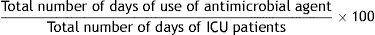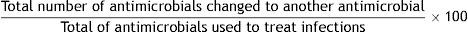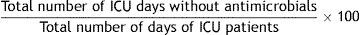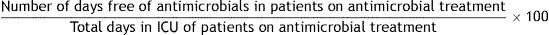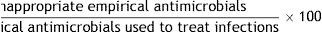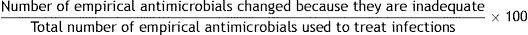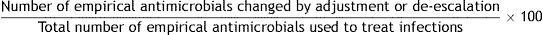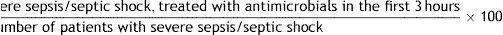Quality indicators have been applied to many areas of health care in recent years, including intensive care. However, they have not been specifically developed and validated for antimicrobial use in critically ill patients. Antimicrobials play a key role in intensive care units not only in the prognosis of each individual patient, but also in the development of resistance and changes in the flora in this setting. Evaluating the use of these agents is complex in the intensive care unit, however, because the indications vary greatly and antimicrobial treatment is often changed during admission.
We designed and developed specific quality indicators regarding the use of antimicrobials in critically ill patients admitted to the intensive care unit. These indicators are proposed as a tool for application in intensive care units to detect problems in the use of antimicrobials. Future trials are needed, however, to validate these indicators in a large population over time.
Los indicadores de calidad se han aplicado a muchas áreas de la atención sanitaria en los últimos años, incluyendo el área de cuidados intensivos. Sin embargo, no se han desarrollado y validado indicadores específicos para el uso de antimicrobianos en pacientes críticos. Los antimicrobianos desempeñan un papel clave en las unidades de cuidados intensivos no sólo en el pronóstico de cada paciente individual, sino también en el desarrollo de resistencias y los cambios en la flora bacteriana. La evaluación del uso de estos fármacos es compleja en las unidades de cuidados intensivos debido a la variedad de indicaciones y a los cambios en el tratamiento antimicrobiano durante el ingreso.
Hemos diseñado y desarrollado un conjunto de indicadores de calidad específicos en relación con el uso de antimicrobianos en pacientes críticos ingresados en las unidades de cuidados intensivos. Estos indicadores se proponen como una herramienta para su aplicación en las unidades de cuidados intensivos para detectar problemas en el uso de antimicrobianos. Serán necesarios posteriormente, ensayos para validar estos indicadores en una población grande y a lo largo del tiempo.
Quality indicators are monitoring systems that can be defined as quantitative criteria for evaluating and monitoring the quality and efficiency of health systems. They aim to provide useful information about deviations from standard practice, and to facilitate decision-making by objectively assessing what is being done in a health system.1 An indicator is the basic element that periodically assesses and measures an important aspect of health care. Indicators are required to meet three characteristics to ensure their usefulness. First, they must be valid, so as to detect problems in quality. Second, they must be sensitive, to detect all instances where there is a quality problem. And third, they must be specific, to detect only those cases that have a quality problem.2
The process of developing quality indicators follows a series of steps. The first step is to define the area to be monitored and to identify the most relevant aspects to be studied. Next, each indicator needs to be specifically designed, including the description of the aspects that guarantee its validity. Once the indicators are defined, they must be systematically measured and the results should be compared with the reference value. On continuation, the results must be analysed in order to detect differences with the reference value and to identify a possible problem regarding quality. If a problem is detected, improvements in quality can be planned. Re-evaluation of the indicator can then test whether the plan is effective and whether the problem is solved.3,4
Quality in critical care is of maximum significance as patients in Intensive Care Units (ICUs) are vulnerable and their physiological response mechanisms are altered. They also require life support with drugs and devices that make their treatment more complex, rendering them more susceptible to complications. Therefore, in this group of patients it is essential to have tools that help ensure quality care.5
In 2005, the Spanish Society of Intensive Care Medicine and Coronary Units published a document with 120 quality indicators relating to critical patient care. This document was reviewed in 2011 and has recently been accepted for inclusion in the National Quality Measures Clearinghouse (NQMC), and the Agency for Healthcare Research and Quality (AHRQ) in the United States. Of the 120 indicators, two refer to the use of antimicrobials in hospitals, but none refer specifically to the use of antimicrobials in the ICU.2
Infections play a major role in the morbidity and mortality of critically ill patients.6 It has been shown that early administration of appropriate antimicrobial improves the outcome of critically ill patients. At the same time, however, bacterial resistance to antimicrobials used to treat infections in hospitalized patients is increasing. As this problem is more acute in ICU patients, sound knowledge of the therapeutics and pharmacokinetics of antimicrobials is essential for their selection and adjustment during a patient's admission.7,8
Studying the use of antimicrobials in the ICU is difficult. One reason is that antimicrobial agents can be administered for several purposes, either as prophylaxis or as treatment for a wide variety of indications. When determining which antimicrobial to use as treatment, many factors must be taken into account. It is necessary to consider the source of the infection, its form of presentation and its location. Another reason is that antimicrobials often need to be changed during ICU stay in view of microbiological results, the patient's clinical course, possible adverse effects, multiresistant pathogens, and de-escalation.9
Although many recommendations have been proposed to optimize antimicrobial use,10,11 quality indicators have not yet been defined and validated in respect to their use in the ICU setting. Recently, the Group Coordinator of the ENVIN-HELICS (“National Study of Nosocomial Infection Surveillance” in Spain and “The Hospitals in Europe Link for Infection Control through Surveillance”) proposed quality indicators for the use of antimicrobials in the ICU and they retrospectively determined the value of these indicators in a sample of patients admitted to ICUs in Spain in 2005 and 2006.12 To date, however, there are no document published that define the fundamental aspects of each indicator.
ObjectivesThe aim of this work was to develop a set of quality indicators for antimicrobial use in critically ill patients admitted to the ICU. The indicators were defined to assess relevant aspects regarding selection and change of antimicrobials, such as global consumption, adequacy of treatment, and duration. These indicators would be a useful tool for health care professionals to assess antimicrobial use in critically ill patients and to detect quality problems for misuse of these drugs.
Materials and methodsThe process of developing quality indicators follows three phases.
- 1.
Development of a set of preliminary quality indicators based on a literature review.
- 2.
Field study and validation of these quality indicators.
- 3.
Analysis and compilation of a definitive quality indicator set by the expert panel.
The present study describes the first step of the process. To develop a set of preliminary quality indicators on the use of antimicrobials in critically ill patients, we selected quality indicators that the Spanish Working Group of Infectious Diseases (Grupo de Trabajo de Enfermedades Infecciosas, GTEI) have proposed at their annual meetings since 2005. The design of each quality indicator includes a description of items to ensure their validity and reliability. These items are listed in Table 1. We reviewed various documents relating to quality control in the field of health through indicators, major clinical practice guidelines on the use of antimicrobials, and protocols and consensus documents in the field of major infections in critically ill patients.
Definition of sections defined for each quality indicator.
| Section | Definition |
| Name | Brief description |
| Formula | Quantitative expression to measure the indicator. It is typically expressed as a percentage or as a mean |
| Type of indicator | Classification of indicators from the evaluation approach. The main types are:• Structure: indicators that measure aspects of the resources needed for health care. Such resources may be technological, organizational or human.• Process: indicators that assess the way in which health care is developed, according to available resources, the best scientific evidence and the protocols.• Outcome: indicators that measure the impact of health care, in terms of complications, missed opportunities, failures of circuits, quality of life, etc. |
| Justification | Explanation of the usefulness of the indicator |
| Population | Definition of unit of study that will be measured |
| Definition of terms | Explanation of all the components of the formula |
| Data source | Explanation about the origin of the information and data collection sequence needed to quantify the indicator |
| Standard available | Required level of good practice given the scientific evidence |
| References | Main available scientific evidence on which is based the indicator described |
We developed ten quality indicators: six of process, three of result and one of structure, to evaluate the quality of care provided to critically ill patients receiving antimicrobials during their stay in the intensive care unit. These indicators are set out in Table 2.1. Antimicrobial use in the intensive care unit
Quality indicators on the use of antimicrobials in critically ill patients.
| 1. Antimicrobial use in the intensive care unit |
| 2. Non-empirical antimicrobial use |
| 3. Changes in antimicrobials used as treatment |
| 4. Days without antimicrobial use in ICU |
| 5. Days free of antimicrobials in patients on antimicrobial treatment |
| 6. Number of days of antimicrobials for surgical prophylaxis |
| 7. Inappropriate empirical antimicrobial treatment |
| 8. Empirical antimicrobials changed because they are inadequate |
| 9. Empirical antimicrobial changed for de-escalation |
| 10. Patients with severe sepsis/septic shock treated with antimicrobials in the first three hours |
Formula:
Type of indicator: Process.
Justification: Patients admitted to the ICU have intrinsic and extrinsic risk factors to present episodes of infection. Proper selection and duration of antimicrobial treatment directly affects the effectiveness of infection control and lowers the risk of bacterial resistance. The indicator expresses the overall weight of antimicrobial use. Because some patients receive several antimicrobials for many days, the rate may exceed the total number of patient-days and can therefore be greater than 100.
This indicator varies greatly between ICUs depending on the characteristics of the patients they serve (coronary unit, medical, surgical or traumatic). Periodic evaluation of this indicator can be a tool to determine the use of an antimicrobials and its impact on emerging flora and the emergence of multidrug-resistant microorganisms. The indicator can be considered in intervention programs to reduce antimicrobial use.
Population: All patients admitted to the ICU for more than 24h.
Definition of terms: Numerator: total number of days each antimicrobial was used during each patient's stay in the ICU. Denominator: total number of days of ICU patients or the sum of days of ICU admission of each patient admitted.
Data source: Clinical documentation.
Standard available: <100.
References: 13–17.2. Non-empirical antimicrobial use
Formula:
Type of indicator: Structure.
Justification: Antimicrobials can be administered empirically without knowing the germ responsible for the infection or as directed treatment when the causative organism is known. One way to limit the use of antimicrobials in an ICU is to have the results of the clinical microbiology as soon as possible. This allows the ICU team to initiate directed antimicrobial treatment, thereby decreasing side effects and costs. The availability of rapid diagnostic techniques such as real-time PCR (polymerase chain reaction) in the critical patient environment will provide targeted treatments, so that the evolution of this indicator is a good marker of quality.
Population: All antimicrobials administered in the ICU.
Definition of terms: Numerator: total number of all antimicrobials used in the treatment of infections, in a targeted or directed manner. Denominator: total number of all antimicrobials used to treat infections, whether or not they are used in a targeted manner like those used empirically. Antimicrobials indicated as prophylaxis are excluded.
Data source: Clinical documentation.
Standard available: >30%.
References: 18–21.3. Changes in antimicrobials used as treatment
Formula:
Type of indicator: Process.
Justification: Critically ill patients who develop an infection are treated with antimicrobials. Throughout their clinic course, the antimicrobials may be modified for several reasons, some of which are related to the antimicrobial itself and its activity against the flora responsible for infection, while others are patient-dependent. The main reasons for changes in antimicrobial agent are findings in microbiological cultures, therapeutic de-escalation, toxicity, clinical response, and adverse effects. This indicator summarizes the complexity of using antimicrobials. It is the sum of positive reasons, such as therapeutic de-escalation, and negative reasons, such as the positive microbiological result not covered by the selected antimicrobial. Due to this variability, a value of less than 35% is considered the benchmark for this indicator.
Population: All antimicrobials administered in the ICU for treatment of an infection.
Definition of terms: Numerator: sum of all antimicrobials changed in the treatment of infections in ICU patients, regardless of the reason for change. Denominator: sum of all antimicrobials used for treatment of infections in ICU patients.
Data source: Clinical documentation.
Standard available: <35%.
References: 22–24.4. Days without antimicrobial use in ICU
Formula:
Type of indicator: Process.
Justification: This indicator reflects the number of days on which a patient is free of antimicrobials. It is an indicator of the overall weight of the use of antimicrobials in the ICU. It includes patients receiving antimicrobials and patients not receiving antimicrobials. Its value also depends significantly on the characteristics of the population attended in each unit; the results in a coronary care unit will differ notably from those in a surgical ICU.
Wise use of antimicrobials in ICU is a priority to ensure proper treatment of critically ill patients and prevent the development of bacterial resistance. The emergence of multiresistant bacteria is a growing concern in hospitals and more specifically in the critical care areas. However, data regarding the use of antimicrobials vary widely, not only because of different policies for each hospital but also because of the different measurement methods. The indicator is a way to measure how many days a patient is in the ICU without antimicrobials. Exposure to broad spectrum antimicrobials has been directly associated with the development of resistance. Reducing duration of antimicrobial treatment is a specific goal that could be assessed by this indicator.
Population: All patients admitted to the ICU for more than 24h.
Definition of terms: Numerator: for each patient admitted to the ICU, the sum of days he/she received no antimicrobial treatment. Denominator: sum of days of admission to the ICU of all patients admitted to the ICU for more than 24h.
Data source: Clinical documentation.
Standard available: 30–40%.
References: 25,26.5. Days free of antimicrobials in patients on antimicrobial treatment
Formula:
Type of indicator: Process.
Justification: The development of infections in ICU patients is a common problem, associated with increased hospital stay and mortality, and increased spending. Proper selection, dosage and duration of antimicrobial treatment have a direct impact on infection control and risk of bacterial resistance. The recommended duration of antimicrobial treatment remains controversial as many factors must be considered, such as the anatomical location of infection, the type of bacteria implied, individual idiosyncrasies, and characteristics of the drugs themselves. Several studies have shown that short treatments are as effective as longer treatments. It is difficult to evaluate the exact duration of each antimicrobial treatment so this indicator gives an overview only. It indirectly allows us to quantify how many days patients who are treated with antimicrobials during their ICU admission do not receive antimicrobial treatment.
Population: All patients admitted to the ICU for more than 24h who receive antimicrobials during their stay.
Definition of terms: Numerator: sum of days that patients who are treated with antimicrobials during their ICU admission do not receive antimicrobials. Denominator: sum of days of admission to the ICU of all patients treated with antimicrobials. Patients who do not receive any antimicrobial treatment during ICU admission are excluded.
Data source: Clinical documentation.
Standard available: <15–20%.
References: 27–30.6. Number of days of antimicrobials for surgical prophylaxis
Formula:
Type of indicator: Process.
Justification: Prophylactic antimicrobial treatment is indicated to prevent infection in cases of high risk, such as in surgical procedures where natural protective barriers are broken, or after injuries such as skull base fractures or open wounds. There is no consensus because the indications are diverse. The duration of prophylaxis related to surgery is clearer than other indications. It is recommended that prophylactic treatment around a surgical procedure should last 1–2 days. This quality indicator shows the duration of the antimicrobial treatments. It can alert to overuse of antimicrobials. Reducing antimicrobial use even by one day is important, not so much for the individual patient, but for overall exposure of critical patients to antimicrobials.
Population: All antimicrobials used for surgical prophylaxis in ICU.
Definition of terms: Numerator: sum of the days that a patient has taken antimicrobials for surgical prophylaxis. Denominator: sum of the patients who have taken surgical prophylactic treatment. This indicator is applied to each antimicrobial used in prophylaxis.
Data source: Clinical documentation.
Standard available: <1–2.
References: 31,32.7. Inappropriate empirical antimicrobial treatment
Formula:
Type of indicator: Result.
Justification: Empirical therapy is inappropriate in any of the following cases: 1. Culture results confirm that no antimicrobials have activity against the microorganism identified according to accepted standards, or that the microorganism identified is resistant to the antimicrobial administered. 2. The antimicrobial has not been administered properly, due to any incorrect dose, incorrect route of administration, or poor penetration into the source of infection. If antimicrobials are used in combination, at least one of them must not be inappropriate.
Patients admitted to the ICU with severe infections have high mortality. Initial administration of a broad spectrum empirical antimicrobial and its correct administration directly correlate with control of infection. Antibiotics should be individually tailored to the needs of each patient. Administration of inappropriate treatment has a direct impact on the evolution of the critical patient. This indicator therefore shows whether the election of the antimicrobial is correct.
Population: All antimicrobials administered in ICU empirically, as treatment of an infection.
Definition of terms: Numerator: sum of all empirical antimicrobials which are not appropriate, according to the previous definition. Denominator: sum of all antimicrobials administered empirically to treat infections. All infections in which no microbiological cultures have been performed or in which cultures are negative are excluded.
Data source: Clinical documentation.
Standard available: <10%.
References: 33–35.8. Empirical antimicrobials changed because they are inadequate
Formula:
Type of indicator: Result.
Justification: Empirical antimicrobial treatment is changed because it is inadequate. Inadequate antimicrobial treatment is defined mainly by microbiological identification of an infection that is not being treated effectively. Factors contributing to inadequate treatment in ICU patients include prior exposure to antibiotics, the use of broad-spectrum antibiotics, prolonged stay, prolonged mechanical ventilation, and the use of invasive devices. Empiric treatment should be initiated according to the individual characteristics of each patient, and the predominant local flora and its susceptibility. Broad spectrum empirical treatment improves mortality and outcome of critically ill patients while inadequate empirical treatment increases overall mortality and mortality. This indicator shows the proportion of antimicrobials which have to be changed due to lack of initial success. This indicator is a tool that can help in the process of periodic review of the empirical antimicrobial treatment protocols in an ICU. An increase in inappropriate treatments can indicate a need for change in empirical treatment protocols.
Population: All antimicrobials administered empirically in the ICU.
Definition of terms: Numerator: sum of all empirical antimicrobials modified because they are inadequate. Denominator: sum of all antimicrobials administered empirically to treat infections.
Data source: Clinical documentation.
Standard available: <10%
References: 34,36–38.9. Empirical antimicrobial changed for de-escalation
Formula:
Type of indicator: Process.
Justification: Mortality in patients with sepsis, severe sepsis, or septic shock varies in series between 27% and 54%. Broad spectrum treatment aims to provide adequate initial antimicrobial treatment and reduce mortality. However, as there is a risk of antimicrobial overuse, a strategy of de-escalation has been proposed to modify broad-spectrum antimicrobial use in accordance with microbiological results. Treatment should be changed when antimicrobial treatment that has a narrower spectrum, less toxicity, or lower cost is an option. Such change should be made between the second and third days of treatment. De-escalation is essential to minimize the development of resistance during treatment. The applicability of this strategy has been evaluated primarily in critical patients with nosocomial pneumonia or septic shock. Findings to date suggest that the strategy of initiating a broad-spectrum treatment early and trying to adjust it as soon as possible reduces inappropriate treatment and minimizes the development of resistance.
Population: All antimicrobials empirically administered in an ICU as treatment for infection.
Definition of terms: Numerator: sum of all empirical antimicrobials that are changed by adjustment or de-escalation. Denominator: sum of all antimicrobials administered empirically to treat infections.
Data source: Clinical documentation.
Standard available: >20%.
References: 39,40.10. Patients with severe sepsis/septic shock treated with antimicrobials in the first three hours
Formula:
Type of indicator: Result.
Justification: Sepsis is defined as the presence of infection associated with systemic signs and symptoms of infection. Severe sepsis is defined as sepsis with acute organ dysfunction and septic shock is defined as severe sepsis plus hypotension persisting after adequate fluid resuscitation. They are major healthcare problems because of their incidence and mortality. The administration of appropriate treatment in the initial hours after sepsis develops, influence the outcome.
The administration of appropriate antimicrobial agents as soon as possible and within the first hour of recognition of septic shock and severe sepsis should be the goal of therapy. Many studies have shown an increase in mortality with each hour of delay in treatment administration. The “Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012” include the recommendation of early administration of antibiotics among the bundles to be completed in the first three hours.
Population: All patients with severe sepsis or septic shock.
Definition of terms: Numerator: sum of all the patients with severe sepsis or septic shock that received antimicrobial treatment during the first three hours since the diagnosis. Denominator: sum of all the patients with severe sepsis or septic shock.
Data source: Clinical documentation.
Standard available: 100%.
References: 41–45.
Discussion and conclusions- 1.
Quality indicators are quantitative criteria for evaluating and monitoring quality. Applied to health care, they provide useful information about situations and deviations related to standard practice.
- 2.
This work defines a set of quality indicators for antimicrobial use in ICUs. For use in clinical practice, they must be validated. Validation must be performed through field study. The quality indicators must then be analysed and a definitive quality indicator should be compiled by an expert panel.
- 3.
A systematically evaluated set of quality indicators allows us to compare the results with established standards to identify suboptimal situations and assess their evolution over time.
- 4.
When a suboptimal situation is detected, results must be interpreted. If a situation that could be improved is detected, actions for improvement should be proposed. These actions should then be implemented and the quality indicator should be re-measured to evaluate whether the measures are effective or not.
- 5.
Some of the indicators are complementary to each other and each unit will decide which indicators apply, depending on their characteristics. The proposed indicators do not take into account the structural characteristics of the different ICUs or the characteristics of the patients they serve. These aspects will be analysed in the future.
- 6.
Finally, we believe that the quality indicators proposed in this work will be a useful tool to understand and improve the use of antimicrobials in the ICU. The next step of this study is to validate the proposed indicators.
This article does not have any economic support.
Conflict of interestThe authors declare no potential conflict of interest relevant to this article.
We thank Carolyn Newey for editing the manuscript.