Pulmonary alveolar proteinosis (PAP) is a rare syndrome characterized by a progressive accumulation of a lipoprotein material in the pulmonary alveoli composed mainly by alveolar surfactant, causing respiratory failure, risk of pulmonary infection and/or fibrosis. PAP can occur in the setting of a variety of disorders that affect production or clearance of the surfactant, most frequently considered an autoimmune disease.1 Despite of the development of new therapeutic targets, whole-lung lavage (WLL) remains the gold standard treatment of PAP.2 Sometimes, patient respiratory situation precludes this therapy.
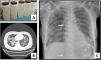
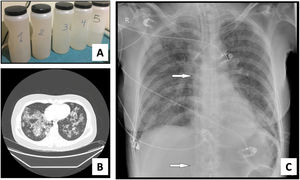
We present a series of three consecutive cases of extracorporeal membrane oxygenation (ECMO) support to perform WLL in patients at risk of complications.Case 1 A 71-year-old woman consulted for worsening dyspnea and supplement oxygen therapy requirement. An interstitial pattern in chest radiography was noted and severe primary PAP diagnosis confirmed by core needle biopsy. Pulmonary function tests showed a restrictive pattern with moderate decrease in diffusion capacity (forced vital capacity [FVC] 1674 mL [72% of predicted value], forced expiratory volume [FEV1] 1575 mL [87%], FEV1/FVC 94%, diffusing capacity of the lung for carbon monoxide [DLCO] 56%, total lung capacity [TLC] 81%). A first WLL was attempted, complicated with progressive respiratory worsening and cardiorespiratory arrest. Procedure was rescheduled after stabilization to be carried out under respiratory support with veno-venous extracorporeal membrane oxygenation (V-V ECMO). After anesthetic induction and intubation with double-lumen endotracheal tube, V-V ECMO was implanted (drainage cannula through left femoral vein [21F] and return cannula through right jugular vein [17F]) (Maquet Cardiopulmonary GmbH, Rastatt, Germany) and maintained with these parameters: flow 3 L/min with FiO2 = 1 and sweep gas of 3 L/min. WLL procedure was completed in a single session by instilling 10 L of saline (1 L aliquots at 37 °C) (Fig. 1A). Ultraprotective ventilation was carried out (volume-limited assist control ventilation with tidal volume 190 mL, respiratory rate 12 bpm, positive end-expiratory pressure [PEEP] 10 cmH2O, FiO2 = 0.9), and no hemodynamic instability occurred. Clinical improvement was observed at 48 h achieving V-V ECMO weaning. Patient was extubated at 72 h and discharged 9 days later, with oxygen discontinuation and radiological improvement. A. Characteristic fluid extracted during WLL, initially with a milky appearance and progressive clearing. B. High-resolution chest CT. Lung parenchyma with extensive ground-glass opacities and associated septal thickening (typical “crazy paving” pattern). C. Chest radiograph. Extensive bilateral alveolointerstitial infiltrates; ECMO cannulae (arrows) properly positioned, with separation >10 cm to minimize recirculation phenomenon. A 35-year-old woman, former smoker, was admitted for progressive dyspnea and respiratory failure. High-resolution chest computed tomography (CT) found extensive “crazy paving” foci suggestive of PAP (Fig. 1B); bronchoalveolar lavage and transbronchial biopsy confirmed the diagnosis. Respiratory function tests showed a normal spirometric pattern with moderately decreased diffusion (FVC 2890 mL [90%], FEV1 2650 mL [98%], FEV1/FVC 92%, DLCO 54%). WLL procedure was scheduled under V-V ECMO. Introducer sheaths were placed in both femoral veins under local anesthesia and sedation, prior to anesthetic induction; after that ECMO was implanted (23F left femoral drainage cannula, 17F right femoral tip-perforated 50-cm return cannula). Extracorporeal support (flow 3 L/min with FiO2 = 1 and sweep gas 2 L/min) and protective one-lung ventilation (volume-limited assist control ventilation with tidal volume 150 mL, respiratory rate 15 bpm, PEEP 8 cmH2O, FiO2 = 0.5) via double-lumen tube were started, performing bilateral lung lavage with saline at 37 °C, through 10 aliquots of 900 mL in each lung. ECMO weaning was attained 12 h later and extubation 48 h after the procedure. ICU stay was 3 days, being discharged home 10 days after the WLL without the need for home oxygen therapy. A 51-year-old woman with previous diagnosis of PAP required ICU admission with respiratory failure. Respiratory function tests presented mild restrictive pattern and severe diffusion impairment (FVC 1570 mL [62%], FEV1 1539 mL [71%], FEV1/FVC 97%, DLCO 28%, TLC 76%). Anesthetic induction with double-lumen tube intubation was performed, prior to implantation of ECMO cannulae (21F drainage cannula through left femoral vein and 50 cm tip-perforated 17 F return cannula through right femoral vein) (Fig. 1C). ECMO parameters were flow 2.6 L/min with FiO2 = 1 and sweep gas 2 L/min. Ultra-protective mechanical ventilation parameters were established (pressure-limited assist control ventilation, maintaining a driving pressure of 10 cmH2O and initial PEEP of 5 cmH2O), and WLL was performed with 10 aliquots of 800 mL in each lung. The procedure was uneventful, achieving ECMO support weaning and extubation 48 h after the procedure. The ICU stay was 5 days, and she was discharged home 8 days after the WLL, without chronic oxygen therapy requirements.
WLL is the treatment of choice for PAP and is indicated considered in the presence of symptoms, hypoxemia, or a deterioration in pulmonary function tests. Observational data have reported an improvement of symptoms and exercise tolerance, radiological infiltrates, respiratory function tests and oxygenation. Despite a safe and well-tolerated procedure, complications as pneumothorax, hydrothorax, pulmonary infection, and respiratory distress can occur.1,2 In the largest cohort described to date, WLL had to be finished prematurely in 9% of the cases, most frequent due to refractory hypoxemia.3
WLL can be performed in a single or two sessions (one per lung). General anesthesia involves high risk of complications because of low functional reserve and poor tolerance to apnea. Vulnerable moments are induction and placement of the double-lumen tube for lung isolation, single-lung ventilation, and extubation.4 Additionally, while performing a single session technique, the procedure on the second lung must be done by ventilating a lung that has just received the lavage, still containing part of the saline that has not been able to be eliminated, which alters diffusion. Removing alveolar surfactant also makes lung easily collapsible, increasing the shunt effect and atelectrauma.5
ECMO support has initially been proposed as a rescue option in the form of veno-arterial ECMO in case of complications. However, according to our series, the use of veno-venous ECMO as perioperative respiratory support for the WLL procedure in the presence of high-risk factors make possible to establish a lung protection strategy and perform simultaneous lavage of both lungs in a single procedure.4,6–10
Our series aims to add information about pre-lavage evaluation and performance under ECMO support. WLL performing centers should have protocols for lung protection through ECMO support in patients at risk, as well as emergent cannulation plans in case of acute complication.
Declaration of interestThe authors declare no conflict of interest.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.