Infection by the SARS-CoV-2 virus, known as COVID-19 (COronaVIrus Disease-19) was initially detected in China in December 2019, and has subsequently spread rapidly throughout the world, to the point that on March 11 the World Health Organization (WHO) reported that the outbreak could be defined as a pandemic. COVID-19 disease ranges from mild flu-like episodes to other serious and even life-threatening conditions, mainly due to acute respiratory failure. These patients are frequently admitted to our Intensive Care Units in relation to acute respiratory distress syndrome (ARDS). The lack of a treatment based on scientific evidence has led to the use of different management guidelines, in many cases with rapid changes in the applied protocols. Recent reviews in reputed journals have underscored the lack of proven therapies and the need for clinical trials to establish clear and objective treatment guidelines. The present study provides an update on the currently applied treatment, and intends to offer help in relation to daily care, without seeking to replace the protocols adopted in each individual center.
La infección por el virus SARS-CoV-2, denominada COVID-19 (COronaVIrus Disease 19), fue detectada inicialmente en China en diciembre 2019, y posteriormente se ha diseminado rápidamente por todo el mundo, hasta el punto de que el 11 de marzo la OMS declaró que el brote podría definirse como pandemia. La COVID-19 presenta un cuadro que oscila desde episodios leves pseudogripales a otros graves e incluso potencialmente mortales debido, sobre todo, a insuficiencia respiratoria aguda. Es frecuente el ingreso de estos pacientes en nuestros Servicios de Medicina Intensiva en relación con un Síndrome de Distrés Respiratorio Agudo (SDRA). La falta de un tratamiento con evidencia científica ha llevado al empleo de diferentes pautas terapéuticas, en muchas ocasiones, con modificaciones rápidas de los protocolos. Recientes revisiones en revistas de prestigio han destacado la falta de terapias probadas y la necesidad de ensayo clínicos que permitan establecer pautas de tratamiento claras y objetivas. Este documento tiene por objeto ofrecer una actualización de la terapia que se está aplicando en la actualidad, y una ayuda en la asistencia diaria, sin pretender sustituir los protocolos adoptados en cada centro.
Infection due to SARS-CoV-2 virus, the so-called COVID-19 (COronaVIrus Disease 19), was initially detected in China back in December 2019,1 and it subsequently spread quickly throughout the world, to the extent that on March 11th the WHO declared this outbreak a global pandemic.2 In our country, the SEMICYUC has devised a Contingency Plan to arrange medical care for critically ill patients with COVID-19.3 SARS-CoV-2 causes clinical signs that go from mild flu-like symptoms to severe and even potentially deadly episodes, especially, acute respiratory distress. These patients are often admitted to our Intensive Care Unit with acute respiratory distress syndrome (ARDS).4–6 The lack of a treatment backed with enough scientific evidence has led to the use of different therapeutic guidelines and quick modifications of the protocols. Recent reviews and editorials have looked into the absence of proven therapies and need to conduct clinical trials to be able to establish evidence-based clinical practice guidelines.7–9
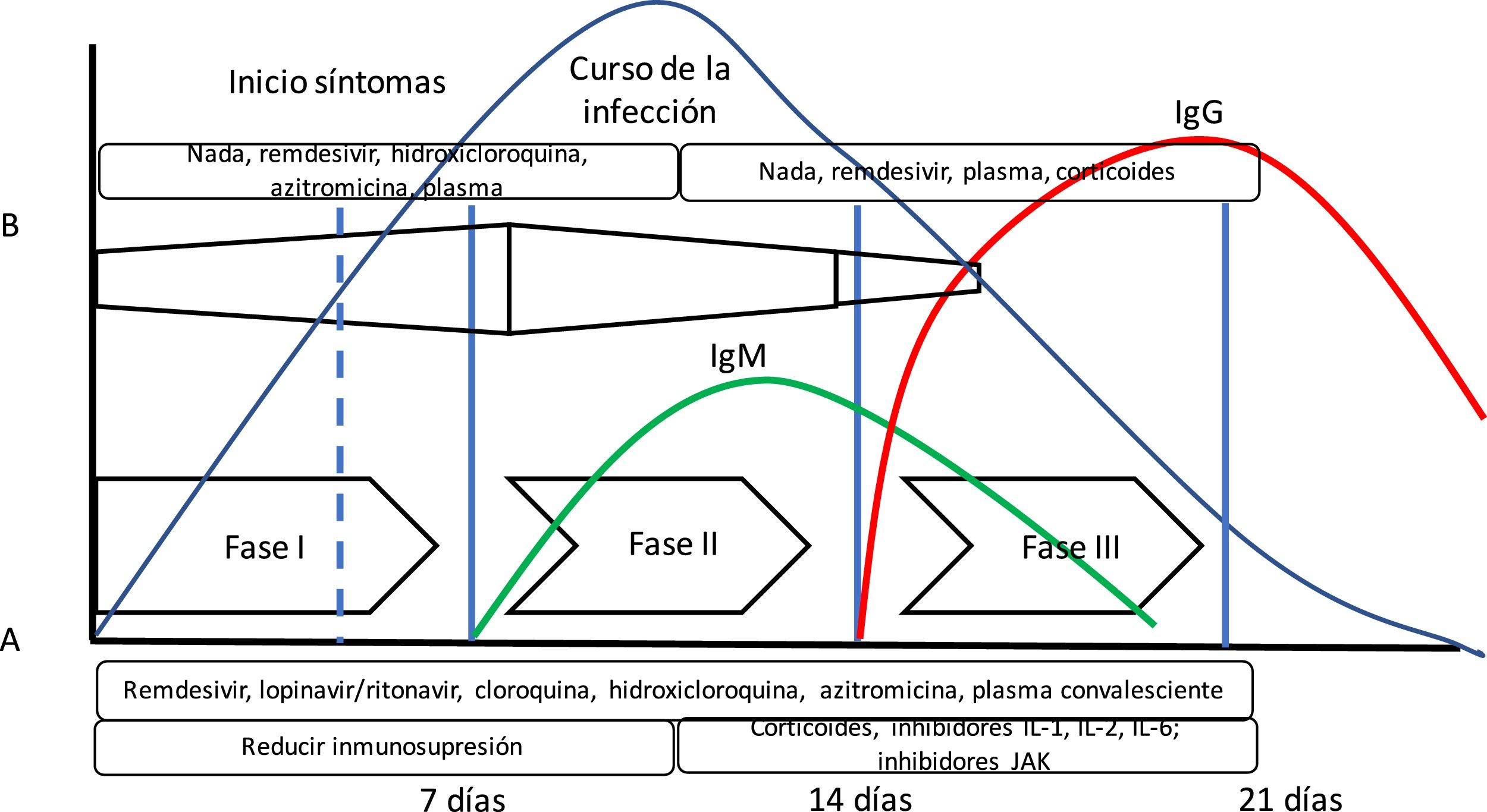
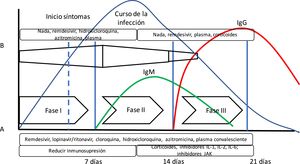
The theory that COVID-19 disease goes through several stages of progression has been suggested. The initial stage would be characterized by a high viral load; therefore, this would be the best time to administer effective antiviral therapies. In a second stage, the inflammatory response is predominant (we could even talk about a cytokine storm syndrome) where anti-inflammatory drugs play an important role.10 (Fig. 1). However, we have not been able to confirm this theory yet.
Evolution of SARS-CoV-2 infection. The diagram shows the arrangement of the course of SARS-CoV-2 infection and the immune response measured by antibodies. Also, the following has been added: (A) Siddiqi’s and Mehra’s10 proposal of clinical stages and the evolution of adaptative immune response mediated by antibody production and the evolution of a common viral infection. Siddiqi and Mehra propose a clinical staging system in 3 different stages to facilitate the use of a uniform nomenclature. Thus, they propose a stage i or early infection stage, a stage ii or pulmonary stage, and a stage iii or hyperinflammatory stage together with potential treatment in each stage. However, this is somehow different from the body’s immune response to viral infection (B). In this bodily response to a viral infection, the innate immune response kicks in right at the beginning of the infection until the adaptative immune response is built with the production of antibodies just a few days later. Thus, some drugs used to treat COVID-19 block interleukin-1 that activates T cells or interleukin-6 that participates in B-cell maturation, the cells that will eventually build the antibodies.
In order to write this document, a bibliographic search was conducted on PubMed using the keywords COVID-19 or SARS-CoV-2 or coronavirus and treatment or therapy. Of the different drugs used, some not very much used today have not been reviewed like ribavirin or ivermectin. The objective of this document is to provide an update on the therapy used today and help in the routine daily care without having to replace the protocols adopted at each center.
Antiviral therapyLopinavir/ritonavirLopinavir is a protease inhibitor used for the management of the human immunodeficiency virus that shows in vitro activity against SARS-CoV-1 virus, causative agent of the severe acute respiratory syndrome (SARS) back in 2003.11 The combination with ritonavir extends its half-life. It also shows activity against the coronavirus that causes the MERS-CoV (Middle East respiratory syndrome).12
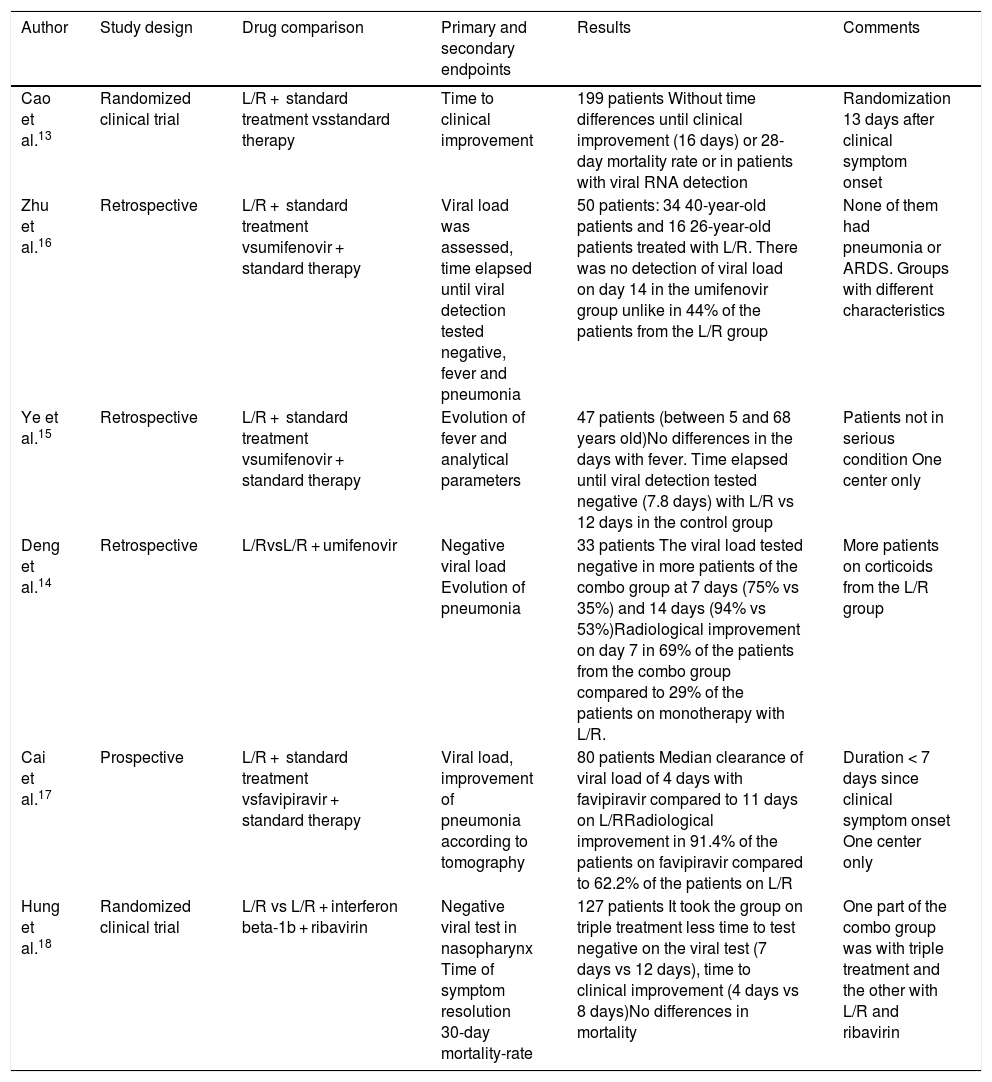
Based on its activity against other coronaviruses, it has been suggested that it could be effective against SARS-CoV-2. However, in the first random clinical trial (that included 199 patients) that has been published recently, its use does not seem to show any improvement compared to the standard therapy.13 A total of 199 patients were included (around 15% received high-flow oxygen therapy [HFOT] or non-invasive mechanical ventilation [NIMV], and only 2 invasive mechanical ventilation [MV]). These patients were randomized with a median clinical progression of 13 days (in the subgroup of less than 12 days no differences were seen either). The different studies are shown on Table 1.13–18
Studies with lopinavir/ritonavir.
| Author | Study design | Drug comparison | Primary and secondary endpoints | Results | Comments |
|---|---|---|---|---|---|
| Cao et al.13 | Randomized clinical trial | L/R + standard treatment vsstandard therapy | Time to clinical improvement | 199 patients Without time differences until clinical improvement (16 days) or 28-day mortality rate or in patients with viral RNA detection | Randomization 13 days after clinical symptom onset |
| Zhu et al.16 | Retrospective | L/R + standard treatment vsumifenovir + standard therapy | Viral load was assessed, time elapsed until viral detection tested negative, fever and pneumonia | 50 patients: 34 40-year-old patients and 16 26-year-old patients treated with L/R. There was no detection of viral load on day 14 in the umifenovir group unlike in 44% of the patients from the L/R group | None of them had pneumonia or ARDS. Groups with different characteristics |
| Ye et al.15 | Retrospective | L/R + standard treatment vsumifenovir + standard therapy | Evolution of fever and analytical parameters | 47 patients (between 5 and 68 years old)No differences in the days with fever. Time elapsed until viral detection tested negative (7.8 days) with L/R vs 12 days in the control group | Patients not in serious condition One center only |
| Deng et al.14 | Retrospective | L/RvsL/R + umifenovir | Negative viral load Evolution of pneumonia | 33 patients The viral load tested negative in more patients of the combo group at 7 days (75% vs 35%) and 14 days (94% vs 53%)Radiological improvement on day 7 in 69% of the patients from the combo group compared to 29% of the patients on monotherapy with L/R. | More patients on corticoids from the L/R group |
| Cai et al.17 | Prospective | L/R + standard treatment vsfavipiravir + standard therapy | Viral load, improvement of pneumonia according to tomography | 80 patients Median clearance of viral load of 4 days with favipiravir compared to 11 days on L/RRadiological improvement in 91.4% of the patients on favipiravir compared to 62.2% of the patients on L/R | Duration < 7 days since clinical symptom onset One center only |
| Hung et al.18 | Randomized clinical trial | L/R vs L/R + interferon beta-1b + ribavirin | Negative viral test in nasopharynx Time of symptom resolution 30-day mortality-rate | 127 patients It took the group on triple treatment less time to test negative on the viral test (7 days vs 12 days), time to clinical improvement (4 days vs 8 days)No differences in mortality | One part of the combo group was with triple treatment and the other with L/R and ribavirin |
L/R, lopinavir/ritonavir.
The most common adverse effects are diarrhea, nausea, vomiting, hypertriglyceridemia, and hypercholesterolemia. Patients can also present with pancreatitis or a prolonged QT interval. We should take into consideration that both elements are inhibitors of cytochrome P450 (CYP450) isoforms, which favors interactions with different drugs commonly used at the ICU setting.
RemdesivirRemdesivir is a prodrug of the group of nucleotide analogs. It is metabolized intracellularly into an active triphosphate adenosine analog that inhibits viral polymerase RNA. It has broad-spectrum activity against viruses of the filovirus family (Ebola virus, Marburg virus), coronaviruses (SARS-CoV-1, MERS-CoV), and paramyxovirus (respiratory syncytial virus) among others. Remdesivir has also been evaluated as a possible prophylaxis in animal models against MERS and SARS infections.19
Remdesivir shows fewer interactions compared to other antiviral drugs with a safety profile proven in stage 1 studies in over 500 patients with Ebola virus infection.
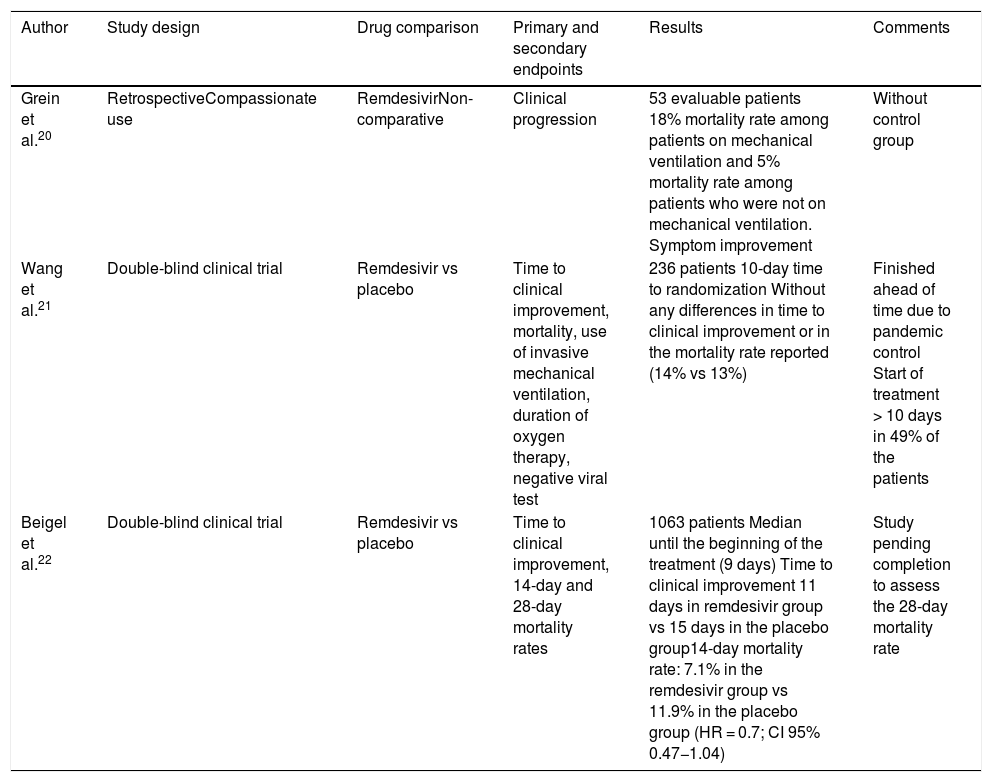
Recently, several studies have been published on the use of remdesivir to treat COVID-19 (Table 2). The first one evaluated the compassionate use of remdesivir to treat patients with COVID-19.20 A total of 57% of the 53 patients analyzed received MV compared to 8% who received ECMO. The overall mortality rate was 13% (18% among patients on MV and 5% among those who were not ventilated).
Studies with remdesivir.
| Author | Study design | Drug comparison | Primary and secondary endpoints | Results | Comments |
|---|---|---|---|---|---|
| Grein et al.20 | RetrospectiveCompassionate use | RemdesivirNon-comparative | Clinical progression | 53 evaluable patients 18% mortality rate among patients on mechanical ventilation and 5% mortality rate among patients who were not on mechanical ventilation. Symptom improvement | Without control group |
| Wang et al.21 | Double-blind clinical trial | Remdesivir vs placebo | Time to clinical improvement, mortality, use of invasive mechanical ventilation, duration of oxygen therapy, negative viral test | 236 patients 10-day time to randomization Without any differences in time to clinical improvement or in the mortality rate reported (14% vs 13%) | Finished ahead of time due to pandemic control Start of treatment > 10 days in 49% of the patients |
| Beigel et al.22 | Double-blind clinical trial | Remdesivir vs placebo | Time to clinical improvement, 14-day and 28-day mortality rates | 1063 patients Median until the beginning of the treatment (9 days) Time to clinical improvement 11 days in remdesivir group vs 15 days in the placebo group14-day mortality rate: 7.1% in the remdesivir group vs 11.9% in the placebo group (HR = 0.7; CI 95% 0.47−1.04) | Study pending completion to assess the 28-day mortality rate |
Among the randomized clinical trials conducted, Wang et al. evaluated remdesivir vs placebo in 237 adult patients in Hubei, China.21 They randomized patients with symptoms of up to 12-day duration since symptom onset (median, 11 days). Concomitant use of lopinavir-ritonavir, interferon or corticoids was allowed. Treatment with remdesivir was not associated with clinical improvement.
Beigel et al.22 randomized 1059 patients to receive remdesivir or placebo with a median of 9 days since the symptom onset. Recovery time was shorter in patients treated with remdesivir (11 days vs 15 days), although this difference was not seen in patients on MV. The 14-day mortality rate was 7.1% with remdesivir and 11.9% with placebo (0.47−1.04). The assessment of the mortality rate after 28 days is still pending. On the other hand, a study that compared treatment with remdesivir in 5 or 10-day clinical courses (in patients without MV) did not show any differences in their clinical condition on day 14.23
Adverse effects have been detected such as anemia or a lower glomerular filtration rate in up to 28.8% of the patients, with a similar incidence in the group that received placebo. The appearance of arterial hypotension during infusion has also been observed.22
The Spanish Ministry of Health has recently approved its use in patients with COVID-19who remain hospitalized in serious condition.24
Hydroxychloroquine and azithromycinHydroxychloroquine is a 4-aminoquinoline antimalarial drug that has proven to have in vitro activity against several RNA viruses including SARS-CoV-2.25 However, the potential effect of this drug in vivo is still unknown today.
It is believed that hydroxychloroquine acts through multiple mechanisms:26 viral entry inhibition, viral release inhibition into the host cell, blocking the activation of endosomal proteases, reducing viral infectivity, and immune modulation.
Compared to chloroquine, in in vitro studies, hydroxychloroquine has proven to be more powerful inhibiting COVID-19.27,28 It has been confirmed that, using a safe hydroxychloroquine sulfate dose (6–6.5 mg/kg/day) serum levels of 1.4–1.5 μM are reached in humans, which is theoretically enough to inhibit infections due to SARS-CoV-2.29
Despite the theoretical benefits, the clinical trials published to date contribute evidence that is still inconsistent. In several controlled clinical trials conducted in Chinese hospitals, compared to controls, treatment with chloroquine has stopped the development of pneumonia, improved lung radiological imaging, speed up viral negativization, and shortened the course of the disease.30–32 However, these studies have important methodological limitations, and their results are still under discussion.
Azithromycin has also been used in association with hydroxychloroquine. A French study with a small sample size and other methodological shortcomings claimed that treatment with hydroxychloroquine sped up the virus conversion to the seronegative state. Also, that this situation improved when combined when used concomitantly with azithromycin.33
A multicenter, retrospective study compared hydroxychloroquine and azithromycin, both in combination or none of them.34 In this study, with 1438 hospitalized patients, treatment with hydroxychloroquine, azithromycin or a combination of both was not associated with an improved in-hospital mortality. Therefore, it necessary to generate more evidence on this regard, especially considering that both hydroxychloroquine and azithromycin are associated with a prolonged QT interval.
Currently, the effectiveness of hydroxychloroquine is being evaluated in, at least, 30 clinical trials.
Today the Spanish Ministry of Public recommends using hydroxychloroquine in the context of clinical trials.24
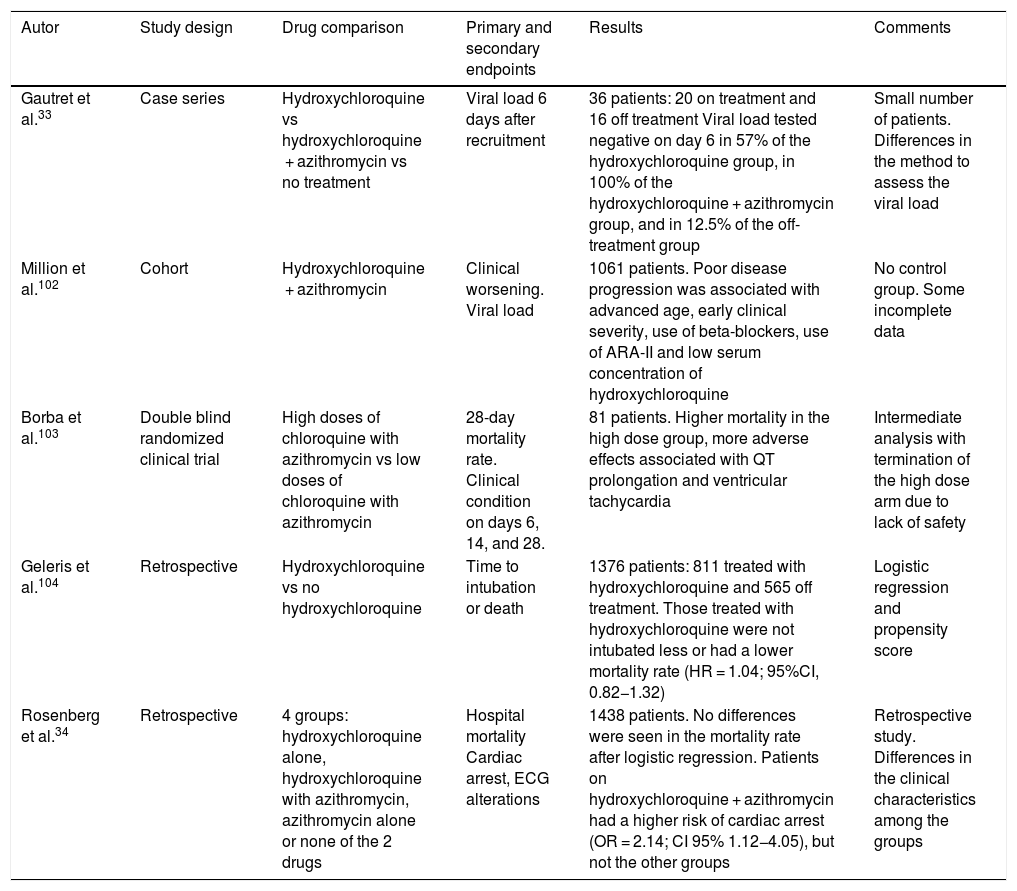
The use of hydroxychloroquine is contraindicated in concomitant treatment with natalizumab (used to treat multiple sclerosis) and agalsidase α or β (indicated to treat Fabry disease). Similarly, doses of hypoglycemic agents, digoxin, beta-blockers, and antipsychotic drugs (chlorpromazine, levomepromazine) should be adjusted since this promotes its effect. It should be used with caution in cases of myasthenia gravis, porphyria, retinal pathology, epilepsy (it reduces the seizure threshold), liver damage, kidney failure, 6-P-deshydrogenase deficiency. Extreme caution is required when co-administered with drugs that prolong the QT interval. Table 3 shows studies conducted with hydroxychloroquine and azithromycin therapies.
Studies with hydroxychloroquine and azithromycin.
| Autor | Study design | Drug comparison | Primary and secondary endpoints | Results | Comments |
|---|---|---|---|---|---|
| Gautret et al.33 | Case series | Hydroxychloroquine vs hydroxychloroquine + azithromycin vs no treatment | Viral load 6 days after recruitment | 36 patients: 20 on treatment and 16 off treatment Viral load tested negative on day 6 in 57% of the hydroxychloroquine group, in 100% of the hydroxychloroquine + azithromycin group, and in 12.5% of the off-treatment group | Small number of patients. Differences in the method to assess the viral load |
| Million et al.102 | Cohort | Hydroxychloroquine + azithromycin | Clinical worsening. Viral load | 1061 patients. Poor disease progression was associated with advanced age, early clinical severity, use of beta-blockers, use of ARA-II and low serum concentration of hydroxychloroquine | No control group. Some incomplete data |
| Borba et al.103 | Double blind randomized clinical trial | High doses of chloroquine with azithromycin vs low doses of chloroquine with azithromycin | 28-day mortality rate. Clinical condition on days 6, 14, and 28. | 81 patients. Higher mortality in the high dose group, more adverse effects associated with QT prolongation and ventricular tachycardia | Intermediate analysis with termination of the high dose arm due to lack of safety |
| Geleris et al.104 | Retrospective | Hydroxychloroquine vs no hydroxychloroquine | Time to intubation or death | 1376 patients: 811 treated with hydroxychloroquine and 565 off treatment. Those treated with hydroxychloroquine were not intubated less or had a lower mortality rate (HR = 1.04; 95%CI, 0.82−1.32) | Logistic regression and propensity score |
| Rosenberg et al.34 | Retrospective | 4 groups: hydroxychloroquine alone, hydroxychloroquine with azithromycin, azithromycin alone or none of the 2 drugs | Hospital mortality Cardiac arrest, ECG alterations | 1438 patients. No differences were seen in the mortality rate after logistic regression. Patients on hydroxychloroquine + azithromycin had a higher risk of cardiac arrest (OR = 2.14; CI 95% 1.12−4.05), but not the other groups | Retrospective study. Differences in the clinical characteristics among the groups |
Interferon-β 1b has antiviral and immunoregulatory activity, and it is used to treat multiple sclerosis. Interferon-β 1b has proven to have in vitro activity against SARS-CoV and MERS.35,36 Also, a reduction of MERS viral load in animal models has been confirmed.37 It has been used as a monotherapy or in combination with lopinavir/ritonavir. A stage 2 clinical trial compared the combined use of lopinavir/ritonavir and ribavirin and interferon-β 1b vs lopinavir/ritonavir in 127 patients in Hong Kong.18 This trial proved that this combo triggered a negative viral C-reactive protein (CRP) test much faster. This study was designed so that interferon-β 1b would not be administered during the period of greater inflammation due to its proinflammatory effects.
It has been described that interferon reduces the activity of cytochrome P450. Therefore, possible drug interactions should be considered.38 The most common adverse effects are flu-like symptoms with fever, chills, headache, joint or muscle pain. Hypoglycemia, diarrhea, high transaminase levels, anemia or thrombocytopenia, among others, have also been reported.39
Anti-inflammatory therapyCorticoidsThe patient’s immune response seems to play an important role in the pathophysiology of both acute lung injury and ARDS. Patients with COVID‐19, especially those with pneumonia and ARDS, have high levels of pro-inflammatory cytokines and other inflammatory biomarkers.40 That is why some authors advocate for the use of steroids in this group of patients. However, the results obtained in other viral infections reveal that their systemic use can be beneficial and also associated with an increased viral replication and dissemination.41–43 On the other hand, it is still unclear whether the results of clinical trials on corticoids in patients with ARDS can be extrapolated to patients with COVID-19. The reason is that these studies also include patients with ARDS of extrapulmonary causes or without an infectious origin.44
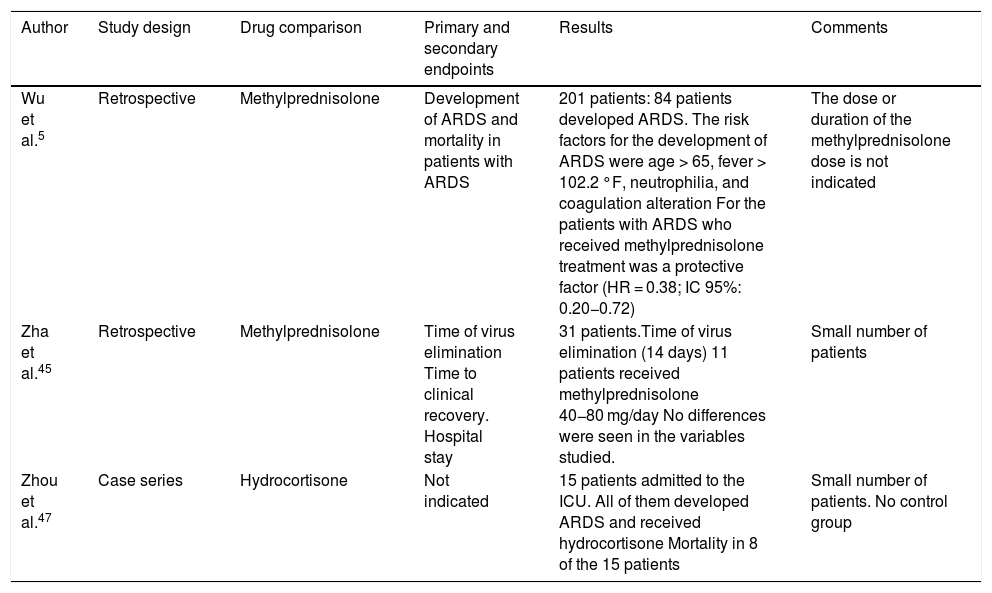
The current evidence about the utility of systemic corticoids in patients with COVID-19infection is very limited. The work conducted by Wu et al.5 is a retrospective single-center study that included 201 patients with COVID-19induced pneumonia. A total of 84 of these patients had ARDS. In this group, the use of methylprednisolone was associated with a significant reduction of mortality (HR = 0.38; 95%CI, 0.20−0.72). Other studies are shown on Table 4.45–47
Studies with corticoids.
| Author | Study design | Drug comparison | Primary and secondary endpoints | Results | Comments |
|---|---|---|---|---|---|
| Wu et al.5 | Retrospective | Methylprednisolone | Development of ARDS and mortality in patients with ARDS | 201 patients: 84 patients developed ARDS. The risk factors for the development of ARDS were age > 65, fever > 102.2 °F, neutrophilia, and coagulation alteration For the patients with ARDS who received methylprednisolone treatment was a protective factor (HR = 0.38; IC 95%: 0.20−0.72) | The dose or duration of the methylprednisolone dose is not indicated |
| Zha et al.45 | Retrospective | Methylprednisolone | Time of virus elimination Time to clinical recovery. Hospital stay | 31 patients.Time of virus elimination (14 days) 11 patients received methylprednisolone 40−80 mg/day No differences were seen in the variables studied. | Small number of patients |
| Zhou et al.47 | Case series | Hydrocortisone | Not indicated | 15 patients admitted to the ICU. All of them developed ARDS and received hydrocortisone Mortality in 8 of the 15 patients | Small number of patients. No control group |
Regarding institutional recommendations, both the WHO and the U.S. Centers for Disease Control and Prevention (CDC) do not recommend the use of corticoids beyond the context of clinical trials or specific treatment of patients with COVID-19and septic shock or in baseline conditions that may require them like exacerbated COPD or asthma. The American Thoracic Society experts committee does not give any recommendations on the use of corticoids either.48 On the other hand, the Surviving Sepsis Campaign (SSC) clinical guidelines suggest the use of systemic corticoids in adult patients with COVID-19and ARDS on MV with a weak degree of recommendation.49
On the doses to be used, the regimens used in China were mainly IV methylprednisolone (40−80 mg daily for 3–6 days).50 Equivalent doses of dexamethasone (7−15 mg/day) could stimulate less fluid retention since dexamethasone has less mineralocorticoid activity.51
Immunomodulatory therapyAs it happens with other diseases caused by coronaviruses such as SARS, where the presence of very high serum levels of proinflammatory cytokines like interleukin-6 (IL-6), tumor necrosis factor, and IL-12 has been confirmed;52 and MERS, where the high production of IL-6, IL-1b, and IL-853 has been confirmed too; the cytokine storm seems like the main mechanism responsible for the death of patients with COVID-19in whom the following findings have been made: high levels of cytokines like IL-6, IL-2, IL-7, IL-10, granulocyte colony-stimulating factor, interferon gamma-inducible protein (IPI0), macrophage inflammatory protein 1a (MIP1A), monocyte chemoattractant protein (MCP1), and tumor necrosis factor-α.1 The rapid activation of monocytes and T cells triggers a reaction in which IL-6 and the granulocyte colony-stimulating factor play a crucial role causing an inflammatory response that could be responsible for the altered gas exchange between the alveoli and the capillaries and for progression to pulmonary fibrosis and organ failure.54 The levels of the cytokines mentioned above seem to be associated with the severity and prognosis of the disease.
The diagnosis of the cytokine storm can be based on the presence of ARDS and changes of analytical parameters such as ferritin, CRP, D-dimer, lactate dehydrogenase, lymphocyte count, and IL-6. To this date, there is not such a thing as a validated scale to diagnose the COVID-19 induced cytokine storm. Therefore, the recommendation is to take serial measurements of these markers and of these patients’ respiratory condition in order to select those patients who can benefit from the administration of anti-inflammatory drugs; the HScore scale (Appendix BTable 1 of the supplementary data) can be useful although it is not sensitive enough and can’t be used early enough.55,56
The following are among the most important therapeutic options proposed:
TocilizumabTocilizumab is a monoclonal recombinant antibody that binds and blocks both the soluble and the membrane bound IL-6 receptors. It is often used for the management of rheumatoid arthritis (RA). Also, it is part of the treatment of the cytokine release syndrome after CAR-T therapy (Chimeric Antigen Receptor T cell therapy).57 Since it acts on the receptor and not on the circulating IL-6, the IL-6 levels are not useful for monitoring the response to treatment since they can actually go up after its administration.
Currently, there are several clinical trials recruiting patients with COVID-19with different degrees of severity. However, to this date we only have the results of 2 small retrospective studies.
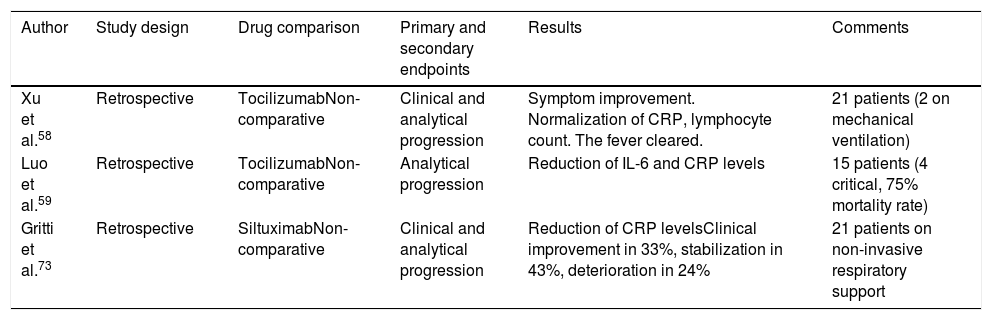
The first one is a retrospective analysis of 21 patients with COVID-19treated in 2 Chinese hospitals. A total of 17 of these patients met the criteria for serious disease (≥ 30 breaths per minute, SaO2 ≤ 93% breathing room air or a PaO2/FiO2 ratio ≤ 300) while 4 met the criteria for critical disease (need for MV, shock or admission to the ICU). The 21 patients received lopinavir and methylprednisolone and a 400 mg dose of IV tocilizumab. Also, 3 of these patients 3 received a second dose at the 2 -h mark (due to persistent fever).
Two of the patients received MV, another one non-invasive mechanical ventilation, and 9 patients received HFOT. In the 21 patients with high IL-6 levels, favorable clinical (temperature normalization, symptom release, and oxygenation), analytical (normalization of lymphocyte count and CRP), and radiological results were reported. In this study no adverse reactions to the drug were seen.58
In another retrospective series of patients with COVID-19 (15 patients: 2 with moderate disease, 6 with serious disease, and 7 with critical disease) from the same Chinese hospital (8 of whom received treatment with methylprednisolone and all of them with high IL-6 levels) had somehow good analytical results (reduced CRP and IL-6 levels). However, in 4 out of the 7 critical patients a favorable analytical response was not obtained, and 3 of them died.59 Some studies with immunomodulatory agents are shown on Table 5.
Studies with immunomodulators.
| Author | Study design | Drug comparison | Primary and secondary endpoints | Results | Comments |
|---|---|---|---|---|---|
| Xu et al.58 | Retrospective | TocilizumabNon-comparative | Clinical and analytical progression | Symptom improvement. Normalization of CRP, lymphocyte count. The fever cleared. | 21 patients (2 on mechanical ventilation) |
| Luo et al.59 | Retrospective | TocilizumabNon-comparative | Analytical progression | Reduction of IL-6 and CRP levels | 15 patients (4 critical, 75% mortality rate) |
| Gritti et al.73 | Retrospective | SiltuximabNon-comparative | Clinical and analytical progression | Reduction of CRP levelsClinical improvement in 33%, stabilization in 43%, deterioration in 24% | 21 patients on non-invasive respiratory support |
The administration of tocilizumab is contraindicated in the context of serious active infections. The side effects more often associated with the administration of tocilizumab are upper respiratory tract infections, nasopharyngitis, headache, high blood pressure, and elevated liver transaminase levels. The most serious adverse reactions were severed infections, complications of diverticulitis, and reactions of hypersensitivity.60
Due to lack of conclusive results, the SSC and the American Thoracic Society experts committee do not give any recommendations on the use of tocilizumab.48,49 However, the recommendations established by the Chinese National Health Commission and other experts recommend its use in critical patients with high IL-6 levels.61,62
The current recommendation of the Spanish Ministry of Health is to administer it during those stages of the disease when it is highly likely that stopping the inflammatory cascade will have an effect on the need for ventilation24 (see Appendix BTable 2 of the supplementary data).
Exceptionally, a second infusion can be assessed 12 h after the first infusion in patients who experience a spike in the analytical parameters after a first favorable response.
SarilumabSarilumab is another IL-6 receptor antagonist, also used to treat RA, that is being studied in patients with COVID-19 with different degrees of severity in several clinical trials (NCT04357808, NCT04315298, NCT04327388, NCT04324073, NCT04322773). However, we still do not have the clinical results.63 It is not available outside the clinical trial setting.
AP-2 associated protein kinase 1 inhibitorsBaricitinib, fedratinib, sunitinib, and erlinitib are AP-2 associated protein kinase 1 (AAK1) inhibitors. The AAK1 is the regulator of clathrin-mediated endocytosis through which most viruses enter the cell. Among these, fedratinib, sunitinib, and erlinitib are associated with serious side effects, which casts doubts on their utility in patients with COVID-19. On the other hand, baricitinib also inhibits the cyclin G-associated kinase (another endocytosis regulator) on top of being a janus kinase (JAK) inhibitor as well; therefore, its use has been suggested to reduce both virus cell entry and the associated inflammatory response.64,65 However, there are doubts about its possible effectiveness, since, at the same time, it reduces the interferon-mediated antiviral response.66
The adverse reactions more often reported in patients treated with baricitinib were higher LDL cholesterol levels (33.6%), upper respiratory tract infections (14.7%), and nausea (2.8%).67
To this day, we do not have any results on the use of baricitinib in patients with COVID-19. However, some ongoing clinical trials are already recruiting patients (NCT04390464, NCT04346147) and 2 more about to be started.
AnakinraAnakinra is an IL-1 recombinant receptor antagonist used to treat RA and Still’s disease.
The analysis of the subgroup of patients with macrophage activation syndrome from a clinical trial that studied the administration of anakinra in patients with sepsis and multiorgan failure revealed a lower mortality rate at 28 days vs placebo.68 The macrophage activation syndrome, a subgroup of hemophagocytic lymphohistiocytosis, occurs as a cytokine storm that triggers multiorgan failure that is often deadly in a short period of time. It is usually associated with rheumatic diseases, but it can be triggered by viral infections. High IL-1, IL-6, IL-18, soluble IL-2 receptor, FNT, and IFN-gamma levels have been observed. It has been proposed that anakinra can be part of the treatment of macrophage activation syndrome. Actually, some authors suggest its use to treat the COVID-19 induced cytokine storm.69
The most common adverse effects of anakinra therapy are local injection site reactions, the appearance of serious infections, and neutrophil decrease.70
Currently, different clinical trials are being conducted in patients with COVID-19 with different degrees of severity (NCT04364009, NCT04324021, NCT04357366, NCT04339712, and NCT04330638). However, we still do not have any clinical results to be able to give any recommendations on the use of anakinra.
RuxolitinibRuxolitinib (RXT) is a selective inhibitor of the Janus associated kinase family (JAK1 and JAK 2), the mediators involved in hematopoiesis and immune function (they participate in the transduction of other proinflammatory and anti-inflammatory cytosines). It is used for the management of RA, myelofibrosis, and polycythemia vera and it has been postulated as a possible candidate to reduce the infamous inflammatory cytokine storm.71 Currently, it is being evaluated in 2 clinical trials for the management of COVID-19 (NCT04362137, NCT04377620, NCT04334044, NCT04338958, and NCT04348695) but no data are available yet.
The Spanish Ministry of Health includes it among the COVID-19 treatment options, either as compassionate use or through a clinical trial.24 The appropriate use criteria and contraindications of RXT are shown on Appendix BTable 3 of the supplementary data.
The adverse reactions most often reported were thrombocytopenia, neutropenia, and anemia. The 3 most often non-hematological adverse reactions were hematomas (21.3%), dizziness (15.3%), and headache (14.0%). Patients treated with RXT suffered serious bacterial, mycobacterial, fungal, viral infections as well as other opportunistic infections.72
Siltuximab (STX)Siltuximab (SXT) is an IL-6 inhibitor used for the management of Castleman disease in patients who test negative for the human immunodeficiency virus and herpes-8 virus.
The data on 21 patients with COVID-19 with pneumonia/ARDS who received SXT were published in Italy. They received an IV dose of 11 mg/kg (for 1 h) followed by a second dose at the discretion of the attending physician received by 5 patients. Thirty-three per cent of the 21 patients improved their clinical condition, in 43% of the patients the situation stabilized, and in 24% it got worse including 1 death. We should mention that these 21 patients were on non-invasive respiratory support.73
The Spanish Ministry Health recommends its use in the same situations as RXT.24
The appropriate use criteria and contraindications of STX are shown on Appendix BTable 3 of the supplementary data.
The most common side effects regarding the use of STX are infections (including upper respiratory tract infections), pruritus, rash, joint pain, and diarrhea. The most serious adverse reaction associated with the use of siltuximab was the anaphylactic reaction.74
Antithrombotic prophylaxis and anticoagulationMany patients with COVID-19 who remain in critical condition especially those who end up dying develop coagulopathy.75–79 It has been described as a condition of disseminated intravascular coagulation,79,80 and as a state of different hypercoagulability as assessed by thromboelastography followed by high inflammation markers.81 Immobilization, inflammatory response, hypoxia, and the development of disseminated intravascular coagulation increase these patients’ thrombotic risk and there has been speculation on the role that microvascular thrombosis plays in hypoxemia and multiorgan failure.
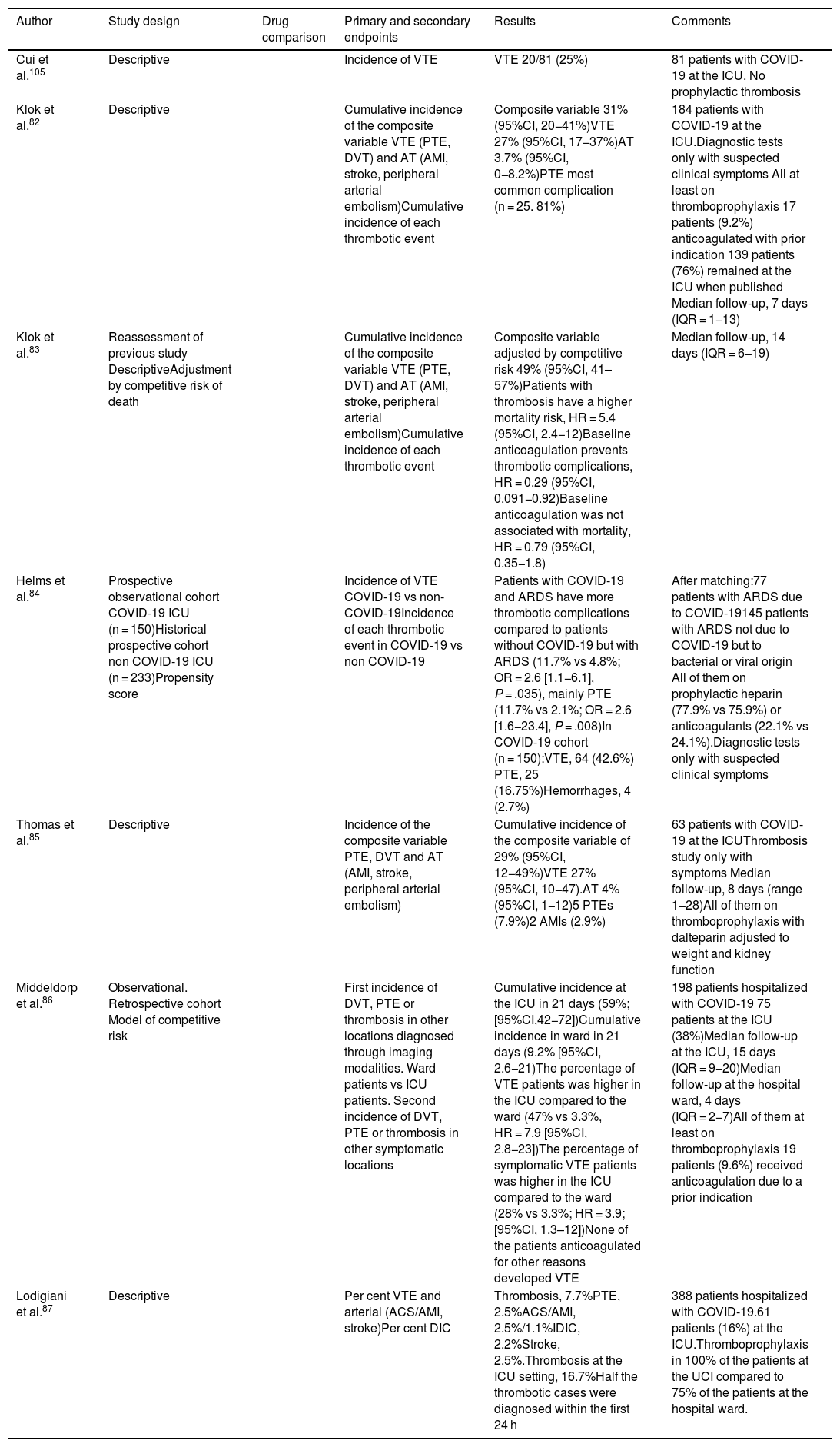
The incidence of thrombotic complications in the critical patient goes from 25% to 100%,82–87 based on the intensity of its search and the treatment administered. A higher mortality rate has been reported in patients with thrombosis (HR = 5.4; 95%CI, 2.4−12), and also that baseline anticoagulation prevents thrombotic complications (HR = 0.29; 95%CI, 0.091−0.92).83
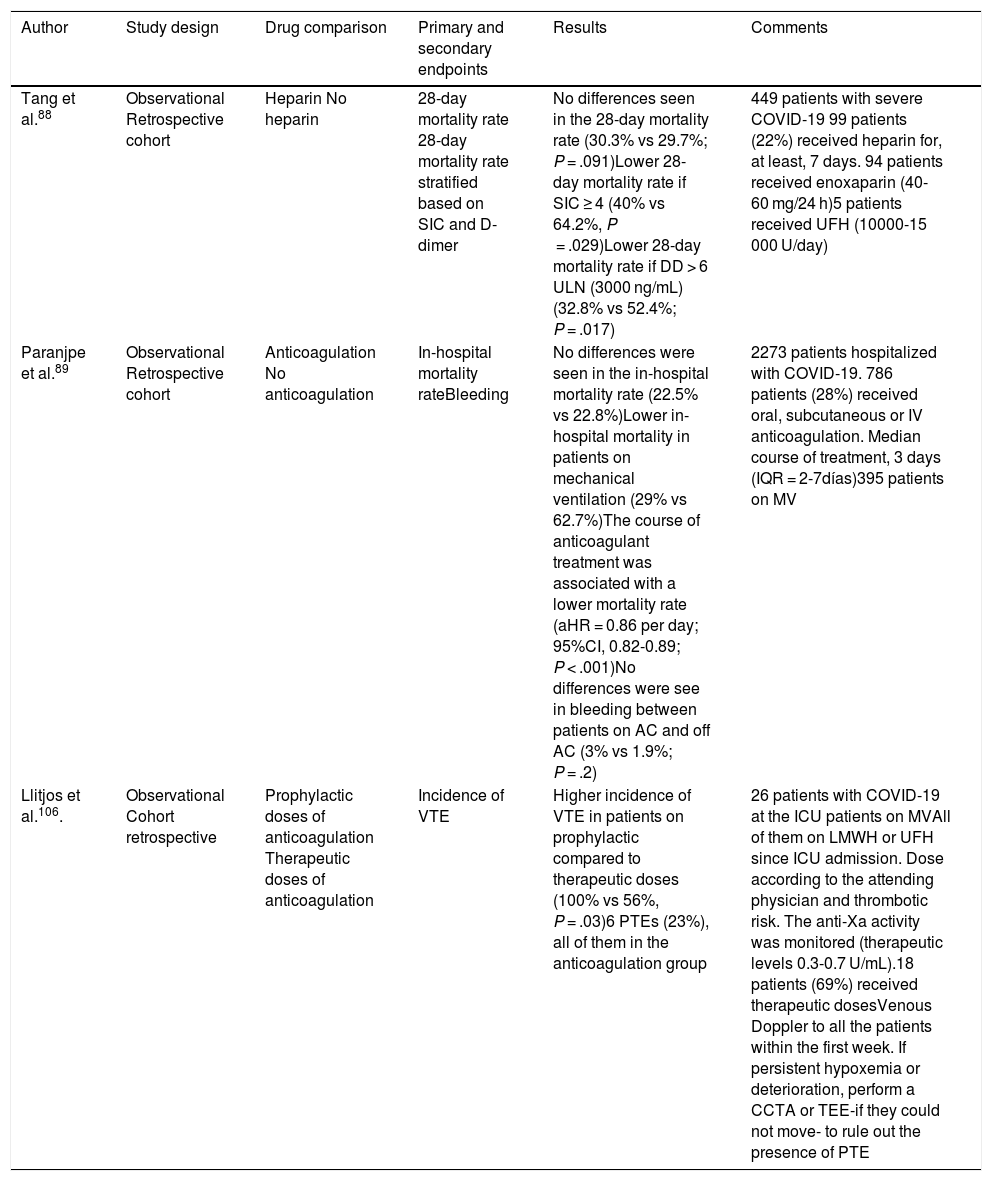
To this day, only 2 observational studies have studied the association between thromboprophylaxis-anticoagulation and mortality. A recent study did not find any significant differences in the 28-day mortality rate among patients treated with prophylactic doses of heparin (40−60 mg/24 h of enoxaparin for at least 7 days) compared to patients who did not receive the drug (30.3% vs 29.7%; P = .91).79 However, in the subgroups of patients with SIC ≥ 4 and D-dimer levels > 3000 ng/mL, the 28-day mortality rate was significantly lower (40% vs 64.2%; P = .029, and 32.8% vs 52.4%, P = .017, respectively) in patients treated with heparin.88 Paranjpe et al.89 did not find any differences in the in-hospital mortality either (22.5% vs 22.8%) between patients who received oral, IV or subcutaneous anticoagulation compared to those who were not anticoagulated. However, the in-hospital mortality rate decreased in the subgroup of anticoagulated patients on mechanical ventilation (29% vs 62.7%), and the duration of anticoagulant therapy was associated with a lower mortality rate (aHR = 0.86 per day, 95%CI, 0.82−0.89; P < .001). We should mention that there were no differences in hemorrhagic complications between anticoagulated and non-anticoagulated patients (3% vs 1.9%; P = 0.2).
Randomized clinical trials are necessary to indicate antithrombotic and anticoagulant therapy in these patients.
Tables 6 and 7 show the clinical trials conducted on thrombosis, anticoagulation, and thromboprophylaxis in patients with COVID-19.
Studies on thrombosis in patients with COVID-19.
| Author | Study design | Drug comparison | Primary and secondary endpoints | Results | Comments |
|---|---|---|---|---|---|
| Cui et al.105 | Descriptive | Incidence of VTE | VTE 20/81 (25%) | 81 patients with COVID-19 at the ICU. No prophylactic thrombosis | |
| Klok et al.82 | Descriptive | Cumulative incidence of the composite variable VTE (PTE, DVT) and AT (AMI, stroke, peripheral arterial embolism)Cumulative incidence of each thrombotic event | Composite variable 31% (95%CI, 20−41%)VTE 27% (95%CI, 17−37%)AT 3.7% (95%CI, 0−8.2%)PTE most common complication (n = 25. 81%) | 184 patients with COVID-19 at the ICU.Diagnostic tests only with suspected clinical symptoms All at least on thromboprophylaxis 17 patients (9.2%) anticoagulated with prior indication 139 patients (76%) remained at the ICU when published Median follow-up, 7 days (IQR = 1−13) | |
| Klok et al.83 | Reassessment of previous study DescriptiveAdjustment by competitive risk of death | Cumulative incidence of the composite variable VTE (PTE, DVT) and AT (AMI, stroke, peripheral arterial embolism)Cumulative incidence of each thrombotic event | Composite variable adjusted by competitive risk 49% (95%CI, 41–57%)Patients with thrombosis have a higher mortality risk, HR = 5.4 (95%CI, 2.4−12)Baseline anticoagulation prevents thrombotic complications, HR = 0.29 (95%CI, 0.091−0.92)Baseline anticoagulation was not associated with mortality, HR = 0.79 (95%CI, 0.35−1.8) | Median follow-up, 14 days (IQR = 6−19) | |
| Helms et al.84 | Prospective observational cohort COVID-19 ICU (n = 150)Historical prospective cohort non COVID-19 ICU (n = 233)Propensity score | Incidence of VTE COVID-19 vs non-COVID-19Incidence of each thrombotic event in COVID-19 vs non COVID-19 | Patients with COVID-19 and ARDS have more thrombotic complications compared to patients without COVID-19 but with ARDS (11.7% vs 4.8%; OR = 2.6 [1.1−6.1], P = .035), mainly PTE (11.7% vs 2.1%; OR = 2.6 [1.6−23.4], P = .008)In COVID-19 cohort (n = 150):VTE, 64 (42.6%) PTE, 25 (16.75%)Hemorrhages, 4 (2.7%) | After matching:77 patients with ARDS due to COVID-19145 patients with ARDS not due to COVID-19 but to bacterial or viral origin All of them on prophylactic heparin (77.9% vs 75.9%) or anticoagulants (22.1% vs 24.1%).Diagnostic tests only with suspected clinical symptoms | |
| Thomas et al.85 | Descriptive | Incidence of the composite variable PTE, DVT and AT (AMI, stroke, peripheral arterial embolism) | Cumulative incidence of the composite variable of 29% (95%CI, 12−49%)VTE 27% (95%CI, 10−47).AT 4% (95%CI, 1−12)5 PTEs (7.9%)2 AMIs (2.9%) | 63 patients with COVID-19 at the ICUThrombosis study only with symptoms Median follow-up, 8 days (range 1−28)All of them on thromboprophylaxis with dalteparin adjusted to weight and kidney function | |
| Middeldorp et al.86 | Observational. Retrospective cohort Model of competitive risk | First incidence of DVT, PTE or thrombosis in other locations diagnosed through imaging modalities. Ward patients vs ICU patients. Second incidence of DVT, PTE or thrombosis in other symptomatic locations | Cumulative incidence at the ICU in 21 days (59%; [95%CI,42−72])Cumulative incidence in ward in 21 days (9.2% [95%CI, 2.6−21)The percentage of VTE patients was higher in the ICU compared to the ward (47% vs 3.3%, HR = 7.9 [95%CI, 2.8−23])The percentage of symptomatic VTE patients was higher in the ICU compared to the ward (28% vs 3.3%; HR = 3.9; [95%CI, 1.3–12])None of the patients anticoagulated for other reasons developed VTE | 198 patients hospitalized with COVID-19 75 patients at the ICU (38%)Median follow-up at the ICU, 15 days (IQR = 9−20)Median follow-up at the hospital ward, 4 days (IQR = 2−7)All of them at least on thromboprophylaxis 19 patients (9.6%) received anticoagulation due to a prior indication | |
| Lodigiani et al.87 | Descriptive | Per cent VTE and arterial (ACS/AMI, stroke)Per cent DIC | Thrombosis, 7.7%PTE, 2.5%ACS/AMI, 2.5%/1.1%IDIC, 2.2%Stroke, 2.5%.Thrombosis at the ICU setting, 16.7%Half the thrombotic cases were diagnosed within the first 24 h | 388 patients hospitalized with COVID-19.61 patients (16%) at the ICU.Thromboprophylaxis in 100% of the patients at the UCI compared to 75% of the patients at the hospital ward. |
Studies on anticoagulation and thromboprophylaxis in patients with COVID-19.
| Author | Study design | Drug comparison | Primary and secondary endpoints | Results | Comments |
|---|---|---|---|---|---|
| Tang et al.88 | Observational Retrospective cohort | Heparin No heparin | 28-day mortality rate 28-day mortality rate stratified based on SIC and D-dimer | No differences seen in the 28-day mortality rate (30.3% vs 29.7%; P = .091)Lower 28-day mortality rate if SIC ≥ 4 (40% vs 64.2%, P = .029)Lower 28-day mortality rate if DD > 6 ULN (3000 ng/mL) (32.8% vs 52.4%; P = .017) | 449 patients with severe COVID-19 99 patients (22%) received heparin for, at least, 7 days. 94 patients received enoxaparin (40-60 mg/24 h)5 patients received UFH (10000-15 000 U/day) |
| Paranjpe et al.89 | Observational Retrospective cohort | Anticoagulation No anticoagulation | In-hospital mortality rateBleeding | No differences were seen in the in-hospital mortality rate (22.5% vs 22.8%)Lower in-hospital mortality in patients on mechanical ventilation (29% vs 62.7%)The course of anticoagulant treatment was associated with a lower mortality rate (aHR = 0.86 per day; 95%CI, 0.82-0.89; P < .001)No differences were see in bleeding between patients on AC and off AC (3% vs 1.9%; P = .2) | 2273 patients hospitalized with COVID-19. 786 patients (28%) received oral, subcutaneous or IV anticoagulation. Median course of treatment, 3 days (IQR = 2-7días)395 patients on MV |
| Llitjos et al.106. | Observational Cohort retrospective | Prophylactic doses of anticoagulation Therapeutic doses of anticoagulation | Incidence of VTE | Higher incidence of VTE in patients on prophylactic compared to therapeutic doses (100% vs 56%, P = .03)6 PTEs (23%), all of them in the anticoagulation group | 26 patients with COVID-19 at the ICU patients on MVAll of them on LMWH or UFH since ICU admission. Dose according to the attending physician and thrombotic risk. The anti-Xa activity was monitored (therapeutic levels 0.3-0.7 U/mL).18 patients (69%) received therapeutic dosesVenous Doppler to all the patients within the first week. If persistent hypoxemia or deterioration, perform a CCTA or TEE-if they could not move- to rule out the presence of PTE |
AC, anticoagulated; ACS, acute coronary syndrome; aHR, adjusted hazard ratio; AMIa, acute myocardial infarction; AT, arterial thrombosis; CI, confidence interval; DD, D-dimer; DIC, disseminated intravascular coagulation; DVP, deep vein thrombosis; HR, hazard ratio; IQR, interquartile range; LMWH, low-molecular weight heparin; MV, mechanical ventilation; PTE, pulmonary thromboembolism; SIC, sepsis-induced coagulopathy; UFH, unfractionated heparin; ULN, upper limit of normal; VTE, venous thromboembolism.
Based on the current scientific evidence available and the recommendations established by other international medical societies,90–95 the therapeutic schemes shown on tables 8 and 9 have been suggested.
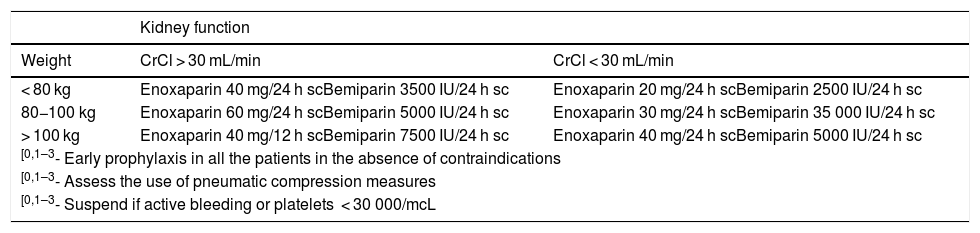
Antithrombotic prophylaxis dosing.
| Kidney function | ||
|---|---|---|
| Weight | CrCl > 30 mL/min | CrCl < 30 mL/min |
| < 80 kg | Enoxaparin 40 mg/24 h scBemiparin 3500 IU/24 h sc | Enoxaparin 20 mg/24 h scBemiparin 2500 IU/24 h sc |
| 80−100 kg | Enoxaparin 60 mg/24 h scBemiparin 5000 IU/24 h sc | Enoxaparin 30 mg/24 h scBemiparin 35 000 IU/24 h sc |
| > 100 kg | Enoxaparin 40 mg/12 h scBemiparin 7500 IU/24 h sc | Enoxaparin 40 mg/24 h scBemiparin 5000 IU/24 h sc |
| [0,1–3- Early prophylaxis in all the patients in the absence of contraindications | ||
| [0,1–3- Assess the use of pneumatic compression measures | ||
| [0,1–3- Suspend if active bleeding or platelets < 30 000/mcL | ||
CrCl, creatinine clearance.
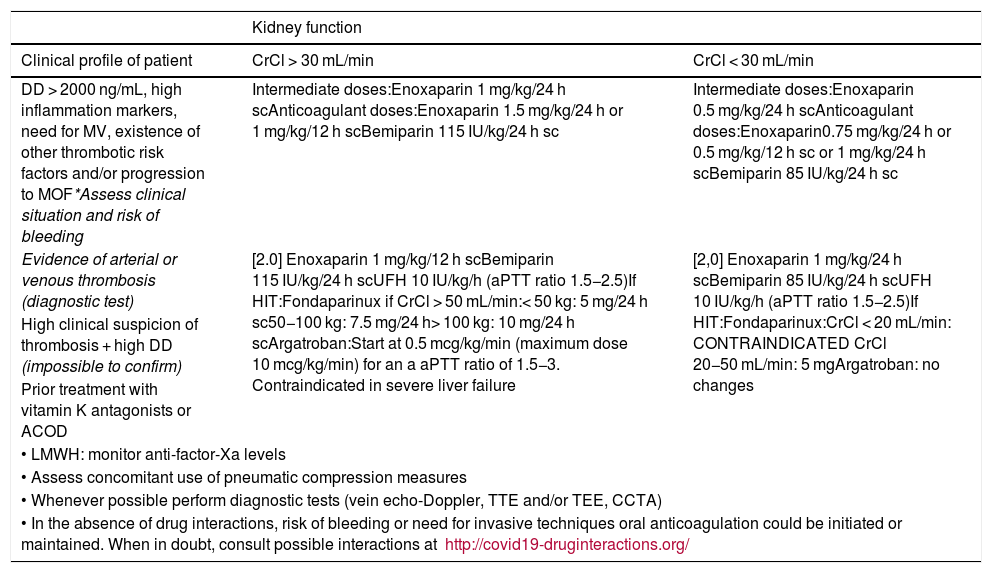
Anticoagulation doses and recommendations.
| Kidney function | ||
|---|---|---|
| Clinical profile of patient | CrCl > 30 mL/min | CrCl < 30 mL/min |
| DD > 2000 ng/mL, high inflammation markers, need for MV, existence of other thrombotic risk factors and/or progression to MOF*Assess clinical situation and risk of bleeding | Intermediate doses:Enoxaparin 1 mg/kg/24 h scAnticoagulant doses:Enoxaparin 1.5 mg/kg/24 h or 1 mg/kg/12 h scBemiparin 115 IU/kg/24 h sc | Intermediate doses:Enoxaparin 0.5 mg/kg/24 h scAnticoagulant doses:Enoxaparin0.75 mg/kg/24 h or 0.5 mg/kg/12 h sc or 1 mg/kg/24 h scBemiparin 85 IU/kg/24 h sc |
| Evidence of arterial or venous thrombosis (diagnostic test) | [2.0] Enoxaparin 1 mg/kg/12 h scBemiparin 115 IU/kg/24 h scUFH 10 IU/kg/h (aPTT ratio 1.5−2.5)If HIT:Fondaparinux if CrCl > 50 mL/min:< 50 kg: 5 mg/24 h sc50−100 kg: 7.5 mg/24 h> 100 kg: 10 mg/24 h scArgatroban:Start at 0.5 mcg/kg/min (maximum dose 10 mcg/kg/min) for an a aPTT ratio of 1.5−3. Contraindicated in severe liver failure | [2,0] Enoxaparin 1 mg/kg/24 h scBemiparin 85 IU/kg/24 h scUFH 10 IU/kg/h (aPTT ratio 1.5−2.5)If HIT:Fondaparinux:CrCl < 20 mL/min: CONTRAINDICATED CrCl 20−50 mL/min: 5 mgArgatroban: no changes |
| High clinical suspicion of thrombosis + high DD (impossible to confirm) | ||
| Prior treatment with vitamin K antagonists or ACOD | ||
| • LMWH: monitor anti-factor-Xa levels | ||
| • Assess concomitant use of pneumatic compression measures | ||
| • Whenever possible perform diagnostic tests (vein echo-Doppler, TTE and/or TEE, CCTA) | ||
| • In the absence of drug interactions, risk of bleeding or need for invasive techniques oral anticoagulation could be initiated or maintained. When in doubt, consult possible interactions at http://covid19-druginteractions.org/ | ||
aPTT, activated partial thromboplastin time; CCTA, coronary computed tomography angiography; CrCl, creatinine clearance; DD, D-dimer; HIT, heparin-induced thrombocytopenia; LMWH, low-molecular weight heparin; MOF, multiorgan failure; MV, mechanical ventilation; sc, subcutaneous; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram; UFH, unfractionated heparin.
Traditionally, convalescent plasma therapy (CPT) has been used to prevent and treat infectious diseases. It was successfully used to treat SARS, MERS, and the 2009 H1N1 pandemic. In a 32-study meta-analysis of infections due to SARS coronavirus and the influenza virus, it was confirmed that there is a statistically significant lower mortality rate in patients treated with CPT compared to placebo and no treatment at all (OR = 0.25; 95%CI, 0.14−0.45).96
Duan et al. measured the effectiveness of PCT in 10 patients with severe COVID-19.97 They transfused 200 mL of plasma from recovered patients with high antibody titers (1:640). The effects seen with the PCT transfusion were: 1) improvement of clinical symptoms and oxygenation parameters, which allowed the de-escalation from MV to HFOT and from HFOT to conventional oxygen therapy; 2) fewer pulmonary lesions; 3) improved laboratory parameters (lymphopenia, PCR, transaminases); 4) increase of antibody titers and disappearance of SARS-CoV-2 RNA; 5) better prognosis (3 hospital discharges and 7 patients with clinical improvement) compared to the control group (3 deaths, 6 cases of stabilization and 1 clinical improvement) (P < .001). There were no serious adverse events in any of the patients. They conclude that PCT could be a safe bailout therapeutic option readily available for patients with severe COVID-19. The optimal dose and the best time to perform the transfusion are still to be defined in more powerful randomized clinical trials. Shen et al. obtained similar results after they recruited 5 cases of patients with severe COVID-19 on MV.98 Overall, the 2 series show a 0% mortality rate in the transfusion group compared to 30% in the control group and no adverse effects.
In a randomized clinical trial conducted by Li et al.99 comparing standard therapy with and without the addition of CPT in 103 patients did not show any clinical benefits at the 28-day mark or in the mortality rate reported (15.7% in treatment group vs 24% in the placebo group; OR = 0.65 (95%CI, 0.29–1.46) (P = 0.3). This study has important limitations, such as the time from symptom onset to randomization (about 30 days), and an early termination, when the pandemic ended in China, which may be limiting the validity of the results. However, as pointed out in the accompanying editorial, there may be a benefit in the subgroup of patients whose condition was most severe.100
Based on the series of Duan et al.97 and Shen et al.,98 the Infectious Diseases Society of America (IDSA) recommends the transfusion of CPT in the context of a clinical trial.8
The SSC49 clinical guidelines point out that there is not enough evidence to suggest or recommend the indiscriminate use of plasma from convalescent patients.
IV immunoglobulinIV immunoglobulin (IVIg) has been used as adjuvant therapy to treat a variety of pathogens as a combined product and as a more concentrated pathogen-specific antibody (hyperimmune). The possibility that protective antibodies are present in the combined product is greater.
Cao et al. published a series of 3 cases that received high doses of IVIg at the beginning of ARDS with a satisfactory clinical and radiographic recovery.101 High IVIg doses were used (0.3−0.5 g/kg/day) for 5 days and zero adverse events were reported. In the 3 patients a rapid clinical improvement was seen after the administration of IVIg. The moment IVIg is administered is of paramount importance. Actually, there may not be any benefits if systemic damage has already occurred.
According to the clinical guidelines published by the IDSA,8 at this moment the possible utility of IVIg for the management of SARS-CoV-2 is still unknown. The routine use of IVIg is ill-advised according to the SSC.49 Therefore, there is not enough evidence to indicate or recommend the indiscriminate use of immunoglobulins.
Las dosis de los fármacos y las recomendaciones de las principales sociedades y organismos oficiales se encuentran recogidas en el Appendix Banexo I y la tabla 4 del material adicional.
Drug doses and the recommendations established by the main official medical societies and organs are summed up in Appendix B annex I and Table 4 of the supplementary data.
FundingThe authors declared that they received no funding while writing this manuscript.
Conflicts of interestNone declared.
Please cite this article as: Díaz E, Amézaga Menéndez R, Vidal Cortés P, Escapa MG, Suberviola B, Serrano Lázaro A, et al. Tratamiento farmacológico de la COVID-19: revisión narrativa de los Grupos de Trabajo de Enfermedades Infecciosas y Sepsis (GTEIS) y del Grupo de Trabajo de Transfusiones y Hemoderivados (GTTH). Med Intensiva. 2021;45:104–121.