To determine whether the prior usage of the flu vaccine is a risk factor for bacterial co-infection in patients with severe influenza.
DesignThis was a retrospective observational cohort study of subjects admitted to the ICU. A propensity score matching, and logistic regression adjusted for potential confounders were carried out to evaluate the association between prior influenza vaccination and bacterial co-infection.
Settings184 ICUs in Spain due to severe influenza.
PatientsPatients included in the Spanish prospective flu registry.
InterventionsFlu vaccine prior to the hospital admission.
ResultsA total of 4175 subjects were included in the study. 489 (11.7%) received the flu vaccine prior to develop influenza infection. Prior vaccinated patients were older 71 [61–78], and predominantly male 65.4%, with at least one comorbid condition 88.5%. Prior vaccination was not associated with bacterial co-infection in the logistic regression model (OR: 1.017; 95%CI 0.803–1.288; p=0.885). After matching, the average treatment effect of prior influenza vaccine on bacterial co-infection was not statistically significant when assessed by propensity score matching (p=0.87), nearest neighbor matching (p=0.59) and inverse probability weighting (p=0.99).
ConclusionsNo association was identified between prior influenza vaccine and bacterial coinfection in patients admitted to the ICU due to severe influenza. Post influenza vaccination studies are necessary to continue evaluating the possible benefits.
Determinar si el uso previo de la vacuna antigripal es un factor de riesgo para coinfección bacteriana en pacientes con influenza grave.
DiseñoEste fue un estudio de cohorte observacional retrospectivo de sujetos ingresados en la UCI. Se realizó un emparejamiento por puntuación de propensión y una regresión logística ajustada para posibles factores de confusión para evaluar la asociación entre el antecedente de vacunación contra la gripe y la coinfección bacteriana.
ÁmbitoCiento ochenta y cuatro ingresos en UCI españolas por gripe grave.
PacientesPacientes incluidos en el registro prospectivo español de gripe.
IntervencionesVacuna antigripal previa al ingreso hospitalario.
ResultadosSe incluyó en el estudio un total de 4.175 sujetos. Recibieron la vacuna contra la influenza antes de desarrollar la infección por influenza 489 (11,7%). Los pacientes previamente vacunados eran mayores de 71 años (RIC 61-78), predominantemente varones (65,4%) y con al menos una condición comórbida (88,5%). La vacunación previa no se asoció con la coinfección bacteriana en el modelo de regresión logística (OR: 1,017; IC95% 0,803-1,288; p=0,885). Después del emparejamiento, el efecto promedio del tratamiento del antecedente de vacuna contra la influenza sobre la coinfección bacteriana no fue estadísticamente significativo cuando se evaluó mediante el emparejamiento por puntuación de propensión (p=0,87), por emparejamiento del vecino más cercano (p=0,59) y mediante la ponderación de probabilidad inversa (p=0,99).
ConclusionesNo se identificó asociación entre el antecedente de vacuna antigripal y coinfección bacteriana en pacientes ingresados en UCI por influenza severa. Más estudios para evaluar los efectos de la vacunación contra la gripe son necesarios para continuar evaluando los posibles beneficios.
Influenza infection is the leading cause of infectious dead worldwide; annual epidemics are estimated to result in about 3–5 million severe illness cases and about 290,000–650,000 deaths.1 This highly transmissible respiratory disease is caused by the influenza virus of the orthomyxovirus family2; this virus can easily adapt due to its high mutation rates that allow it to evade the immune system and reassort their segmented genomes to create antigenically novel pandemic strains.3 Importantly, the “Great Influenza” pandemic of 1918 remains the worst outbreak of an infectious disease in recent history, and influenza infection continues affecting millions of people every year around the globe.4
The influenza vaccine is the most effective way to prevent influenza infection.5 Moreover, several influenza vaccines are currently available in the market. Inactive influenza vaccines (IIV), recombinant hemagglutinin influenza vaccine (RIV) and live attenuated influenza vaccine (LAIV) have been approved by the United States Food and Drug Administration (FDA).5,6 One of the main presentations of influenza infection is severe pneumonia; importantly, most patients who died due to influenza pneumonia develop bacterial co-infection. Influenza virus alters host susceptibility to different bacterial strains such as Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes and Hemophilus influenza among others.7 Influenza-associated bacterial co-infections contribute to severe disease and mortality (i.e., 49–95%) during pandemic and seasonal influenza.8
In addition, it has been seen that LAIV, given as a nasal spray, stimulates immune responses and interaction between bacterial pathogens, increasing the bacterial carriage and density of S. pneumoniae, Moraxella catarrhalis, H. influenzae and S. aureus.7 These phenomena switch the bacterial niche that affects transmission dynamics and bacterial transmission to other contacts, not vaccinated unintentionally.9,10 Importantly, this effect was observed in a randomized controlled trial published in 2015, where researchers selected a total of 151 healthy children to receive trivalent LAIV and evaluate the effects of viral infection and bacterial carriage and how bacterial density increases 6 times at 28-days after vaccination compared to the control group.11 Some studies has demonstrated that all kind of vaccine induced immunity is not always effective at blocking infection and pathogen transmission,12 once the host has been vaccinated, the vaccine can induce a mild or moderate infection can leave the susceptible to a coinfection.13,14 Therefore, we it is unknow the role of flu vaccines in bacterial co-infection in patients admitted to the hospital due to severe influenza infection.
We hypothesize that the influenza vaccine can influence changes in the upper respiratory tract's microbiota that increases the risk of bacterial co-infection in patients with severe influenza infection. Therefore, this study's objective is to determine whether prior vaccination against influenza can be considered a risk factor for bacterial co-infection in patients with severe influenza infection hospitalized in the ICU.
Patients and methodsStudy designThis was a retrospective observational cohort study of subjects admitted to 184 ICUs due to severe influenza infection in Spain between June 2009 and June 2019.15 The patients were included in a voluntary registry created by Spanish Society of Critical and Intensive Medicine and Coronary Units (SEMICYUC).16 Data was collected by the attending physicians through review of medical records, laboratory data and radiological records. This research was categorized as risk-free, and the requirement of informed consent was waived due to the observational nature of the study; the protocol was approved by the Institutional Review Board of the Universidad de la Sabana (IRBref#11,809).
ParticipantsThe cohort includes patients hospitalized in the ICU due to severe influenza; defined as a patient with fever (>38°C), presence of respiratory symptoms such are: cough, sore throat, myalgia or influenza-like illness, radiography with alveolar infiltrates (pneumonia), and that required additional management due to shock (vasopressor requirement), acute respiratory failure and/or multiple organ failure due to influenza infection. The viral diagnosis was carried out by reverse transcription-polymerase chain reaction (rt-PCR) upon admission to the ICU at each hospital and following,17,18 for the microbiological confirmation of influenza A or B virus. All patients included in the cohort were analyzed in this study.16
Data collectionThe following variables were recorded during ICU admission: demographic data, comorbidities and prior vaccination against flu during the corresponding season, presence or absence of bacterial co-infection upon admission to the ICU, and microbiological isolation. Disease severity was determined by Acute Physiology and Chronic Health Evaluation (APACHE II score),19 and organ failure was assessed using the Sequential Organ Failure Assessment (SOFA score).20
Study definitionsBacterial co-infection was defined as the isolation of respiratory bacterial pathogens in a respiratory sample (e.g., bronchoalveolar lavage, sputum, or pleural fluid) and/or blood culture within the first 24h of ICU admission.15,17,18
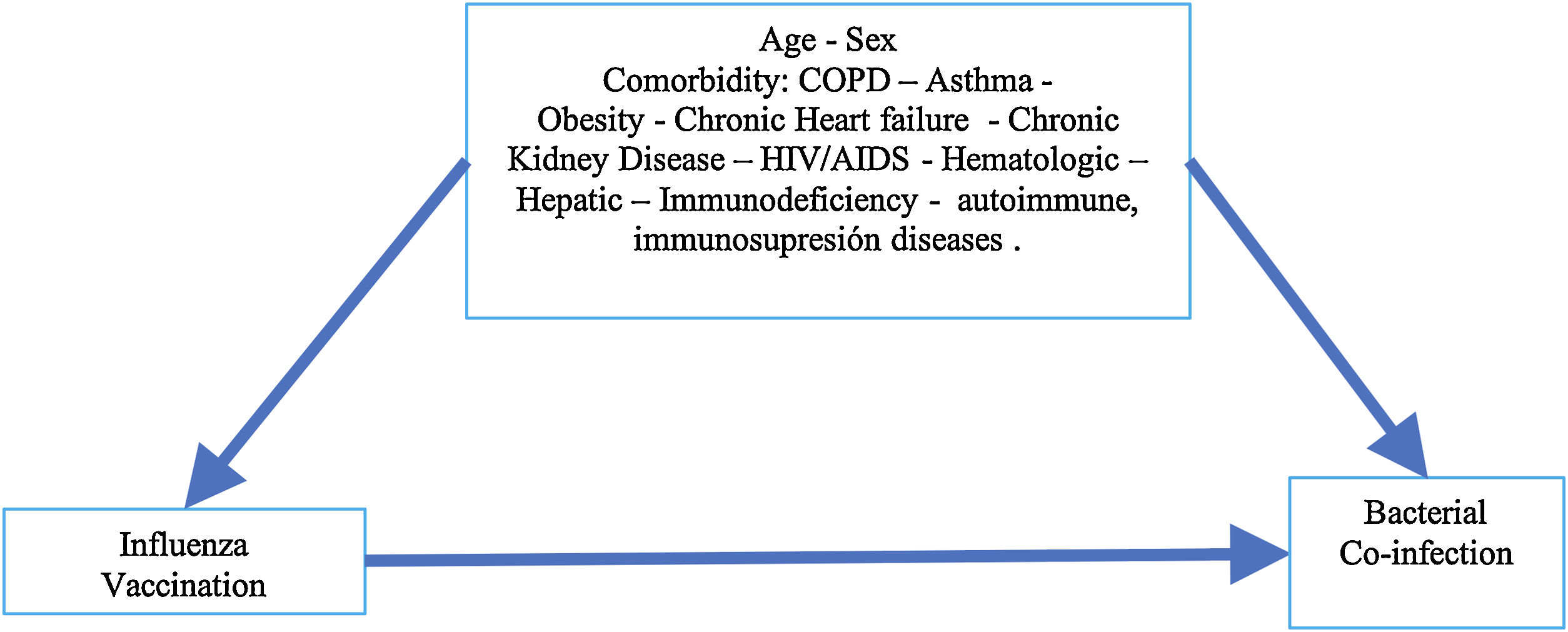
Statistical analysisDiscrete variables were expressed as percentages, and continuous variables with normal distribution were expressed as means (standard deviation), variables with no normal distribution were expressed as median (interquartile ranges, percentile 25 and 75, p25/p75). Categorical variables are presented in counts (percentages) and were evaluated through the Chi-square test. For continuous variables with normal distribution, the t Student test was performed, and for variables with no normal distribution Wilcoxon-Mann–Whitney test was used. Our hypothesis system contemplates that vaccination against influenza is a risk factor for bacterial co-infection, as presented in an acyclic diagram graph (Fig. 1); where the variables that could be possible confounders in the causal pathway of bacterial co-infection were identified in patients with severe influenza exposed to influenza vaccination before hospital admission. Multivariable logistic regression analysis and propensity score matching (PSM) were carryout to evaluate this association.21
A multivariate logistic regression model was developed to evaluate influenza vaccination's association in patients with severe influenza and bacterial co-infection (dependent variable). The explanatory variables included demographic (e.g., sex and age), comorbid conditions (e.g., asthma, chronic obstructive pulmonary disease (COPD), heart failure, chronic kidney disease, hematologic disease, pregnancy, obesity, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), hepatic, neuromuscular, autoimmune, immunodeficiency disease) and evidence of prior influenza vaccination. Variables with a p<0.25 in the initial bivariate analysis were included in the logistic regression model, then the best model was evaluated in terms of AUC, and the goodness of fit for the model was assessed with the Hosmer Lemeshow test.
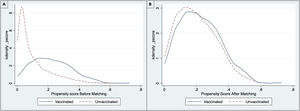
A PSM analysis was then developed to reduce selection bias from different baseline characteristics between individuals vaccinated and unvaccinated prior to hospitalization. First, the balance of the variables between the treated group (vaccinated) and untreated (not vaccinated) was evaluated through standardized means. Then, a propensity score was estimated through a logistic regression model, and the matching was performed using PSM, nearest neighbor matching (NNM), kernel method, and inverse probability weighting (IPW).22,23 The variables used to calculate the propensity score were obtained from the acyclic diagram graph (Fig. 1), which was also used for the logistic regression model variables. The balance pre- and post-matching was compared using the standardized mean differences and the Rubin index to ensure a good balance of the vaccinated and not vaccinated groups.22,24 After matching the subjects, a calculation of average treatment effect (ATE) and moderate treatment effects among treated subjects (ATET) were made with their corresponding 95% confidence intervals (95%CI) estimated by the Robust Method. Statistical significance was set at p<0.05; All statistical analysis was carried out in the R studio 1.3.1056 statistical package for macOS and STATA 14.
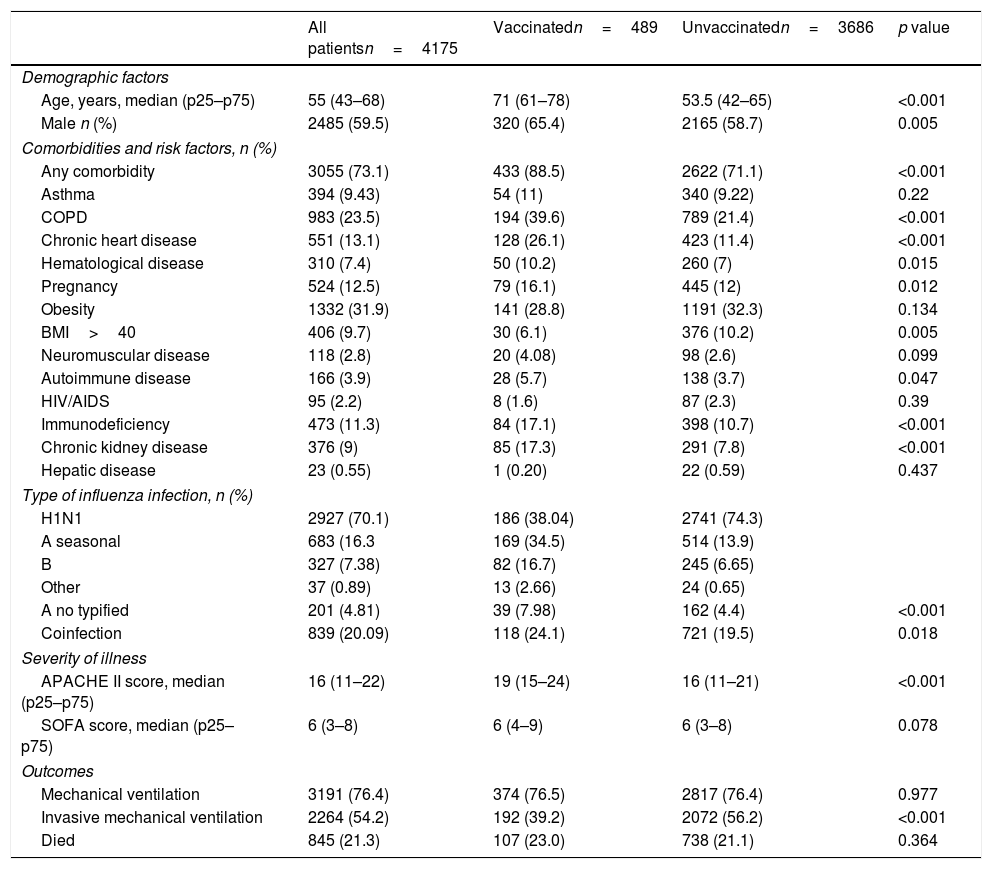
ResultsA total of 4175 subjects were analyzed in this study (Table 1). The median (p25–p75) age was 55 years (43–68), and 59.5% were male. Additionally, 73.1% of the subjects have at least one comorbid condition; being obesity 31.9% the most frequent chronic condition, followed by COPD 23.5% and chronic heart disease 13.1% (Table 1). Bacterial co-infection was observed in 20.09% of the subjects. Notably, one patient might have several bacterial isolations; thus, the final number of bacterial pathogens was 1833 (Table 2). Regarding disease severity, the median (p25–p75) APACHE II and SOFA scores were 16 (11–22) and 6 (3–8), respectively. Of all patients in the cohort, 76.4% required mechanical ventilation, 54.2% required invasive mechanical ventilation, and 21.3% of patients died.
Baseline characteristics of the cohort.
| All patientsn=4175 | Vaccinatedn=489 | Unvaccinatedn=3686 | p value | |
|---|---|---|---|---|
| Demographic factors | ||||
| Age, years, median (p25–p75) | 55 (43–68) | 71 (61–78) | 53.5 (42–65) | <0.001 |
| Male n (%) | 2485 (59.5) | 320 (65.4) | 2165 (58.7) | 0.005 |
| Comorbidities and risk factors, n (%) | ||||
| Any comorbidity | 3055 (73.1) | 433 (88.5) | 2622 (71.1) | <0.001 |
| Asthma | 394 (9.43) | 54 (11) | 340 (9.22) | 0.22 |
| COPD | 983 (23.5) | 194 (39.6) | 789 (21.4) | <0.001 |
| Chronic heart disease | 551 (13.1) | 128 (26.1) | 423 (11.4) | <0.001 |
| Hematological disease | 310 (7.4) | 50 (10.2) | 260 (7) | 0.015 |
| Pregnancy | 524 (12.5) | 79 (16.1) | 445 (12) | 0.012 |
| Obesity | 1332 (31.9) | 141 (28.8) | 1191 (32.3) | 0.134 |
| BMI>40 | 406 (9.7) | 30 (6.1) | 376 (10.2) | 0.005 |
| Neuromuscular disease | 118 (2.8) | 20 (4.08) | 98 (2.6) | 0.099 |
| Autoimmune disease | 166 (3.9) | 28 (5.7) | 138 (3.7) | 0.047 |
| HIV/AIDS | 95 (2.2) | 8 (1.6) | 87 (2.3) | 0.39 |
| Immunodeficiency | 473 (11.3) | 84 (17.1) | 398 (10.7) | <0.001 |
| Chronic kidney disease | 376 (9) | 85 (17.3) | 291 (7.8) | <0.001 |
| Hepatic disease | 23 (0.55) | 1 (0.20) | 22 (0.59) | 0.437 |
| Type of influenza infection, n (%) | ||||
| H1N1 | 2927 (70.1) | 186 (38.04) | 2741 (74.3) | |
| A seasonal | 683 (16.3 | 169 (34.5) | 514 (13.9) | |
| B | 327 (7.38) | 82 (16.7) | 245 (6.65) | |
| Other | 37 (0.89) | 13 (2.66) | 24 (0.65) | |
| A no typified | 201 (4.81) | 39 (7.98) | 162 (4.4) | <0.001 |
| Coinfection | 839 (20.09) | 118 (24.1) | 721 (19.5) | 0.018 |
| Severity of illness | ||||
| APACHE II score, median (p25–p75) | 16 (11–22) | 19 (15–24) | 16 (11–21) | <0.001 |
| SOFA score, median (p25–p75) | 6 (3–8) | 6 (4–9) | 6 (3–8) | 0.078 |
| Outcomes | ||||
| Mechanical ventilation | 3191 (76.4) | 374 (76.5) | 2817 (76.4) | 0.977 |
| Invasive mechanical ventilation | 2264 (54.2) | 192 (39.2) | 2072 (56.2) | <0.001 |
| Died | 845 (21.3) | 107 (23.0) | 738 (21.1) | 0.364 |
APACHE: Acute Physiology and Chronic Health Evaluation; BMI: body mass index; COPD: chronic obstructive pulmonary disease; HIV/AIDS: human immunodeficiency virus/acquired immunodeficiency syndrome; SOFA: Sequential Organ Failure Assessment.
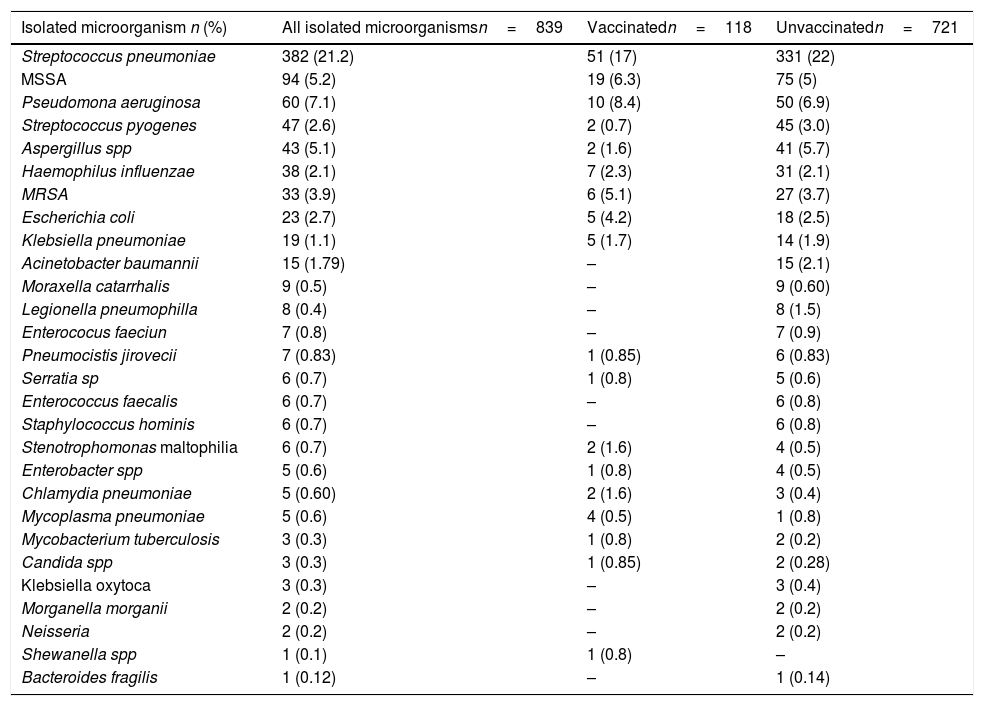
Isolated microorganisms.
| Isolated microorganism n (%) | All isolated microorganismsn=839 | Vaccinatedn=118 | Unvaccinatedn=721 |
|---|---|---|---|
| Streptococcus pneumoniae | 382 (21.2) | 51 (17) | 331 (22) |
| MSSA | 94 (5.2) | 19 (6.3) | 75 (5) |
| Pseudomona aeruginosa | 60 (7.1) | 10 (8.4) | 50 (6.9) |
| Streptococcus pyogenes | 47 (2.6) | 2 (0.7) | 45 (3.0) |
| Aspergillus spp | 43 (5.1) | 2 (1.6) | 41 (5.7) |
| Haemophilus influenzae | 38 (2.1) | 7 (2.3) | 31 (2.1) |
| MRSA | 33 (3.9) | 6 (5.1) | 27 (3.7) |
| Escherichia coli | 23 (2.7) | 5 (4.2) | 18 (2.5) |
| Klebsiella pneumoniae | 19 (1.1) | 5 (1.7) | 14 (1.9) |
| Acinetobacter baumannii | 15 (1.79) | – | 15 (2.1) |
| Moraxella catarrhalis | 9 (0.5) | – | 9 (0.60) |
| Legionella pneumophilla | 8 (0.4) | – | 8 (1.5) |
| Enterococus faeciun | 7 (0.8) | – | 7 (0.9) |
| Pneumocistis jirovecii | 7 (0.83) | 1 (0.85) | 6 (0.83) |
| Serratia sp | 6 (0.7) | 1 (0.8) | 5 (0.6) |
| Enterococcus faecalis | 6 (0.7) | – | 6 (0.8) |
| Staphylococcus hominis | 6 (0.7) | – | 6 (0.8) |
| Stenotrophomonas maltophilia | 6 (0.7) | 2 (1.6) | 4 (0.5) |
| Enterobacter spp | 5 (0.6) | 1 (0.8) | 4 (0.5) |
| Chlamydia pneumoniae | 5 (0.60) | 2 (1.6) | 3 (0.4) |
| Mycoplasma pneumoniae | 5 (0.6) | 4 (0.5) | 1 (0.8) |
| Mycobacterium tuberculosis | 3 (0.3) | 1 (0.8) | 2 (0.2) |
| Candida spp | 3 (0.3) | 1 (0.85) | 2 (0.28) |
| Klebsiella oxytoca | 3 (0.3) | – | 3 (0.4) |
| Morganella morganii | 2 (0.2) | – | 2 (0.2) |
| Neisseria | 2 (0.2) | – | 2 (0.2) |
| Shewanella spp | 1 (0.1) | 1 (0.8) | – |
| Bacteroides fragilis | 1 (0.12) | – | 1 (0.14) |
MSSA: methicillin susceptible Staphylococcus aureus; MSRA: methicillin resistant Staphylococcus aureus.
The most frequent isolated microorganism was S. pneumoniae 21.2% followed by methicillin-susceptible S. aureus (MSSA) 5.2%. Other isolated microorganisms were Klebsiella pneumoniae 1.1%, Legionella pneumophila 0.4%, H. influenzae 2.1% and M. catarrhalis 0.50%.
Comparing the vaccinated and unvaccinated groupsA total of 489 vaccinated (prior to hospital admission) subjects and 3686 non-vaccinated subjects were included in the study. Patients in the vaccinated group were older (71 [61–78] vs 53.5 [42–65] years; p<0.001) and predominantly male (65.4% vs 58.7%; p=0.005), subjects with at least one comorbid condition were more frequently identified in the vaccinated group (88.5% vs 71.1%; p<0.001).
Vaccinated subjects were more frequently coinfected compared to unvaccinated subjects (24.1% vs 19.5% p=0.020) in the bivariate analysis. About the isolated microorganisms in subjects with bacterial co-infection unvaccinated group had a higher proportion of S. pneumoniae (22% vs 17% p=0.052) in contrast, MSSA isolations were more frequent in vaccinated subjects (6.3% vs 5% p=0.339).
Importantly, bacterial co-infection was associated with an increased risk of death (relative risk (RR):1.848 [95%CI: 1.552–2.200], p<0.001), the need for mechanical ventilation (RR:1.341 [95%CI:1.112–1.619], p=0.002), and invasive mechanical ventilation (RR:1.526 [95%CI:1.306–1.782], p<0.001). Regarding severity scores, APACHE score in bacterial co-infection subjects was higher median (p25–p75) (19 [14–24] vs 15 [11–21], p<0.001), as well as SOFA score for bacterial co-infection subjects (7 [4–10] vs 5[3–8], p<0.001).
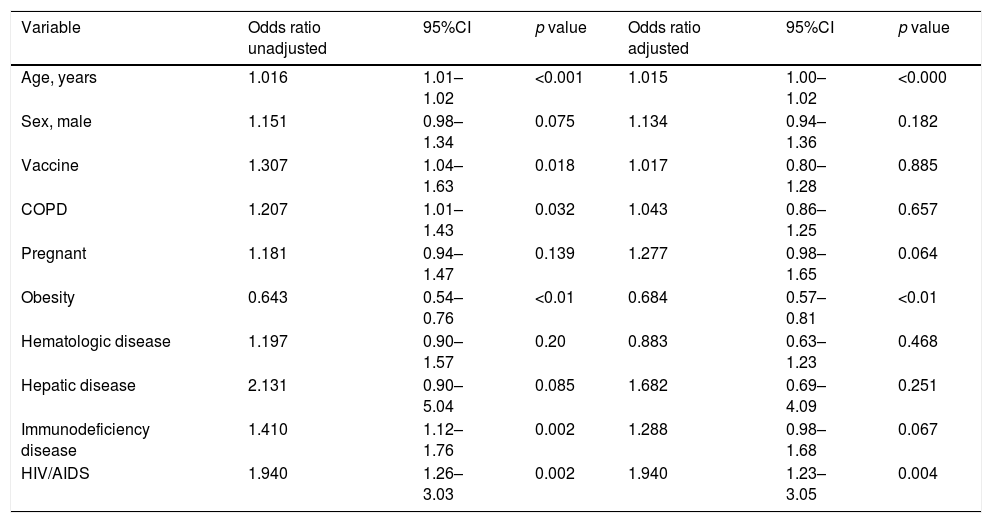
Influenza vaccination and secondary bacterial co-infection, using multivariable logistic regressionThe variables included in the model were age (OR:1.015; 95%CI: 1.00–1.02), obesity (OR:0.684; 95%CI: 0.574–0.816), and HIV (OR:1.940; 95%CI:1.2–3.051). Prior vaccination was not associated with bacterial co-infection in the logistic regression model (OR: 1.017; 95%CI: 0.803–1.288; p=0.885) (Table 3) Hosmer Lemeshow test p=0.698.
Unadjusted and adjusted risk factors for co-infection in patients with influenza infection.
| Variable | Odds ratio unadjusted | 95%CI | p value | Odds ratio adjusted | 95%CI | p value |
|---|---|---|---|---|---|---|
| Age, years | 1.016 | 1.01–1.02 | <0.001 | 1.015 | 1.00–1.02 | <0.000 |
| Sex, male | 1.151 | 0.98–1.34 | 0.075 | 1.134 | 0.94–1.36 | 0.182 |
| Vaccine | 1.307 | 1.04–1.63 | 0.018 | 1.017 | 0.80–1.28 | 0.885 |
| COPD | 1.207 | 1.01–1.43 | 0.032 | 1.043 | 0.86–1.25 | 0.657 |
| Pregnant | 1.181 | 0.94–1.47 | 0.139 | 1.277 | 0.98–1.65 | 0.064 |
| Obesity | 0.643 | 0.54–0.76 | <0.01 | 0.684 | 0.57–0.81 | <0.01 |
| Hematologic disease | 1.197 | 0.90–1.57 | 0.20 | 0.883 | 0.63–1.23 | 0.468 |
| Hepatic disease | 2.131 | 0.90–5.04 | 0.085 | 1.682 | 0.69–4.09 | 0.251 |
| Immunodeficiency disease | 1.410 | 1.12–1.76 | 0.002 | 1.288 | 0.98–1.68 | 0.067 |
| HIV/AIDS | 1.940 | 1.26–3.03 | 0.002 | 1.940 | 1.23–3.05 | 0.004 |
COPD: chronic obstructive pulmonary disease; HIV/AIDS: human immunodeficiency virus/acquired immunodeficiency syndrome.
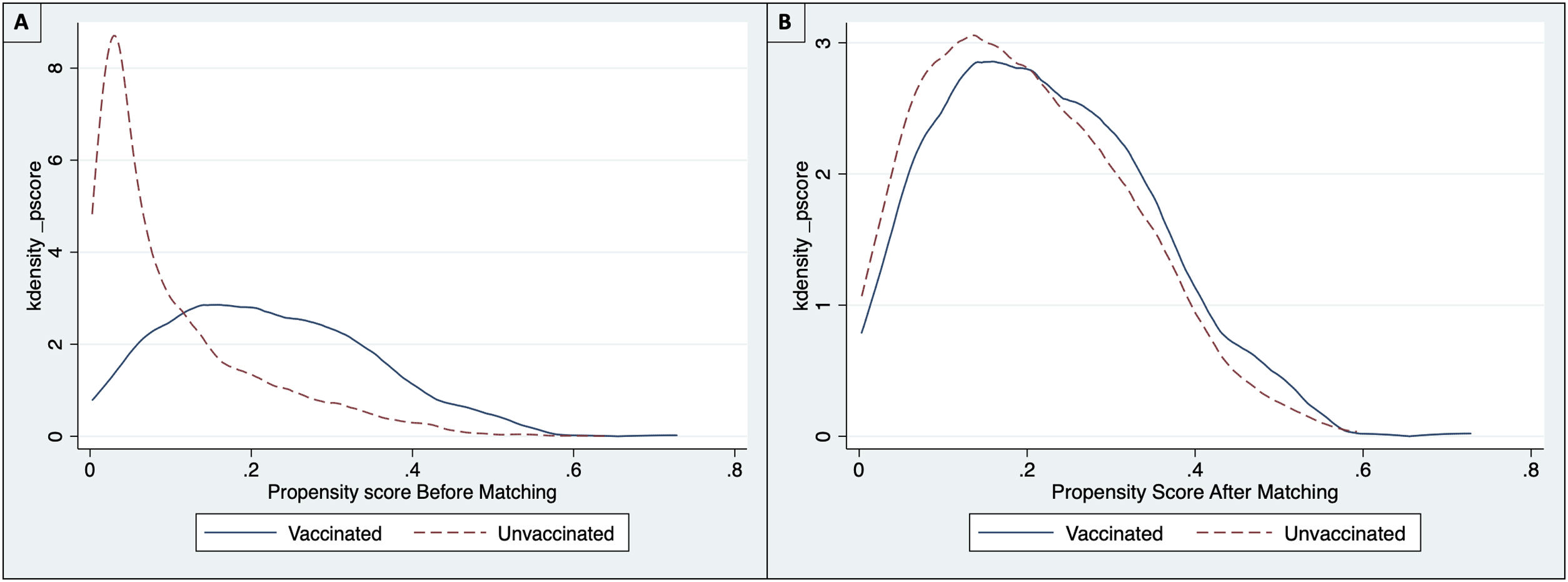
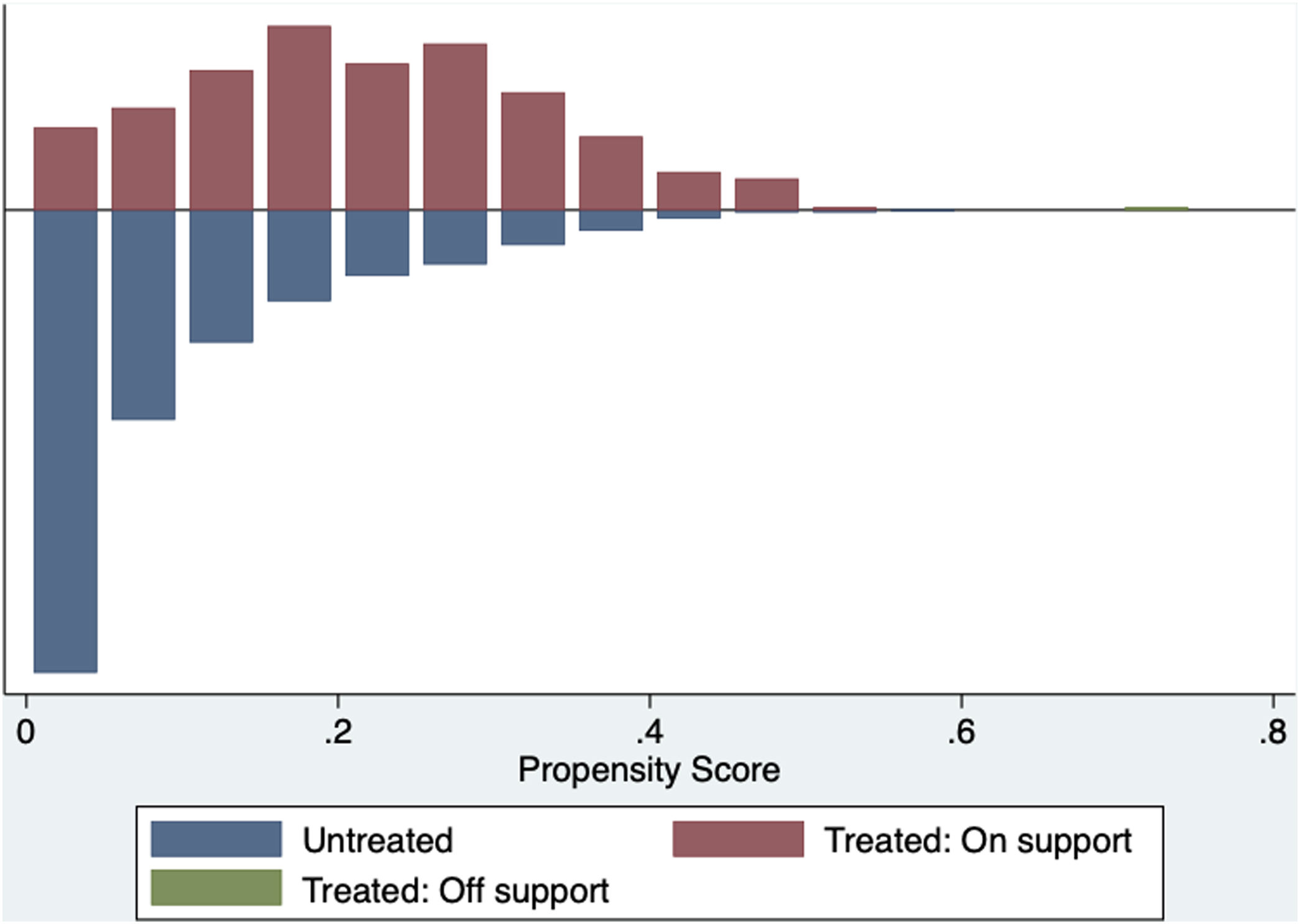
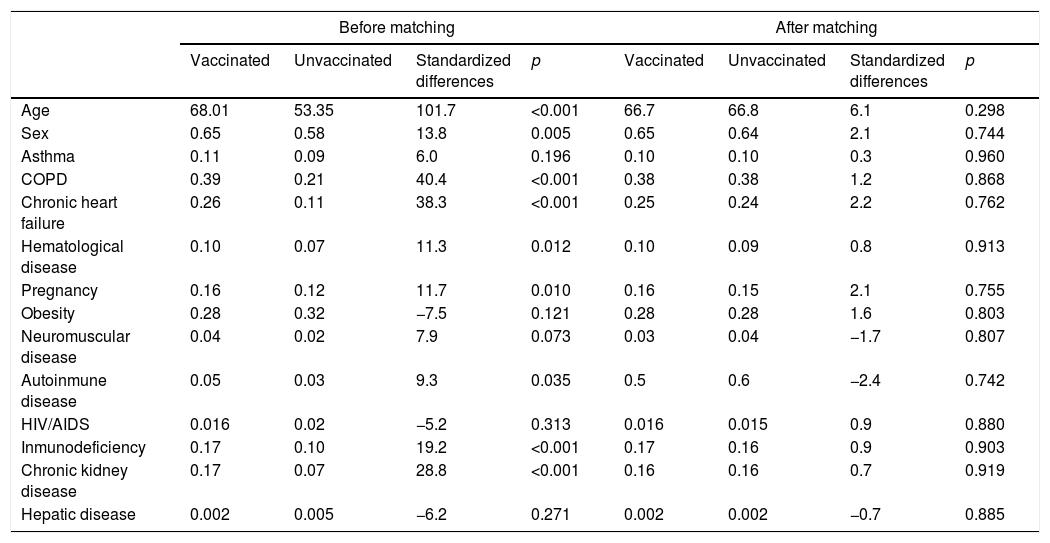
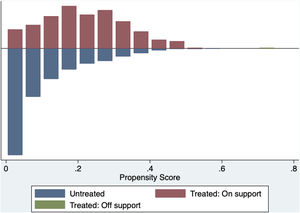
The common support area allows to include a large number of vaccinated and non-vaccinated subjects. (Fig. 2) Before matching age, COPD, chronic heart failure, immunodeficiency and chronic kidney disease were unbalanced among the groups; however, these differences were less than 10% after the balancing by propensity score (Table 4). The Rubin index decreased from 114.2 before matching to 9.1 after matching, obtaining a balance of variables and adequate bias reduction.18 Also, matching was graphically evaluated. (Fig. 3) The average effect on the treated (ATT) was 0.005, difference that decreased after matching.
Balance of selected variables before and after matching.
| Before matching | After matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | Standardized differences | p | Vaccinated | Unvaccinated | Standardized differences | p | |
| Age | 68.01 | 53.35 | 101.7 | <0.001 | 66.7 | 66.8 | 6.1 | 0.298 |
| Sex | 0.65 | 0.58 | 13.8 | 0.005 | 0.65 | 0.64 | 2.1 | 0.744 |
| Asthma | 0.11 | 0.09 | 6.0 | 0.196 | 0.10 | 0.10 | 0.3 | 0.960 |
| COPD | 0.39 | 0.21 | 40.4 | <0.001 | 0.38 | 0.38 | 1.2 | 0.868 |
| Chronic heart failure | 0.26 | 0.11 | 38.3 | <0.001 | 0.25 | 0.24 | 2.2 | 0.762 |
| Hematological disease | 0.10 | 0.07 | 11.3 | 0.012 | 0.10 | 0.09 | 0.8 | 0.913 |
| Pregnancy | 0.16 | 0.12 | 11.7 | 0.010 | 0.16 | 0.15 | 2.1 | 0.755 |
| Obesity | 0.28 | 0.32 | −7.5 | 0.121 | 0.28 | 0.28 | 1.6 | 0.803 |
| Neuromuscular disease | 0.04 | 0.02 | 7.9 | 0.073 | 0.03 | 0.04 | −1.7 | 0.807 |
| Autoinmune disease | 0.05 | 0.03 | 9.3 | 0.035 | 0.5 | 0.6 | −2.4 | 0.742 |
| HIV/AIDS | 0.016 | 0.02 | −5.2 | 0.313 | 0.016 | 0.015 | 0.9 | 0.880 |
| Inmunodeficiency | 0.17 | 0.10 | 19.2 | <0.001 | 0.17 | 0.16 | 0.9 | 0.903 |
| Chronic kidney disease | 0.17 | 0.07 | 28.8 | <0.001 | 0.16 | 0.16 | 0.7 | 0.919 |
| Hepatic disease | 0.002 | 0.005 | −6.2 | 0.271 | 0.002 | 0.002 | −0.7 | 0.885 |
COPD: chronic obstructive pulmonary disease; HIV/AIDS: human immunodeficiency virus/acquired immunodeficiency syndrome.
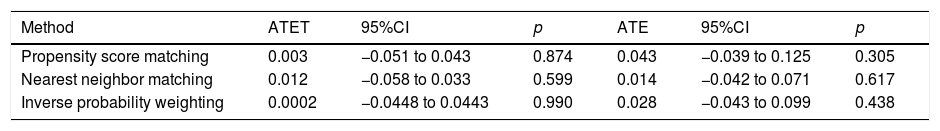
PSM by the NNM method was performed using 8 subjects non-vaccinated for every subject vaccinated. Finally, no differences were found when performing the calculation of average treatment effects among treated subjects (ATET) and average treatment effect (ATE) by PSM, NNM and IPW (Table 5).
Effect of prior influenza vaccine on bacterial co-infection.
| Method | ATET | 95%CI | p | ATE | 95%CI | p |
|---|---|---|---|---|---|---|
| Propensity score matching | 0.003 | −0.051 to 0.043 | 0.874 | 0.043 | −0.039 to 0.125 | 0.305 |
| Nearest neighbor matching | 0.012 | −0.058 to 0.033 | 0.599 | 0.014 | −0.042 to 0.071 | 0.617 |
| Inverse probability weighting | 0.0002 | −0.0448 to 0.0443 | 0.990 | 0.028 | −0.043 to 0.099 | 0.438 |
Propensity Score matching and nearest neighbor matching performed using 8 unvaccinated subjects per vaccinated subject. ATET: average treatment effects among treated; ATE: average treatment effect in population; 95%CI: confidence interval, obtained by the Robust Method.
Our study found that prior influenza vaccine was not associated with the development of bacterial co-infection in patients admitted to the ICU due to severe influenza infection. We observed that non-vaccinated subjects had a higher invasive mechanical ventilation requirement than vaccinated subjects. We also found that vaccinated subjects had a higher proportion of associated comorbidities than non-vaccinated subjects, and the median APACHE II score was superior in this group of patients.
Inactive influenza vaccines (IIV) and live attenuated influenza vaccine (LAIV) have been evaluated in mice studies, where vaccine-induced immunity against influenza virus and improved survival after a bacterial co-infection,6 but did not always yield complete protection. For instance, the flu vaccine can yield a mild infection that may leave the vaccinated host susceptible to a bacterial co-infection.14 Also, It has been observed that the influenza vaccine can modify the host‘s microbiota, increasing the bacterial carriage and density of the upper respiratory tract by microorganism increasing the risk of bacterial co-infection.25 It is possible that changes in the microbiota of vaccinated patients depend on the kind of vaccine used (e.g., IIV, RIV or LAIV).25 Thus, there is a lack of knowledge about this potential unintended effect of the vaccines, because the efficacy of the vaccine is always evaluated as the ability to directly prevent disease from the vaccine target pathogen, it is important to consider positive or negative indirect effects of vaccination as well.6,9,26 However, our results suggest that if there is a change in the colonization of the upper respiratory tract (i.e., microbiome), this does not influence the development of bacterial co-infection in patients with severe influenza. In contrast, Lee et al. estimated protective effects of influenza vaccination against group A streptococcus illness in army recruits during influenza seasons 2002–2006,27 which was not observed in our cohort. The absence of association between prior flu vaccination and bacterial co-infection in our study supports the safety of the flu vaccine and recommendations on the application in adults and children found in multiple studies about vaccination and safety profile.28 Moreover, we also found that there is a potential benefit of prior flu vaccine, by reducing the requirement of mechanical ventilation in patients infected with influenza.
The relation between prior vaccination and bacterial co-infection can be bias in patients with special characteristics where vaccination is more frequent. For example, older patients with comorbidities or vulnerable populations with risk for complications associated with severe influenza.29 Moreover, patients admitted due to severe influenza might have a higher risk of bacterial co-infection due to prior comorbid conditions not related to prior flu vaccination. In our study, 73.1% of the patients had at least one comorbidity, being the most prevalent diseases obesity, COPD, chronic heart disease, and immunodeficiency. These diseases are also considered risk factors for infections by different pathogens (i.e., co-infection) in patients admitted due to influenza infection. For instance, patients over 65 years of age, multiple concomitant diseases or immunosuppression have an increased risk of infection by S. pneumoniae30 or subjects with cardiopulmonary disease have a higher risk of presenting respiratory infections due to Gram-negative bacilli.31,32 Thus, these conditions could be explained by the higher frequency of bacterial coinfection described previously by some studies and not associated with prior vaccination.
Importantly, in our study, the requirement for invasive mechanical ventilation was statistically significantly higher in unvaccinated subjects. Joshi et al., compared a cohort of vaccinated and non-vaccinated subjects, their data suggest that there was no protective effect from prior vaccination in preventing hospital admission, respiratory failure and mortality in a population of older men admitted to the hospital with influenza33; however, several studies have reported influenza vaccine effectiveness in reducing illness severity.34 For example Thompson et al., who reported influenza vaccine effectiveness of 82% in reducing influenza associated ICU admissions among adults.35 Thus, our results builds on the argument that the influenza vaccine may reduce disease severity in patients that develop an influenza infection.
According to the literature, the main isolated microorganisms in subjects with bacterial co-infection are S. pneumoniae and MSSA.15 In our study, these microorganisms had similar proportions between the vaccinated and unvaccinated groups without statistically significant differences. In relation to pneumococcal infection, Influenza virus alters the lungs in a way that predisposes to adherence, invasion and induction of disease by the pneumococcus.36 Importantly, pneumococcal vaccines have been described as a way to reduce the risk of bacterial co-infection or severity of bacterial co-infection in subjects with influenza. Unfortunately, we did not have the information regarding prior pneumococcal vaccination in our cohort.
Our study's main strengths and weaknesses are, first, this is a large and homogenous cohort enrolled during more than ten years; second, this study provides a robust statistical analysis (the logistic regression model and PSM performed getting matching results). However, our study's limitations may be explained by the difficulty of discriminating the kind of vaccine applied to each patient in the cohort, and the differential effect between vaccines cannot be observed. However, most vaccinated subjects receive the IIV, which is approved for any patient other than LAIV.5 In our cohort, we had a small number of people vaccinated; nevertheless, our cohort has 4175 subjects who have been systematically collected for ten years, and it is representative of this kind of population. It is important to highlight that patients with severe influenza that required admission to the ICU frequently develop bacterial coinfection and that it is why we choose this population to carry out this project. However, this constitutes an important selection bias that should be recognized. Finally, pneumococcal vaccines have been described to reduce the risk of bacterial co-infection or diseases severity in subjects with influenza. Unfortunately, we did not have the information regarding prior pneumococcal vaccination in our cohort, which is an important limitation. However, this study aimed to assess the effect of flu vaccine on bacterial colonization, not the pneumococcal co-infection rate.
In summary, no association was found between bacterial co-infection and prior vaccination in patients admitted to the ICU due to influenza infection. These findings support the safety of the flu vaccine and its recommendations for the application. It is necessary to improve the vaccination rate, especially in subjects with comorbidities due to low vaccine coverage. Post influenza vaccination studies are essential to continue evaluating the benefits of influenza vaccination.
FundingThis study used the voluntary registry created and funded by Spanish Society of Critical and Intensive Medicine and Coronary Units (SEMICYUC).
Authors’ contributionAll authors attest they meet the ICMJE criteria for authorship. JP, LFR and AR designed the study. JP and AB performed all the statistical analyses. IML, ED, BS, GM, MB, MN, AE, JSV, EP, JG and AR enrolled patients. JP, LFR, AB, IML, ED, BS, GM, MB, MN, AE, JSV, DC, EP, JG and AR wrote and reviewed the manuscript.
Conflict of interestAll authors have no conflict of interest.
The authors thank the Spanish Society of Critical and Intensive Medicine and Coronary Units (SEMICYUC) for creating the registry and allow us to use the dataset to carry out this project.