A study is made of the influence of preemptive hemodynamic intervention restricting fluid administration upon the development of oleic acid-induced lung injury.
DesignA randomized in vivo study in rabbits was carried out.
SettingUniversity research laboratory.
SubjectsSixteen anesthetized, mechanically ventilated rabbits.
VariablesHemodynamic measurements obtained by transesophageal Doppler signal. Respiratory mechanics computed by a least square fitting method. Lung edema assessed by the ratio of wet weight to dry weight of the right lung. Histological examination of the left lung.
InterventionsAnimals were randomly assigned to either the early protective lung strategy (EPLS) (n=8) or the early protective hemodynamic strategy (EPHS) (n=8). In both groups, lung injury was induced by the intravenous infusion of oleic acid (OA) (0.133mlkg−1h−1 for 2h). At the same time, the EPLS group received 15mlkg−1h−1 of Ringer lactate solution, while the EPHS group received 30mlkg−1h−1. Measurements were obtained at baseline and 1 and 2h after starting OA infusion.
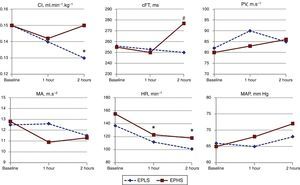
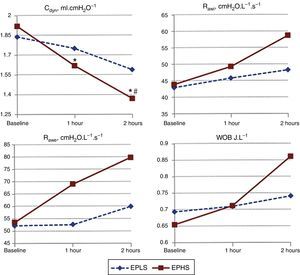
ResultsAfter 2h, the cardiac index decreased in the EPLS group (p<0.05), whereas in the EPHS group it remained unchanged. Lung compliance decreased significantly only in the EPHS group (p<0.05). Lung edema was greater in the EPHS group (p<0.05). Histological damage proved similar in both groups (p=0.4).
ConclusionsIn this experimental model of early lung injury, lung edema progression was attenuated by preemptively restricting the administration of fluids.
Conocer cómo influye una intervención hemodinámica preventiva basada en la restricción de fluidos sobre el desarrollo de la lesión pulmonar inducida por la administración de ácido oleico.
DiseñoEstudio aleatorizado en animales vivos.
LugarLaboratorio universitario de investigación experimental.
VariablesMecánica respiratoria (método de los mínimos cuadrados), medidas hemodinámicas (doppler esofágico), estimación del edema pulmonar (relación peso húmedo/seco del pulmón derecho) y daño histológico del pulmón izquierdo.
IntervencionesOcho animales fueron asignados a un grupo con una estrategia protectora pulmonar (EPP), y otros 8 a otro grupo con una estrategia protectora hemodinámica (EPH). En ambos grupos la lesión pulmonar se desencadenó mediante la administración intravenosa de ácido oleico (0,133mL/kg−1/h−1 durante 2h), recibiendo simultáneamente los animales del grupo EPP 15mL/kg−1/h−1 de Ringer Lactato y los del grupo EPH 30mLKg−1h−1. Se obtuvieron medidas basales, a la hora y a las 2h.
ResultadosTranscurridas las 2h de experimento el índice cardiaco permaneció estable en el grupo EPH, pero disminuyó en el grupo EPP (p<0,05). Por el contrario, la distensibilidad pulmonar disminuyó significativamente solo en el grupo EPH (p<0,05), en el cual el edema pulmonar estimado mediante la relación peso húmedo/seco del pulmón derecho fue mayor (p<0,05). El daño histológico fue similar en ambos grupos (p=0,4).
ConclusionesEn este modelo experimental de lesión pulmonar aguda en fase inicial, la formación del edema pulmonar fue atenuada por la restricción preventiva en la administración de fluidos.
Acute respiratory distress syndrome (ARDS) is a catastrophic form of respiratory failure that has been described in more than 30 entities or triggering conditions.1,2 A common finding in all of them is a delay, which can range from hours to several days, between the time this initial aggression occurs and the subsequent clinical presentation of ARDS.3 During this time gap, further attacks or impacts called “second hits” frequently occur that, although less intense, affect the lungs that are already damaged and sensitized, further aggravating the initial lung inflammation, and may precipitate the clinical onset of ARDS.4,5 Most commonly, second hits are iatrogenic insults, such as transfusions, mechanical ventilations with high tidal volumes, or the inappropriate administration of fluids.4–6 The theory of second hits implies that ARDS may have preclinical stages, offering to clinicians an opportunity to intervene earlier before devastating respiratory failure when clinical criteria of ARDS are present. In fact, a recent interdisciplinary intervention aimed at avoiding large tidal volumes and inappropriate transfusions in mechanically ventilated patients was associated with a decreased frequency of new cases of ARDS.7
Diffuse alveolar damage with severe inflammation and high permeability protein-rich edema in the lungs represents the most characteristic pathological finding in more than half of patients with ARDS.8 In these patients, high permeability pulmonary edema contributes significantly to the development of the disease and represents a potential target for early preventative therapy. Pulmonary edema is determined by the Starling relationship, which predicts the net flow of liquid across a membrane. In high permeability pulmonary edema, the most common mechanism for a rise in the transcapillary filtration of fluids is an increase in capillary permeability. However, experimental studies demonstrated that, at a given increase in capillarity permeability, a modest change in pulmonary vascular pressure greatly modifies the quantity of pulmonary edema.9 This is probably one of the reasons why a strategy of restricting the administration of fluids has demonstrated to be useful in ARDS for decreasing pulmonary edema and improving lung function.10 However, whether this strategy restraining fluid administration may also be useful in preventing the development of ARDS is not well known.
We hypothesized that a preemptive hemodynamic intervention by restricting the administration of fluids, right at the very start of lung injury, would be successful in reducing the formation and the progression of pulmonary edema, decreasing the severity of the resulting respiratory failure. We refer to this strategy as the early protective lung strategy (EPLS) and compare it against another strategy to maintain hemodynamic stability, which we call the early protective hemodynamic strategy (EPHS). In this study, we applied from the very inception of the injury and pulmonary inflammation in a rabbit model of lung injury with the involvement of the cardio circulatory function, induced by continuous intravenous administration of oleic acid (OA).11–13
Materials and methodsSixteen New Zealand rabbits (weight 2.8±0.2kg), supplied by the Reproduction Laboratory of the University of Cadiz, were maintained in individual cages on a 12-hour light/dark cycle with free access to food and water until the time of the experimental procedures. All of the procedures and protocols in this investigation were reviewed and approved by the Ethical Committee for Animal Experimentation of the School of Medicine of the University of Cadiz. The animal care and use procedures conformed to European Ethical Standards (European Union Directive 86/609 and Spanish Royal Decree 1201/2005) for the care and use of laboratory animals for experimental research.
Animal preparation and instrumentationThe animals were anesthetized with xylazine (10mg/kg intramuscularly) and Ketamine (40mg/kg intramuscularly). The animals were tracheotomized, intubated, and mechanically ventilated with volume-controlled ventilation (Siemens Servo 900C ventilator, Siemens-Elema AB, Solna, Sweden) at a tidal volume of 8ml/kg, a positive end expiratory pressure (PEEP) of 3cmH2O, an inspiration to expiration ratio of 1:2, a respiratory rate of 35/minute, and an inspired oxygen fraction of 0.6. The anesthesia was maintained with a continuous intravenous infusion of ketamine (20mgkg−1h−1) and xylazine (6mgkg−1h−1). A muscular blockade was maintained with a rocuronium bromide infusion (0.1mgkg−1h−1).
Respiratory monitoringContinuous non-invasive measurements of flow, pressure, and volume in the animal's airway were measured using a calibrated CO2SMO Plus! monitor (Respironics Novametrix Medical System INC, Wallingford, CT, USA) with a neonatal flow sensor connected directly to the animal's tracheal tube.14 Dynamic compliance (Cdyn), inspiratory and expiratory airway resistance (Rawi, Rawe), dynamic intrinsic PEEP (auto-PEEP), and total inspiratory work of breathing (WOB) were continuously computed by least squares fitting of the flow, volume, and pressure raw waveform data to a simple model.15 Data were downloaded via a RS232 connection to a laptop computer for collection and posterior review using data acquisition software (Analysis+, Novametrix Medical System INC, Wallingford, CT, USA).
Hemodynamic monitoringCommercially available Doppler equipment (Cardio QPTM, Deltex Medical TM, Chichester, UK) with a 4MHz flexible pediatric transesophageal probe (KDP n-Kinder Doppler Probe®) was used for the studies.16 The probe was introduced into the rabbit at mid-esophagus until the aortic blood flow signals were best identified. The optimal position of the probe was suggested by an audible, maximal pitch and a sharply defined velocity waveform with minimal spectral dispersion. Consecutive transesophageal Doppler measurements for 60s at the beginning, at 1h, and at 2h (end of experiment) were completed and averaged to calculate the cardiac index (CI) (cardiac output divided by the weight), the corrected flow time (cFT) (length of time of systolic blood flow adjusted for heart rate, [i.e., divided by the square root of the heart cycle time]), the peak velocity (PV) (maximal velocity during systole), and the mean acceleration (MA) (PV divided by the acceleration time during systole) of the descending aorta velocity waveform.17–19 The arterial pressure was measured by an indwelling femoral artery catheter connected to a pressure transducer (TruWave®, Edwards Lifesciences LLC, Irvine, CA, USA).
Experimental protocolImmediately after the baseline measurement of hemodynamic and lung mechanics, the animals were randomly assigned to the EPLS group (n=8) or the EPHS group (n=8), and the lung injury was induced by continuous intravenous infusion of OA (0.133mlkg−1h−1 for 2h) via the central venous catheter.11 During this period, the EPLS group received 15mlkg−1h−1 of Ringer Lactate, and the EPHS group received 30mlkg−1h−1. Once 2h elapsed, all of the rabbits were killed by an injection of a lethal dose of chloride potassium.
At the end of the experiment, the thorax was opened, and the lungs were removed en bloc. The right lung was weighed and then dried in a microwave oven at a power of 150W for 60min.20 The ratio of wet weight to dry weight (WW/DW) of the right lung was calculated to assess tissue edema. The left lung was excised and immersed in 10% formaldehyde for at least 72h. Tissue samples were dehydrated with graded alcohol, embedded in paraffin, and cut in a series of 4μm-thick slices that were stained with hematoxylin and eosin. A pathologist (JRC) then evaluated these tissue sections in a blinded fashion using the following scoring system to grade the degree of lung injury: 0 (no damage) to 3 (maximal damage). These score were according to the combined assessments of alveolar congestion, alveolar hemorrhage and edema, infiltration/aggregation of the neutrophils in the airspace or vessel wall, the thickness of the alveolar wall, bronchiole epithelial desquamation, and hyaline membrane formation
Statistical analysisUnless otherwise indicated, the results are presented as mean±SD. Data were tested for normal distribution with the Agostino-Pearson test. We used a two-way analysis of variance for repeated measures to determine the statistical significance of group differences in the respiratory parameters and the Doppler transesophageal measurements at different time points. When statistical significance was indicated, it was further examined by a post hoc analysis (Student–Newman–Keuls test). A Student's unpaired t-test was used for single-time point observation. Results from the histological studies are expressed as median and range, and the statistical analysis was performed using the Mann Whitney U-test. Data were analyzed by using MedCalc Statistical Software (version 9.5.2.0; MedCalc Software bvba, Ostend, Belgium). Statistical significance was assumed at a probability <0.05.
ResultsIn 16 rabbits randomly allotted to each of the two groups (EPLS and EPHS), we found that at the beginning of the experiment, each group had similar baseline characteristics for any of the hemodynamic and respiratory variables measured (Tables 1 and 2). The total fluid intake (i.e., sedation, oleic preparation, and fluid therapy) was 19.8mlkg−1h−1 in the EPLS group and 36.2mlkg−1h−1 in the EPHS group (p<0.001).
Baseline hemodynamic parameters in EPLS and EPHS groups.
| EPLS | EPHS | p | |
|---|---|---|---|
| CI, mlmin−1kg−1 | 151±11 | 155±21 | 0.8 |
| cFT, ms | 256±28 | 255±21 | 0.9 |
| MA, ms−2 | 12.5±2.7 | 12.8±3.1 | 0.8 |
| PV, ms−1 | 82±14 | 80±11 | 0.7 |
| HR, min−1 | 137±22 | 155±16 | 0.09 |
| MAP, mmHg | 66±12 | 65±15 | 0.9 |
Data are presented as mean±standard deviation. EPLS, early protective lung strategy; EPHS, early protective hemodynamic strategy; CI, cardiac index; cFT, corrected aortic flow time; MA, mean acceleration of aortic flow; PV, peak velocity of aortic flow; HR, heart rate; MAP, mean arterial pressure (p Values refer to difference between both groups by unpaired t test).
Baseline lung mechanics in EPLS and EPHS groups.
| EPLS | EPHS | p | |
|---|---|---|---|
| Cdyn, mlcmH2O−1 | 1.84±0.22 | 1.92±0.38 | 0.5 |
| Rawi, cmH2OL−1s−1 | 42.9±4.28 | 43.93±10.79 | 0.8 |
| Rawe, cmH2OL−1s−1 | 52.22±6.93 | 53.58±12.4 | 0.7 |
| WOB, J/L | 0.694±0.076 | 0.655±0.068 |
Data are presented as mean±standard deviation. EPLS, early protective lung strategy; EPHS, early protective hemodynamic strategy; Cdyn, dynamic compliance of respiratory system; Rawi, inspiratory airway resistance; Rawe, expiratory airway resistance; WOB, total inspiratory work of breathing (p Values refer to difference between both groups by unpaired t test).
As shown in Fig. 1, both strategies shown different hemodynamic effects. Compared with the baseline, there was a decrease in the CI that reached statistical significance in the animals in the EPLS group; however in the animals in the EPHS group, the CI remained unchanged throughout the experiment. Heart rate decreased significantly in the animals in the EPHS group, and by the end of the experiment, the cFT was significantly higher in the EPHS group (Fig. 1).
Changes over time of hemodynamic parameters as strategy used. EPLS, early protective lung strategy; EPHS, early protective hemodynamic strategy; CI, cardiac index; cFT, corrected aortic flow time; PV, peak velocity of aortic flow; MA, mean acceleration of aortic flow; HR, heart rate; MAP, mean arterial pressure (*p<0.05 compared to baseline by two-way analysis of variance for repeated measures; #p<0.05 comparing values between both groups at the end of experiment by Student's unpaired t-test).
As shown in Fig. 2, the two strategies had different effects on respiratory mechanics. Compared to the baseline, at 1 and 2h (end of experiment), the Cdyn had decreased significantly only in animals in the EPHS group. Other measurements of respiratory mechanics, Rawi, Rawe and WOB, increased more in the EPHS group, although without statistical significance. No animals in either group showed auto-PEEP.
Changes over time of respiratory mechanics as strategy used. EPLS, early protective lung strategy; EPHS, early protective hemodynamic strategy. Cdyn, dynamic compliance of respiratory system; Rawi, inspiratory airway resistance; Rawe, expiratory airway resistance; WOB, total inspiratory work of breathing (*p<0.05 compared to baseline by two-way analysis of variance for repeated measures; #p<0.05 compared to one hour by two-way analysis of variance for repeated measures).
Pulmonary edema estimation by calculating the WW/DW ratio was increased in the two groups, but it was higher in the EPHS group than in the EPLS group (6.8±0.8g in the EPHS group and 5.8±0.8g in the EPLS, p: 0.02; normal value in our laboratory ∼5g).
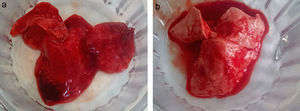
In all of the animals, the lungs exhibited gross damage, including edema with petechial hemorrhages on the surface; however, the lungs of the animals in the EPHS group exhibited more intense damage, especially hemorrhages (Fig. 3). The histological findings, including alveolar congestion, interstitial and alveolar infiltration/aggregation of neutrophils, alveolar membrane focal thickening, alveolar edema or hemorrhage, bronchiole epithelial desquamation, and hyaline membranes were present in similar degrees in both groups (lung injury score 8.57–11 in the EPHS group and 8.56–9 in the EPLS group; p: 0.4).
Gross pathology surface of right lung of representative animals from (a) the EPHS group and (b) the EPLS group. (a) EPHS shows more intense damage, especially hemorrhagic areas. (b) EPLS shows less hemorrhage with most areas of normal pink. EPHS, early protective hemodynamic strategy; EPLS, early protective lung strategy.
The main finding of this experimental study is that a preemptive hemodynamic intervention by restricting the administration of fluids significantly slowed the progression of pulmonary edema and the decreased pulmonary compliance in OA-induced acute lung injury. When fluids were administered more liberally to focus on ensuring the hemodynamic stability, pulmonary edema progression was higher and Cdyn decreased more than when fluids were infused in smaller quantities that were insufficient to prevent hemodynamic deterioration. Therefore, the EPHS proved useful to maintain hemodynamic stability, but it was detrimental to the progression of pulmonary edema; conversely, the EPLS was successful in reducing the progression of pulmonary edema, but it was not effective in preventing hemodynamic deterioration.
ARDS is a catastrophic form of lung injury characterized in more than half of patients by diffuse alveolar damage with severe inflammation and high permeability protein-rich edema in the lungs.8 This syndrome produces results that are notoriously difficult to treat once the diagnosis is made and has an associated mortality approaching 40%.21 Recent studies suggest that the development of lung damage represents a progression of pathology in the lung between the time of insult and the time at which ARDS criteria are met.22,23 During this period of time, referred to by some as the silent phase,24 further attacks or impacts (i.e., second hits) frequently occur. These attacks contribute to the gradual progression from initial mild-moderate lung injury to clinical ARDS.4–7 Most of the time, the second hits are iatrogenic insults, including transfusions, mechanical ventilations with high tidal volumes, delayed treatments of shock and infection, and the inappropriate administration of fluids. Because of the second hits favoring the progression from mild to severe lung injury fulfilling the criteria for ARDS with devastating respiratory failure, there are reasons to believe that the prevention of these hits should result in a decrease in the incidence and severity of ARDS.5 In this regard, we think that our results suggest that fluid administration, even cautiously adjusted to maintain cardiac output, might behave as a second hit influencing the progression of pulmonary edema and favoring the development of ARDS.
The relationship between the administration of fluids and the development of ARDS was already stated over 40 years ago during periods of war. During these times, it was observed that many patients who had suffered severe trauma were strongly raised with crystalloid solutions and were stabilized in the battle field. Later in the hospital, these patients developed a very severe hypoxemic respiratory failure due to pulmonary edema, which often led to death. This entity, called “wet lung”,25,26 at autopsy was characterized by histopathological changes similar to those described later in ARDS.27,28 In the civil stage, several studies have reported a relationship between a positive fluid balance and the development of ARDS29–33; however, to date, there is a widespread opinion that it is difficult to establish whether or not this relationship represents a simple association or a cause-and-effect relationship.34
During the onset of lung injury and the development of diffuse alveolar damage, high permeability pulmonary edema has a central role, acting as a driving force that reduces the airspace available for normal gas exchange and contributes to the disruption of surfactant, which leads to alveolar instability and collapse. Pulmonary edema is determined by the Starling relationship, which predicts the net flow of liquid across a membrane. In acute lung injury, the most common mechanism for a rise in the transcapillary filtration of fluids is an increase in capillary permeability.35 However, experimental studies demonstrated that, at a given increase in capillarity permeability, a modest change in pulmonary vascular pressure greatly modifies the quantity of pulmonary edema.9 That is probably why a strategy of restricting the administration of fluids has demonstrated to be useful in acute lung injury for decreasing pulmonary edema and improving lung function.10 The novelty of our study is that, when this strategy of restricting the administration of fluids was used at the very inception of the injury and pulmonary inflammation, there was less pulmonary edema and less affectation of the respiratory mechanics.
On the other hand, the strategy based on restricting the fluid administration, inadequately maintained cardiac output. The main hemodynamic consequences of OA infusion are a sharp increase in pulmonary vascular resistance with the elevation of pulmonary artery pressure and a significant decline in cardiac output and heart rate.36 Moreover, depending of the doses and the velocity of OA administration, systemic vascular collapse can develop. To reverse the cardiac consequences of OA administration, energetic fluid infusion and/or inotropic drugs are frequently used. For results in the benefits, the fluid administration must increase the heart preload, thereby increasing the systolic volume and the cardiac output. In addition to the cardiac output, transesophageal Doppler measurements include the cFT, a parameter that, although is influenced by the afterload, has been proposed as a value of the preload.18,37 By the end of experiment, the cFT was higher in the EPHS group, and therefore, we believe that the animals in the EPHS group maintained cardiac output, at least during the second hour of the experiment, mainly by increasing the cardiac preload. However, the importance of maintaining the cardiac output during ARDS development is not well known. Although adequate cardiac output is necessary to ensure enough oxygen delivery, high cardiac output can be detrimental for lung injury enhancing transcapillary filtration and pulmonary edema.38 Therefore, it is possible that in our experiment, a slight decrease in the cardiac output in the animals in the EPLS group was useful to protect the lungs.
In addition to the experimental nature of this study, there are some limitations to our study. First, the lung injury model we used. Although OA administered to animals increases pulmonary vascular permeability and produces a condition that pathophysiologically resembles acute lung injury in humans,13 the severity or extensiveness of the injury depends on the doses and the direction of the OA administration: a simple bolus, repeated injections, or a continuous infusion. We chose a model by a slow and steady infusion of OA for 2h11 that provides a better hemodynamic tolerance and allows for the monitoring of hemodynamic and respiratory changes in detail. However, when the OA is administered in this way, lung damage may progress for at least 6h.11 Therefore, it is very likely that the short duration of our study prevented the full development of lung injury in both of the groups. Another important aspect of the study is the way in which the fluids were administered. For the EPHS group, we selected a set amount of fluid to keep CI based on previous preliminary observations we made in our laboratory. However, it is possible that the animals in the EPHS group received an excessive and inadequate amount of fluids, and a component of overhydration was present. Obviously the inclusion of a sham group could have helped to clarify this issue. With respect to the measurement of cardiac output by oesophageal Doppler there is little experience about precision in small animals,39 but we believe that a previous analysis published by our own group40 strongly supports the accuracy of the reported differences in CI between the two groups. Finally, although we graded the histopathologic findings using a scoring system, we did not perform an extensive study. The fact that there was more macroscopic involvement in the lungs of the EPHS group (Fig. 3), raises the question that the histological damage was also more extensive in this group.
In conclusion, the results of our study entrench a cause-and-effect relationship between fluids and the development of pulmonary edema at the beginning of acute lung injury with diffuse alveolar damage and demonstrate that, at the very inception of the injury and the pulmonary inflammation, an early strategy of restricting the infusion of fluids is associated with a reduction in the progression of pulmonary edema and the deterioration of lung mechanics. Thus, we believe that in a clinical setting with patients at risk of developing ARDS a strategy of restricting the administration of fluids should be investigated.
FundingThis study did not receive any funding after being rejected twice by the Ministry of Health of the Government of Andalusia in 2008 calls (PI-0174) and 2009 (PI-0264).
Conflict of interestsAnselmo Gil Cano has received Honoraria from Edwards Lifesciences. Manuel Ignacio Monge García was consultant for Edwards Lifesciences and received travel expenses from Deltex. Manuel Gracia Romero, Pedro Guijo González and José Ruiz Campos have no conflict of interest to declare.
To the Dr. Carlos Costela Villodres, the director of the Center of Experimentation and Animal Production of the University of Cadiz for his advice and collaboration during the development of the study.