad. La mayoría de los pacientes con SDRA requieren apoyo ventilatorio. Esta terapia tiene un impacto directo sobre los resultados de los pacientes y se asocia con importantes efectos secundarios. De ellos, la principal preocupación cuando se aplica esta terapia es la lesión pulmonar asociada a ventilador (LPAV). Los mecanismos fundamentales de la LPAV y su tratamiento se encuentran en constante evolución. En esta revisión, describiremos los mecanismos clásicos de la LPAV y cómo han evolucionado con los recientes hallazgos de estudios patofisiológicos y clínicos para analizar las implicaciones clínicas que se derivan de ellos. Al final de esta revisión, extraeremos una serie de recomendaciones basadas en los conocimientos, las cuales pueden resultar útiles para la terapia con ventilador a pie de cama en pacientes con SDRA.
The acute respiratory distress syndrome (ARDS) is still related to high mortality and morbidity rates.1 In spite of all the knowledge on its pathophysiology, there are no treatments aimed to modify the natural history of the disease. Instead, the treatment of ARDS is based on a delicate equilibrium between restoration of the most basic physiology and avoidance of side effects.
The single strategy with a major impact in ARDS is mechanical ventilation. Most of the patients with ARDS will require ventilatory support, which may restore gas exchange and decrease work of breathing, thus improving the probability of survival. But mechanical ventilation is not exempt from side effects. Among these, the potential of positive pressure ventilation to damage the lungs, included in the concept of ventilator-induced or ventilator-associated lung injury (VILI/VALI, referred to experimental models and patients, respectively), is currently considered one of the key mechanisms related to the outcome.2 The application of strategies aimed to minimize VALI, mainly by using low tidal volumes, has decreased the mortality of the syndrome.3 Even the benefits of other treatments such as prone position4 or neuromuscular blocking agents5 are attributed to its potential to minimize the secondary damage caused by the ventilator.
The impact of ventilator settings on the induction of VALI has been present in the history of ARDS since its first description. Ashbaugh et al. describe in the original report of the syndrome that those patients who were ventilated with PEEP showed a better outcome.6 A large body of evidence since then has demonstrated that the lung damage caused by ventilation is highly dependent on some ventilator variables.7 In other words, different strategies may yield different effects. The ultimate mechanisms behind these differences have evolved over time, and the framework of VALI is under constant evolution.
In this review, we will describe the classical mechanisms of VALI and how they have evolved with the recent findings from pathophysiological and clinical studies, in order to analyze the clinical implications derived from them. Our objective is to extract a series of knowledge-based recommendations that can be helpful for the ventilatory management of ARDS patients at the bedside.
Mechanisms of ventilator-induced lung injuryThe contemporary management of mechanical ventilation is intimately linked to the concept of VALI. Ultimately, VALI is a molecular response to the application of abnormal forces within the lungs that may lead to inflammation, oedema and extracellular matrix remodelling.8 The spread of this mechanism beyond the lungs has been linked to the development of multiple organ failure. Collectively, VALI has been related to the clinical outcome, so its avoidance is a key objective in the ventilated patient.
A large number of molecular pathways are modified during mechanical ventilation and almost any process related to cell homeostasis has been implicated.9 Inflammatory responses, changes in cell survival signalling and processing of the components of the extracellular matrix have been described after mechanical ventilation. The description of these mechanisms at a cellular and chemical level is outside the scope of this article. Instead, we will focus on the pathophysiological mechanisms that trigger VALI.
Classical mechanisms of VALIMechanical ventilation is the cornerstone of the critically-ill patients support, providing better gas exchange conditions while respiratory muscles rest. In 1967, the term “respirator lung” was coined to describe the diffuse alveolar damage and hyaline membranes found in post-mortem studies of patients submitted to positive pressure ventilation.10 During the following decades, studies with experimental models showed the deleterious effects of high positive pressure ventilation and the benefit obtained by the application of positive end-expiratory pressure (PEEP). These pioneering studies allowed to introduce the experimental concept of Ventilator-induced lung injury (VILI) and, later on, its clinical counterpart, Ventilator-associated lung injury (VALI).11,12
Three classical mechanisms responsible for VALI have been described: biotrauma, barotrauma/volutrauma and atelectrauma:
Biotrauma: The mechanical stimulus that involves the application of positive pressure during mechanical ventilation triggers, through a process of mechanotransduction, a biological response characterized by the secretion of proinflammatory cytokines and the emergence of a neutrophilic infiltrate. As a result, there is a release of inflammatory mediators from the ventilated lung that can lead to a systemic dissemination, contributing to the development of the multiple organ dysfunction syndrome.13 The establishment of protective ventilatory strategies and the application of PEEP can attenuate this phenomenon.
The biotrauma contributes to the persistence of the inflammatory process and it is associated with worse prognosis in patients with ARDS.14,15 Therapeutic strategies based in the interference of the pulmonary inflammatory response have obtained successful results in experimental models, but have not been translated to the clinical practice yet.
Barotrauma/volutrauma: Experimental studies in rats submitted to high ventilatory pressures showed alveolar damage by over-stretching, consisting in perivascular and alveolar oedema.11 It is accepted that the use of high volumes can cause breakage of the alveolar walls. The pulmonary over-stretching is increased due to the coexistence of healthy alveoli and non-aerated collapsed areas. This regional heterogeneity can aggravate the lung damage in previously healthy aerated alveoli and in the interface aerated/non-aerated areas, even when low volumes are used for ventilation.10
Atelectrauma: Mechanical ventilation may result in cyclic variations of alveoli aeration, that lead to epithelium damage due to the emergence of shear forces at the interfaces between air and fluid in the injured lung, and the generation of open-collapse alveoli phenomena.16 The application of PEEP minimizes the closing and reopening stress in the alveolar spaces, thus reducing the lung damage.
Knowledge regarding these mechanisms of injury has led to changes in the clinical practice, consisting on the application of PEEP and the use of low tidal volumes, giving rise to the strategy known as “protective ventilation”.3 At the beginning of the century, several studies involving patients with ARDS have shown the importance of minimizing lung damage associated with mechanical ventilation in terms of mortality. Other ventilatory strategies aimed to reduce VALI include the prone position ventilation17 and the use of high PEEP.18 Together with the ventilator settings, pharmacological sedation and neuromuscular blockade have also a role in providing less harmful ventilation.5 Moreover, the use of extracorporeal membrane oxygenation (ECMO) techniques contributes to maintaining an adequate gas exchange until lung damage resolution.19
Functional imaging of injured lung: the baby lung PET visionIn order to better understand the way mechanical ventilation results harmful in the inhomogeneous lung tissue typical of ARDS, functional imaging techniques have been used during the last years. This promising tool has been technically evolving at the same time the need for knowledge regarding this old syndrome has been growing up. In that sense, Positron Emission Tomography (PET) has raised as a powerful image tool,20–23 useful for reaching this objective from a pathophysiological perspective. Indeed, several studies on ARDS and VALI have based their findings on this technique. By measuring the activity of different tracers, it has been used to evaluate the relationship between the distributions of pulmonary ventilation and perfusion,24 the effect of different ventilatory strategies and manoeuvres,25,26 and the regional distribution of inflammation and increased permeability of the endothelial-epithelial barrier.27,28
Focusing on inflammation, Chen DL et al. demonstrated that 18-Fluor-deoxy-glucose (18FDG) PET could be correlated to the neutrophil inflammatory response to endotoxin, although the increased uptake of this analogue of glucose by metabolically activated cells could not be ascribed exclusively to neutrophils.29 Only a couple of years later, Bellani et al. combined lung computed tomography (CT) and 18FDG PET scan in patients with ARDS, and observed significant findings. As expected, the inflammatory activity of the lungs was increased when compared with controls. But, surprisingly, it was augmented through the entire lung density spectrum, suggesting that the inflammatory response is not only present in the collapsed and non-aerated regions, but also in normally aerated lung tissue.30 Following these observations, the uptake of 18FDG in normally aerated lung regions was correlated with plateau pressure, with a marked increased above values of 26cmH2O. Furthermore, increased metabolic activity was also related to the ratio (tidal volume/end expiratory lung volume) in the same regions, which is a surrogate marker of lung tissue strain.31 These findings point to stress and strain as trigger of the inflammatory response within the baby lung.
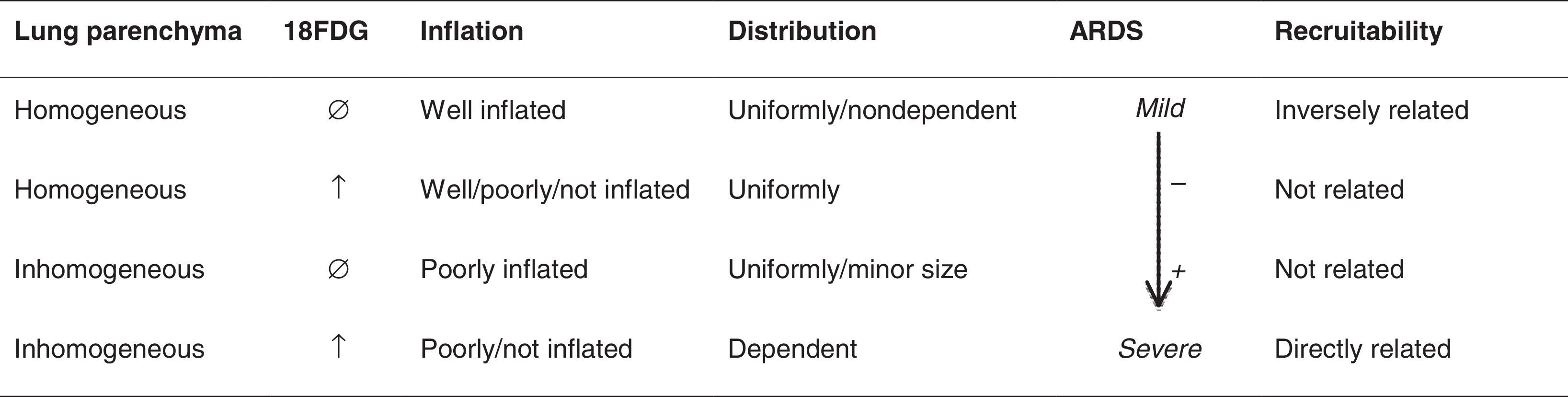
In a recent study that thoroughly describes the pathophysiology of ARDS by using CT and PET scan, and integrating them with clinical and analytical variables, Cressoni et al. arbitrarily divided the lung into four compartments in order to better understand their findings (Table 1): homogeneous lung compartment with normal 18FDG uptake rate, homogeneous lung compartment with increased 18FDG uptake rate (uniformly distributed along the lung and not related to recruitability), inhomogeneous lung compartment with increased 18FDG uptake rates (poorly aerated lung tissue, distributed in the dependent lung regions) and inhomogeneous lung compartment with normal 18FDG uptake rate (representing only a minor portion of lung volume). Interestingly, according to their findings, the inhomogeneous lung compartment with increased 18FDG was always present at the interface between inflated and not inflated tissue and was well related to lung recruitability.32 These data suggest, once again, that mechanical stress in specific tissue areas suffering recruiting and derecruiting phenomena may induce the molecular and chemical cell processes involved in this increased metabolic activity.
All these findings suggest that PET may be useful to assess the application of different ventilatory or pharmacological therapeutic approaches, as the functional counterpart to more anatomical imaging techniques, enlightening or complementing knowledge regarding the classical mechanisms of VALI.
The role of alveolar stabilityAn alternative approach to understand how mechanical ventilation affects the lungs is to consider the deformation of the pulmonary parenchyma in a comprehensive manner. Under normal conditions, the inspired volume is divided among a large number of alveoli. These units share a variety of biophysical mechanisms, conferring them a great stability and leading to minimal changes in their structure during ventilator cycles. The underlying mechanisms (isotropic expansion, deployment of alveolar areas, etc.) for this slight change have not been fully understood.7 Direct visualization of the alveoli, using in vivo microscopy, shows the absence of relevant changes in size during ventilation. Disruptions of these alveolar stability mechanisms or the appearance of parenchyma heterogeneity may induce some degree of instability, altering the tissue mechanical stress and increasing the injury. Available evidence suggests that ventilation with large tidal volumes promotes surfactant deactivation and favours alveolar instability.33,34 Halter et al.35 found that the combination of low tidal volume ventilation plus the application of high PEEP synergistically stabilizes the alveolar units, introducing minimal changes in the biological response attributed to the mechanical stress. These findings have been correlated with in vitro studies with epithelial alveolar cells, in which the cyclic strain, rather than the degree of static overstretching, can actually cause injury.36 This deformation caused by mechanical ventilation, sometimes leading to the rupture of the epithelium integrity, triggers the inflammatory response and the activation of repair and remodelling processes within the cytoskeleton.37
Given the complexity of the alveolar structure, several authors have developed and proposed mathematical and physical models trying to explain these phenomena. In 2007, Kitaoka et al.38 described a 4-D model, validated by in vivo microscopy, that explains how the size and number of aerated alveoli change during ventilation. This model is able to illustrate the alveolar recruitment and de-recruitment mechanisms. Further studies have documented a new level of alveolar heterogeneity. During inspiration, some alveolar walls remain virtually static, while others are subjected to a greater deformation.39 Therefore, it seems that these areas with more distortion and increased mechanical load, could act as seeds of the biological response in the parenchyma.
The consequences of spontaneous breathingIn normal lungs, spontaneous breathing generates a negative pressure along the pleural surface that results in lung inflation. In opposite, mechanical ventilation applies a positive pressure in a very narrow area (the tip of the tracheal tube) that results in a heterogeneous flow predominantly directed towards high compliant zones. By these mechanisms, air distribution is more homogeneous in spontaneous breathing.
In ventilated patients, spontaneous inspiratory efforts may be superimposed to the ventilator-driven breaths. The synchrony between both determines the net result on aeration and tissue stress. In this sense, spontaneous breathing may have beneficial effects. First, ventilation can be more homogeneous, so tissue damage is ameliorated. Second, the diaphragmatic contraction generates local increases in transpulmonary pressures that facilitate aeration and recruitment of the nearby lung areas.40 As these juxtadiaphragmatic segments are usually collapsed in ARDS, the final result is a substantial improvement in aeration, functional residual capacity and gas exchange.
But when asynchronies are present, lung tissue can be exposed to a large stress, resulting from the addition of the pressure driven by the ventilator and the inspiratory effort.41 Double-triggering (an spontaneous inspiration immediately after the end of a ventilator-controlled inspiration) can raise end-inspiratory volume near total lung capacity, thus causing overdistension of aerated alveoli. Wasted inspiratory or expiratory efforts can induce the collapse of poorly-aerated areas and promote cyclic changes in aeration, which, as previously described, triggers tissue inflammation. In fact, the incidence of asynchronies during ventilation has been related to mortality.42 With these concepts in mind, it is not surprising that muscle paralysis during the first days of ARDS, when the lung is more prone to additional injuries caused by ventilation, improved the outcome of ventilated patients.5
Therefore, the combination of spontaneous and mechanical breathing can lead to very different outcomes depending on the patient-machine interaction. The fine tuning of the ventilator settings, avoiding both over- and under-assistance from the ventilator, and the application of ventilatory modes that facilitate this interplay may be the key to take advantage of spontaneous breathing as a tool for recruitment and, ultimately, to improve outcomes in ARDS.
Implications for conventional ventilatory settingsOnce the clinical relevance of VALI has been firmly established and accepted, ventilatory settings should be adjusted with the minimization of lung damage as a main objective. A tidal volume in the range of 6ml/Kg of ideal body weight, with a reasonable level of PEEP is the standard of care for patients with the ARDS. However, optimal levels of tidal volume and PEEP have not been completely established.
Regarding tidal volume, it is now accepted that large tidal volumes cause clinically relevant lung injury, even in healthy lungs.43 Moreover, even a standard approach using a tidal volume of 6ml/kg may not be protective, exposing lungs to cyclic overstretching.44 Therefore, in spite of general recommendations, the individual fine-tuning of tidal volume is a difficult task. Recently, driving pressure (this is, plateau pressure minus PEEP), has been shown as a powerful predictor of outcome in these patients, and values around 15cmH2O are suggested as the upper limit.45 Although driving pressure could offer some advantages over tidal volume, it should be highlighted that this has not been demonstrated in specific clinical trials, and the recent findings could be only an epiphenomenon of reduced tidal volumes and severity. If tidal volumes are fixed at 6ml/kg, driving pressure relies in compliance, which is a marker of severity. In other words, in an ARDS patient with high compliance, could we use a tidal volume of 10–12ml/kg, provided driving pressure is below 15cmH2O?
On the other side, decreasing tidal volume below 6ml/kg faces its own problems. With such low volumes, patient-ventilator asynchronies may be more frequent, and there is an increased risk of atelectasis and hypoxemia.46 The role of the so-called “ultraprotective” approaches, in which extracorporeal support is required to reduce tidal volumes up to 3ml/kg or less, although feasible, is currently under research.47
Advanced monitoring during mechanical ventilationThe increasing knowledge of lung injury mechanisms motivated a growing concern about different parameters of respiratory mechanics, beyond the classic ones, that could be useful as a guide for mechanical ventilation adjustment. For instance, deformation of lung parenchyma, and not only the application of a pressure or volume, is believed to cause tissue damage. The concepts of stress and strain were developed as a more precise approach to the mechanical load transferred from the ventilator to the lungs.48
Stress is the force required to deform the lungs while they are inflated, a concept that may be equivalent to transpulmonary pressure. Strain is the magnitude of the deformation, expressed as a fraction of the baseline situation. Calculation of strain is not as straightforward as stress. The magnitude of the deformation includes both the tidal volume and the increase in volume caused by PEEP. Moreover, depending on the consideration of functional residual capacity (FRC) or end-expiratory lung volume (EELV) in the presence of positive end-expiratory pressure (PEEP) as starting point for the calculation of strain, the application of PEEP seems to produce respectively an increase or decrease of strain.
Therefore, calculation of strain is easy at zero end-expiratory pressure, but the complex effects of PEEP must be taken into account when applied. Overall, PEEP increases strain by promoting inflation of the lung above its resting volume (functional residual capacity). To explain the protective effects of PEEP against VILI, the concepts of dynamic and static strain arose. Whereas the first refers to the cyclic changes in volume (this is, strain induced by tidal volumes), the second is limited to the static increase in volume (this is, strain induced by PEEP). However, in injured, non-aerated lung areas, PEEP can increase the lung volume available for ventilation by recruiting previously non-aerated zones, thus leading to a decrease in strain. In a recent study, these local effects of PEEP on strain were assessed using CT scans. In a sample of ARDS patients and in an animal model, although PEEP did not modify overall strain, high PEEP levels (20cmH2O) virtually abolished dynamic strain, rendering the lung more stable during ventilation.49
A non-invasive, radiation-free, bedside monitoring technique providing real-time information about regional variations in ventilation and perfusion in relation to a reference state could be the key when trying to figure out what PEEP is actually achieving in the lung parenchyma of our patients. We are referring to electrical impedance tomography (EIT). This technique can estimate, from relative changes in local lung impedance, global or regional lung volume. A recently published work by Cinnella et al., performed in a small cohort of patients with mild ARDS, aimed to compare the Open Lung approach with the ARDS Network protocol.50 These two strategies tackle ARDS patients ventilatory management from different points of view: on one side, the ARDS Network protocol, conservative and matching a minimal oxygenation target with the lowest possible PEEP; and on the other side, the more recent, physiologically oriented open lung approach, whose objective is achieving the maximal alveolar recruitment by applying the PEEP level needed to achieve the best compliance of the respiratory system. EIT monitoring revealed that the open lung approach may improve recruitment of dorsal lung regions and obtains a more homogeneous volume distribution, in patients with a high potential for alveolar recruitment. However, overdistension and haemodynamic impairment make the OL approach unsafe in nonresponders. These findings are concordant with those from Camporota et al. reporting the usefulness of EIT to estimate the potential for alveolar recruitment in two cases of severe ARDS. In this setting, EIT may be a useful tool for dynamically monitoring the tidal volume distribution during ventilation, which would permit to assess regional lung aeration changes when applying different lung-protective ventilation parameters, and to evaluate the individual potential recruitability at each patient's bedside.51 Similarly, a severe acute COPD exacerbation published case illustrates how EIT may proportionate information about regional ventilation and optimal PEEP levels in particular clinical scenarios.52
It has to be taken into account that EIT data should be considered in conjunction with all the information available, as the technique is not exempt of many limitations. For instance, EIT data are just estimations crucially dependent on the electrode position and conformational changes of the chest wall, not being able to detect closed lung areas and open lung but not ventilated lung areas. Combined EIT-TC could maybe lighten some of these concerns, but unavoidably losing two of the major advantages of this tool, which are its radiation-free condition and its bedside application.
The role of PEEPThe use of PEEP was first described in 1938 and reported to be useful as a treatment in pulmonary oedema. Its utilization was then widespread since 1967, when Ashbaugh et al. reported an improved oxygenation after PEEP application in patients suffering ARDS.6
PEEP increases EELV, preventing surfactant aggregation, avoiding airway and alveolar collapse, decreasing airway resistance and improving both oxygenation and respiratory system compliance. Thus, capillary-alveoli available area for gas exchange increases and extravascular lung water is displaced from alveolar to peribronchial interstice. Also, ventilation/perfusion mismatch is reduced since the lung becomes more homogeneous. However, PEEP may have complex effects on haemodynamics that must limit its application.53
Basically, in patients with ARDS in whom the lung is completely inhomogeneous, mechanical forces applied during each respiratory cycle may damage the tissue. The use of PEEP minimizes cyclic atelectasis during mechanical ventilation by keeping the alveoli open during expiration. By increasing plateau pressures, it also promotes the opening of collapsed alveoli, adding new pulmonary units available to distribute the tidal volume. This recruitment can decrease alveolar overdistension and increase lung compliance, thus decreasing driving pressure. In other words, the application of PEEP allows the ventilator to increase the baby lung size and makes the lung parenchyma more homogeneous.54,55 This mechanism of action may decrease dynamic strain within the lung and reduce the risk of developing VILI.56,57
However, the optimal level of PEEP in patients with ARDS still remains uncertain.58 A number of clinical trials have suggested that high PEEP levels could be more beneficial in terms of oxygenation, ventilatory mechanics, ventilator-free days and organ dysfunction59; moreover, in the subgroup of patients with moderate to severe ARDS in whom the estimated lung recruitability was higher, the use of high PEEP levels tended to be more beneficial in terms of survival. A substudy of the Lung Open Ventilation Study (LOV Study) showed that the use of recruitment manoeuvres and high levels of PEEP does not increase sedative, opioid or neuromuscular blocker doses in adults with ARDS. Moreover this ventilatory strategy may improve patients’ comfort when compared with lower PEEP strategy and may result in more benefits when applied in prone positioned ARDS patients.60 As always, recommendations based on subgroup analyses must be taken with caution.
Prone positionTogether with the low tidal volume ventilatory strategy and the use of neuromuscular blocking agents, the application of prone position is one of the therapeutic approaches to ARDS patients proven to improve survival.4 It is well known that prone position makes transpulmonary pressure and air distribution more homogeneous throughout the lung, helping to achieve and maintain a significant positional recruitment. Moreover, prone improves shunt and facilitates the resolution of hydrostatic oedema by relieving cardiac compression and moving heart position to a dependent area. The final consequence of these better ventilation/perfusion ratios is a more efficient gas exchange.
The effects of turning a patient prone on ventilation are based on how this manoeuvre affects the local distribution of forces within the lung. Prone position might base its beneficial effects on the way it attenuates VALI by moderating transpulmonary forces, homogenizing aerating lung regions and reducing the aerated/non-aerated interfaces responsible for translating mechanical stress into an acute inflammatory response.
However, only a fraction of patients responds to prone with improvements in oxygenation, even though lung densities redistribute towards dependent ventral regions in all cases. The explanation for these interindividual differences may be highlighted by PET studies demonstrating how perfusion redistribution after turning prone shows significant variability.25 Thus, we could hypothesize that patients responders to prone are those who preserve perfusion in dorsal regions, therefore reducing the regional shunt. This ultimate hypothesis is opposed to the certainly main role of the redistribution of ventilation as responsible for the benefits of prone. May the answer be supplied by future 18FDG PET studies in prone positioned ARDS patients aimed to understand the pathophysiological background for the beneficial effects in terms of attenuation of VALI.
Other alternatives in ARDS managementDespite the large research invested in the treatment of acute lung injury, the mortality in ARDS remains high.1,61 Among the different strategies proposed to improve this poor outcome, only a few were aimed to minimize VILI.
High-frequency oscillatory ventilation (HFOV) has been postulated as a ventilator mode able to maintain gas exchange while reducing secondary damage produced by mechanical ventilation due to atelectrauma.62,63 Some trials had suggested had HFOV may improve outcomes in patients with ARDS, while this technique is unlikely to cause harm.64 Nevertheless, two randomized trials failed to demonstrate a benefit in terms of mortality62; in fact, the Canadian one had to be terminated because of the high mortality observed in the HFOV group.63
Extracorporeal membrane oxygenation (ECMO) was first used in 1967 as a venoarterial bypass for a respiratory assistance.65 Cases reported during the following years were associated with high mortality rates.66–70 During ECMO, blood is removed from the vessels and pumped through a circuit where is oxygenated and CO2 is removed; finally, the blood is returned to the venous or arterial circulation (veno-venous or veno-arterial ECMO respectively).
The development of new circuits and devices made this therapy become safer and more useful, improving outcomes in ARDS so that its application has been widespread to many centres all over the world.61,71 The three advantages that ECMO offers are:
- 1.
It increases PaO2 levels, thus relieving hypoxaemia.
- 2.
CO2 removal allows to reduce tidal volume below 6ml/kg. In this sense, ultraprotective strategies with a volume of 3ml/kg have been demonstrated to be feasible in ARDS patients. The additional benefit of this strategy is to be demonstrated.47,72
- 3.
A venoarterial shunt can alleviate a failing right ventricle.73–76
Nevertheless, there are many complications related to this technique so patients requiring ECMO should be transferred to high case volume ECMO centres where outcomes have been demonstrated to be better.77,78
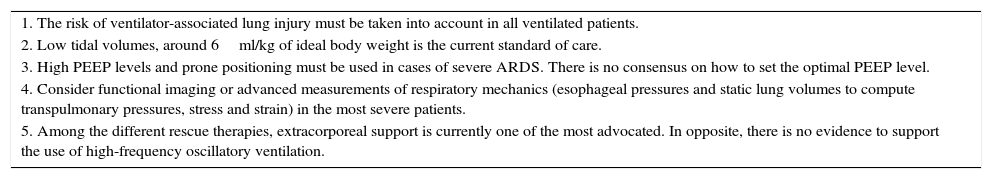
ConclusionsOne of the major goals to achieve during mechanical ventilation is the reduction of the ventilator-associated lung injury. To do so, the clinician must know the mechanisms of VALI and its relationships with ventilatory settings. Although a general approach using low tidal volumes and moderate PEEP levels is currently accepted, finding the optimal settings for a given patient is a difficult task. The emergence of new technologies and developments in classic strategies such as respiratory mechanics may provide some additional help. Table 2 provides some recommendations based on the current evidences. However, the best approach to minimize VALI is yet an unresolved question.
Recommendations on ventilatory settings.
| 1. The risk of ventilator-associated lung injury must be taken into account in all ventilated patients. |
| 2. Low tidal volumes, around 6ml/kg of ideal body weight is the current standard of care. |
| 3. High PEEP levels and prone positioning must be used in cases of severe ARDS. There is no consensus on how to set the optimal PEEP level. |
| 4. Consider functional imaging or advanced measurements of respiratory mechanics (esophageal pressures and static lung volumes to compute transpulmonary pressures, stress and strain) in the most severe patients. |
| 5. Among the different rescue therapies, extracorporeal support is currently one of the most advocated. In opposite, there is no evidence to support the use of high-frequency oscillatory ventilation. |
Supported by Fundación para el fomento en Asturias de la investigación científica aplicada y la tecnología (FICYT, GRUPIN14-089, Consejería de Hacienda, Principado de Asturias, Spain). GMA is the recipient of a grant from Instituto de Salud Carlos III (INT-15/002). Instituto Universitario de Oncología is supported by Fundación Bancaria Caja de Ahorros de Asturias.
Conflict of interestThe authors have no conflict of interest to disclose.