To describe outcomes of critically ill patients with COVID-19, particularly the association of renal replacement therapy to mortality.
DesignA single-center prospective observational study was carried out.
SettingICU of a tertiary care center.
PatientsConsecutive adults with COVID-19 admitted to the ICU.
InterventionRenal replacement therapy.
Main variables of interestDemographic data, medical history, illness severity, type of oxygen therapy, laboratory data and use of renal replacement therapy to generate a logistic regression model describing independent risk factors for mortality.
ResultsOf the total of 166 patients, 51% were mechanically ventilated and 26% required renal replacement therapy. The overall hospital mortality rate was 36%, versus 56% for those requiring renal replacement therapy, and 68% for those with both mechanical ventilation and renal replacement therapy. The logistic regression model identified four independent risk factors for mortality: age (adjusted OR 2.8 [95% CI 1.8–4.4] for every 10-year increase), mechanical ventilation (4.2 [1.7–10.6]), need for continuous venovenous hemofiltration (2.3 [1.3–4.0]) and C-reactive protein (1.1 [1.0–1.2] for every 10mg/L increase).
ConclusionsIn our cohort, acute kidney injury requiring renal replacement therapy was associated to a high mortality rate similar to that associated to the need for mechanical ventilation, while multiorgan failure necessitating both techniques implied an extremely high mortality risk.
Describir los resultados de pacientes críticamente enfermos con COVID-19, especialmente la asociación de la terapia de reemplazo renal con la mortalidad.
DiseñoEstudio observacional, prospectivo y unicéntrico.
ÁmbitoEn la unidad de cuidados intensivos (UCI) de un centro de atención terciaria.
PacientesPacientes adultos con COVID-19 ingresados de forma consecutiva en la UCI.
IntervenciónAdministración de terapia de reemplazo renal.
Variables de interés principalesDatos demográficos, antecedentes médicos, gravedad de la enfermedad, tipo de oxigenoterapia, datos analíticos y uso de terapia de reemplazo renal para generar un modelo de regresión logística que describa factores de riesgo independientes de la mortalidad.
ResultadosDe los 166 pacientes, el 51% recibieron ventilación mecánica (VM) y el 26% requirió terapia de reemplazo renal (TRR). La mortalidad hospitalaria global fue del 36%, frente al 56% en el caso de los pacientes que requirieron TRR y el 68% en el subconjunto de pacientes que necesitó tanto VM como RTT. Un modelo de regresión logística señala cuatro factores de riesgo independientes de la mortalidad: edad (OR ajustada: 2,8 [IC del 95%: 1,8-4,4] por cada incremento de 10 años), ventilación mecánica (4,2 [1,7-10,6]), necesidad de hemofiltración venovenosa continua (HVVC) (2,3 [1,3-4,0]), y proteína C reactiva (1,1 [1,0-1,2] por cada incremento de 10mg/L).
ConclusionesEn nuestra cohorte, la lesión renal aguda que necesita TRR se asocia con una mortalidad similarmente elevada a la de los pacientes que requieren VM, y la insuficiencia multiorgánica que hace necesarias ambas intervenciones se asocia con un riesgo de mortalidad extremadamente alta.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global pandemic that has started to plateau or decline in many regions, while it continues to spread in others – including multiple areas throughout the United States.1,2 While the substantial mortality associated with mechanical ventilation has been established, there is emerging evidence regarding the pathogenesis of acute kidney injury (AKI) and related adverse outcomes.
SARS-CoV-2 is associated with the development of AKI via inflammatory multi-system organ failure or direct viral kidney toxicity.3,4 AKI in SARS-CoV-2 has been associated with worse outcomes5–8 and when severe enough to warrant renal replacement therapy (RRT), it can put a strain on an institution's capacity. Not only are the machines used for RRT a finite resource, but a patient on continuous renal replacement therapy (CRRT) also requires placement of specific venous access, physician and nursing expertise and typically a reduced (and often 1:1) nurse to patient ratio. While there has been a focus on the scarcity of ventilators and ICU bed availability,5,9 the dialysis machines and nursing expertise are typically scarcer, and the required resources are not available at all institutions. While older studies have suggested that RRT may potentially have beneficial effects in disease processes such as sepsis and Acute Respiratory Distress Syndrome (ARDS),10,11 other studies have called this into question12–14 and the use of CRRT was correlated with a higher mortality in during the Middle East Respiratory Coronavirus (MERS-CoV) outbreak.15 A recent meta-analysis showed that 20% of COVID-19 patients may need CRRT (rates varying from 5 to 60%), and so its effect on outcomes is of particular interest.16
Therefore, our goal is to describe the experience and outcomes of critically ill patients with COVID-19 in an urban tertiary care center,17,18 in particular the association of RRT with mortality. Additionally, we explore patient level features that might influence this outcome.
Patients and methodsThis is a prospective observational study of critically ill patients with COVID-19 from Medstar Georgetown University Hospital. The study was approved by the Institutional Review Board of Georgetown University. All consecutive patients were included if treated in the medical intensive care unit (ICU) for laboratory confirmed SARS-COV-2 from our first ICU admission on 3/8/2020 until 5/31/2020. Testing was performed internally by the microbiology laboratory at the hospital.
The following information was recorded: demographics (age, sex, race, BMI), past medical history, ICU length of stay, hospital outcome, type of oxygen therapy administered (high flow nasal cannula (HFNC) or mechanical ventilation (MV)) and for how long, proning, administration of renal replacement therapy (RRT), therapeutics targeted toward COVID-19 (full dose anticoagulation, steroids, tocilizumab, convalescent plasma, remdesivir) and laboratory data thought relevant based on available literature19,20 (absolute lymphocyte count, C-reactive protein, d-dimer, IL-6, troponin). We additionally calculated the sequential organ failure assessment (SOFA) score for the first 24h in the ICU.21–23 Data was obtained directly from the health system's clinical data warehouse and supplemented with direct review of patient records when needed.
Steroid use was recorded as a binary variable (Yes or No) if at least 40mg of methylprednisolone or equivalent were given for a period of at least five days for the purpose of treating inflammation associated with the viral pneumonia. Notably, this practice was empiric and prior to more recent information about the use of steroids for the treatment of COVID-19 pneumonia.24 Anticoagulation (Yes or No) refers to therapeutic dosing of intravenous heparin or subcutaneous enoxaparin administered in the ICU. A relatively robust program for procuring convalescent plasma was developed at the hospital; provision was based on availability and blood type matching as decided by the treating intensivist in cooperation with the blood bank. A limited supply of remdesivir was made available after the publication demonstrating its benefit25 and was used exclusively after that point on a case-by-case basis. The use of HFNC was recorded if newly initiated for respiratory failure, not if extubated from mechanical ventilation to HFNC.
The decision to administer RRT and the choice between continuous veno-venous hemofiltration (CVVH) and intermittent hemodialysis (iHD) is made by the treating intensivist in conjunction with a consulting nephrologist and is done in accordance with society guidelines.26 Generally, this is based on hemodynamics, fluid balance, acid base status, electrolyte derangements and initiation is no sooner than absolutely needed. Our hospital uses NxStage brand hemodialysis machines and electrolyte replacement bags. We defined chronic kidney disease as Kidney Disease: Improving Global Outcomes (KDIGO) stage 3 or higher27 and end stage renal disease (ESRD) as requiring intermittent hemodialysis.
Summary statistics describe the frequency of each categorical variable and either mean (for parametric) or median (for nonparametric) of continuous variables. In comparisons between subjects who survived and died, continuous variables were analyzed using the student t-test for parametric or Wilcoxon rank sum test for nonparametric data. Categorical variables were compared via with Chi-Square or Fischer's Exact test as appropriate. Of note, p-values are not provided for differences in administration of therapeutics (medications and convalescent plasma) as practices regarding their use varied in terms of specific indication, timing of initiation, dosing and length of therapy making hypothesis testing unreliable. Data were only missing for laboratory measurements, and we elected not to impute these (as it is not clear these values were missing at random). We included variables with statistically significant univariate associations as candidates in a logistic regression model with hospital survival as the dependent variable of interest, developed in a stepwise fashion with a stopping rule based on minimum Bayesian Information Criterion (BIC). However, we excluded specific therapeutics (for the reasons above) and laboratory data with >30% missing values. The analysis was performed with the use of JMP Pro 15 (Cary, NC).
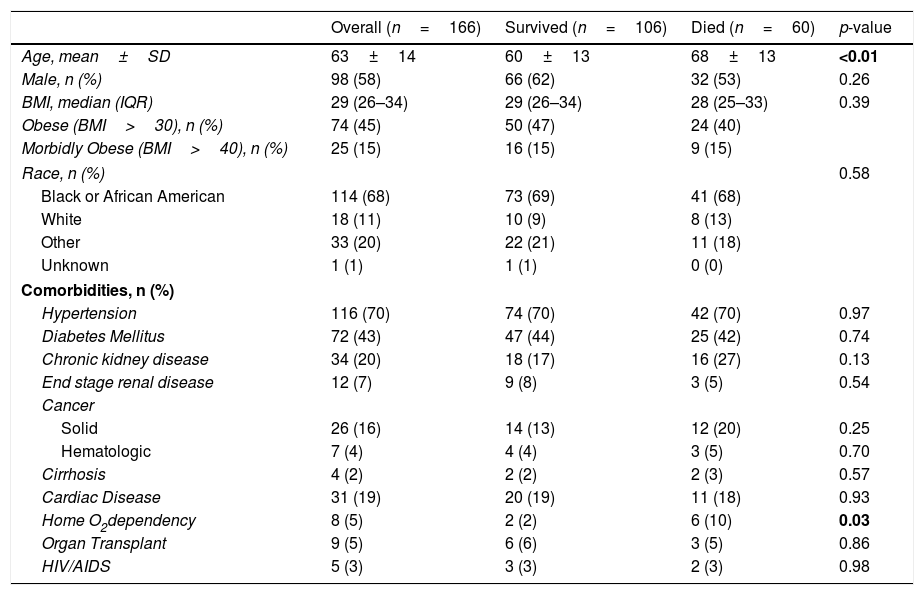
ResultsPatient characteristicsA total of 166 unique admissions to the ICU occurred over the study period, 60 of who died (36%), the remainder discharged from the hospital alive. Demographic features and past medical history are described in Table 1. The patients ranged from 16 to 96 years old, 58% were male and 68% Black. The mortality at the mean age (63 years old) or above was 53% (n=94); below, 23% (n=77).
Patient Demographics.
| Overall (n=166) | Survived (n=106) | Died (n=60) | p-value | |
|---|---|---|---|---|
| Age, mean±SD | 63±14 | 60±13 | 68±13 | <0.01 |
| Male, n (%) | 98 (58) | 66 (62) | 32 (53) | 0.26 |
| BMI, median (IQR) | 29 (26–34) | 29 (26–34) | 28 (25–33) | 0.39 |
| Obese (BMI>30), n (%) | 74 (45) | 50 (47) | 24 (40) | |
| Morbidly Obese (BMI>40), n (%) | 25 (15) | 16 (15) | 9 (15) | |
| Race, n (%) | 0.58 | |||
| Black or African American | 114 (68) | 73 (69) | 41 (68) | |
| White | 18 (11) | 10 (9) | 8 (13) | |
| Other | 33 (20) | 22 (21) | 11 (18) | |
| Unknown | 1 (1) | 1 (1) | 0 (0) | |
| Comorbidities, n (%) | ||||
| Hypertension | 116 (70) | 74 (70) | 42 (70) | 0.97 |
| Diabetes Mellitus | 72 (43) | 47 (44) | 25 (42) | 0.74 |
| Chronic kidney disease | 34 (20) | 18 (17) | 16 (27) | 0.13 |
| End stage renal disease | 12 (7) | 9 (8) | 3 (5) | 0.54 |
| Cancer | ||||
| Solid | 26 (16) | 14 (13) | 12 (20) | 0.25 |
| Hematologic | 7 (4) | 4 (4) | 3 (5) | 0.70 |
| Cirrhosis | 4 (2) | 2 (2) | 2 (3) | 0.57 |
| Cardiac Disease | 31 (19) | 20 (19) | 11 (18) | 0.93 |
| Home O2dependency | 8 (5) | 2 (2) | 6 (10) | 0.03 |
| Organ Transplant | 9 (5) | 6 (6) | 3 (5) | 0.86 |
| HIV/AIDS | 5 (3) | 3 (3) | 2 (3) | 0.98 |
The median number of chronic medical conditions was 2 and interquartile range (1–3). The only medical condition that differed between those that survived and died is underlying pulmonary disease (of any type) severe enough to require home oxygen (Table 1); however, there were only eight such individuals. The total number of chronic medical conditions did not predict the need for mechanical ventilation or mortality.
The median ICU length of stay was 11 days (7–15); it was longer among those who died 12 (8–17) than those who survived 8 (4–13), p=0.03.
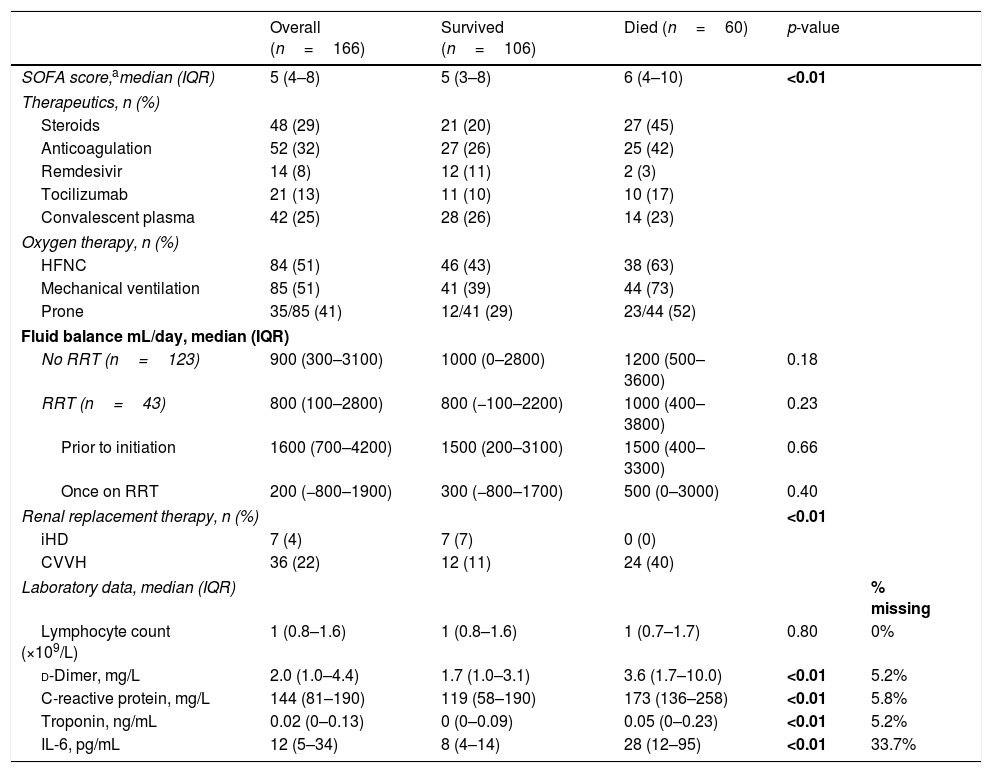
Oxygen therapyEighty-four (51%) of the patients were initiated on high flow nasal cannula (HFNC) and 85 (51%) required mechanical ventilation (MV) (Table 2). Fifty percent (42/84) of those initiated on HFNC ultimately required MV, with a median time on HFNC prior to intubation of 24h (14–65). The median time on HFNC for those who did not require intubation was 114h (55–174). Those on HFNC who ultimately required intubation had a lower nadir PaO2/FiO2 in the first 24h of their ICU stay: 96 vs. 143 (p<0.01).
ICU course.
| Overall (n=166) | Survived (n=106) | Died (n=60) | p-value | ||
|---|---|---|---|---|---|
| SOFA score,amedian (IQR) | 5 (4–8) | 5 (3–8) | 6 (4–10) | <0.01 | |
| Therapeutics, n (%) | |||||
| Steroids | 48 (29) | 21 (20) | 27 (45) | ||
| Anticoagulation | 52 (32) | 27 (26) | 25 (42) | ||
| Remdesivir | 14 (8) | 12 (11) | 2 (3) | ||
| Tocilizumab | 21 (13) | 11 (10) | 10 (17) | ||
| Convalescent plasma | 42 (25) | 28 (26) | 14 (23) | ||
| Oxygen therapy, n (%) | |||||
| HFNC | 84 (51) | 46 (43) | 38 (63) | ||
| Mechanical ventilation | 85 (51) | 41 (39) | 44 (73) | ||
| Prone | 35/85 (41) | 12/41 (29) | 23/44 (52) | ||
| Fluid balance mL/day, median (IQR) | |||||
| No RRT (n=123) | 900 (300–3100) | 1000 (0–2800) | 1200 (500–3600) | 0.18 | |
| RRT (n=43) | 800 (100–2800) | 800 (−100–2200) | 1000 (400–3800) | 0.23 | |
| Prior to initiation | 1600 (700–4200) | 1500 (200–3100) | 1500 (400–3300) | 0.66 | |
| Once on RRT | 200 (−800–1900) | 300 (−800–1700) | 500 (0–3000) | 0.40 | |
| Renal replacement therapy, n (%) | <0.01 | ||||
| iHD | 7 (4) | 7 (7) | 0 (0) | ||
| CVVH | 36 (22) | 12 (11) | 24 (40) | ||
| Laboratory data, median (IQR) | % missing | ||||
| Lymphocyte count (×109/L) | 1 (0.8–1.6) | 1 (0.8–1.6) | 1 (0.7–1.7) | 0.80 | 0% |
| d-Dimer, mg/L | 2.0 (1.0–4.4) | 1.7 (1.0–3.1) | 3.6 (1.7–10.0) | <0.01 | 5.2% |
| C-reactive protein, mg/L | 144 (81–190) | 119 (58–190) | 173 (136–258) | <0.01 | 5.8% |
| Troponin, ng/mL | 0.02 (0–0.13) | 0 (0–0.09) | 0.05 (0–0.23) | <0.01 | 5.2% |
| IL-6, pg/mL | 12 (5–34) | 8 (4–14) | 28 (12–95) | <0.01 | 33.7% |
Represented here are the patient's severity of illness (SOFA score), specific therapeutics and ICU interventions. Of note, p-values are not provided for oxygen therapy (discussed in Results, “Oxygen Therapy”) or therapeutics (varied availability and usage patterns make hypothesis testing unreliable).
Of the 85 mechanically ventilated patients, 44 died (52%) and overall time on the ventilator did not vary among those who died vs. those who survived: 12 days (6–22) vs. 11 days (7–20) respectively (p=0.48). However, those that were proned at least once (n=35, 41%) were on the ventilator significantly longer (for a median of 19 days [11–25] vs. 9 days [5–12], p<0.01)) and had a higher hospital mortality (66% vs. 42%, p=0.03). Ten patients received a tracheostomy (seven underwent a percutaneous bedside procedure performed by the intensivist group, three had an operating room procedure performed by an otolaryngologist), of whom nine were discharged from the hospital alive.
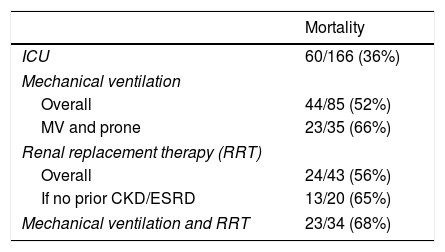
Renal replacement and outcomesA total of 43 patients (26%) required renal replacement (RRT), of whom 31 (19%) did not have pre-existing end-stage renal disease (ESRD) (Table 2). Seven received intermittent hemodialysis (iHD) in the ICU (five of whom had pre-existing ESRD); the remainder received continuous veno-venous hemofiltration (CVVH). Overall mortality associated with RRT was 56% (Table 3); however, excluding those with ESRD already chronically receiving iHD, the mortality associated with acute kidney injury requiring new RRT was 68% (21/31). The median days receiving RRT was 8 (2–15), which did not significantly vary by those who survived vs. died. Similarly, daily fluid balance did not differ by survival (Table 2); however, in the subset who received RRT, once initiated, fluid balance was lower than prior to initiation: 1600mL/day IQR (700–4200) vs. 200 (−800–1900), p<0.01.
Mechanically ventilated patients were more likely to require renal replacement: 34/85 (40%) vs. 9/81 (11%) (p<0.01) and this combination was associated with a mortality of 68% (Table 3). Individuals whose respiratory failure was severe enough to require proning along with receiving RRT had a 76% mortality (n=17).
A multivariate logistic regression model with survival as the outcome finds four independent risk factors for mortality in our cohort: age (adjusted OR 2.8 [95% CI 1.8–4.4] for every 10-year increase), mechanical ventilation (4.2 [1.7–10.6]), need for CVVH (2.3 [1.3–4.0]), and C-rp (1.1 [1.0–1.2] for every increase of 10mg/L). IL-6 was not included as a candidate variable despite the statistically significant univariate association given the number of missing values (33.7%).
DiscussionA defining characteristic of the SARS-CoV2 pandemic has been respiratory failure and the need for mechanical ventilation, stressing health care systems to the limit of available personnel and equipment. Initial reports describe exceptionally high mortality in mechanically ventilated individuals ranging as high as 65–81%5–7,19–22,28–37; more recent show mortality approaching “traditional” ARDS of any cause.5,7,20,33,38,39 While the focus on the management of respiratory failure in the pandemic is crucial, we describe that the need for renal replacement therapy (RRT) is common and carries a similar mortality risk as the need for mechanical ventilation (MV), and the combination carries a significantly higher risk.
The new development of AKI informs the mortality associated with RRT. All seven patients who received intermittent hemodialysis (iHD) in ICU but never needed continuous veno-venous hemofiltration (CVVH) survived; five had a history of end-stage renal disease (ESRD) and therefore required dialysis while hospitalized but did not necessarily have poor hemodynamics necessitating continuous dialysis. Of those that required CVVH, the mortality was higher for the patients that had no history of prior kidney disease (56% vs. 65%). It appears that patients with AKI severe enough to necessitate RRT without a history CKD represent a cohort that developed more severe multi-system organ failure that was ultimately manifested by a higher mortality rate. If the substantial mortality rates associated with the need for MV, proning, and RRT mirrors the experience elsewhere, this information could be used to help guide discussions with families or surrogate decision makers.
In this cohort, comorbid illness, sex, and race did not predict the need for either MV or mortality, in contrast to previous studies.5–7,19,21,28–30,33,36,40 Since this group was composed exclusively of the already critically ill, perhaps these demographic features and past medical history are less predictive of adverse outcomes. This is speculative, however. Although the need for home oxygen is associated with mortality, this finding should be interpreted cautiously given the small number of patients and may be artifactual due to multiple comparisons.
Age, C-rp, and the need for MV or RRT were independent predictors of mortality in a logistic regression model. The association of age with adverse outcomes has been extensively reported, as has the association of specific lab values.5,7,21,22,28,29,34,36,37 Notably, laboratory data in our cohort was not tracked serially and therapies were not protocolized based on values. It remains an open question as to whether or not the measured lab values are part of a causative pathway worthy of therapeutic targeting or monitoring, or if they are mediated by the disease severity. Though the use of specific therapeutics also differed in survivors, this is likely reversely causal as we elected to use our limited supply of remdesivir in those who appear poised to survive, and anticoagulation and steroids for the most severely ill.
Our study is one of the first description of outcomes related specifically to RRT. The vast majority of our patients had a discharge disposition at the time of writing, leading to a more complete description of outcomes than previous studies. Differences in ICU admission criteria may lead to some differences in mortality between centers; for example, our ICU accepts any patient who is initiated on HFNC while at other institutions, these patients may be triaged to an intermediate care unit, altering overall ICU mortality in comparison. Like many institutions, we initially avoided non-invasive ventilation due to fear of spread via aerosolization, and this may have influenced the proportion of patients on MV. There are a handful of notable limitations, however. This is a single center experience, and measurements were made in the routine care of patients rather than accordance with a protocol. Additionally, most patients were cared for prior to recent clinical trials demonstrating the efficacy of remdesivir and dexamethasone. The impact of those therapies on AKI in this disease is as of yet unknown.
In conclusion, at our tertiary care center in a region with a substantial COVID-19 outbreak, the new requirement of RRT for AKI is associated with a similarly high mortality as the need for MV, and multiorgan failure necessitating both carries an extremely high mortality risk. Once intubated and initiated on RRT, the specific predictors of outcomes still remain elusive and require further study. We believe the availability and rational use of dialysis machines is central to the discussion regarding resource utilization during the pandemic.
FundingThere was no funding for this project.
Author contributionsRajiv Sonti, Elena Burke, C. William Pike and Erin Haber all contributed to the design of the study, helped acquire data. Rajiv Sonti interpreted data. Rajiv Sonti, Elena Burke, C. William Pike and Erin Haber helped draft the manuscript and approved its submission.
Conflict of interestThe authors declare there is no conflict of interest.