Ventilator-associated pneumonia (VAP) is related with high mortality, duration of mechanical ventilation and costs. Recent studies have questioned the safety and effectiveness of oral chlorhexidine to prevent VAP. We sought to verify whether the adverse effects of this substance outweigh its benefits. We searched several databases and selected studies that investigated the use of oral chlorhexidine and its impact on mortality. No association between oral chlorhexidine and lower VAP rates was found on meta-analyses of double-blind randomized trials, however significant increase in mortality was reported. It is speculated that chlorhexidine can cause damage to several organic sectors and cytotoxicity. Although it still can be beneficial in specific settings, robust evidence to recommend its routine application for all mechanically ventilated patients is lacking; therefore, given the possibility of harm, it would be better to follow the principle of non-maleficence until more studies becomes available.
La neumonía asociada al respirador (VAP) está relacionada con una elevada mortalidad, mayor duración de la ventilación mecánica y costes elevados. Estudios recientes han cuestionado la seguridad y la eficacia de la clorhexidina oral para prevenir la VAP. Hemos intentado verificar si los efectos adversos de esta sustancia superan sus beneficios. Se realizaron búsquedas en diversas bases de datos y se seleccionaron estudios que habían investigado el uso de la clorhexidina oral y su impacto sobre la mortalidad. En los metaanálisis de los ensayos aleatorizados a doble ciego no se encontró ninguna asociación entre clorhexidina oral y tasas de VAP más bajas; sin embargo, sí se informó de un aumento significativo de la mortalidad. Se especula que la clorhexidina puede causar daño a varias partes del organismo y citotoxicidad. Pese a que todavía podría ser beneficiosa en entornos específicos, no se dispone de evidencias sólidas para recomendar su aplicación rutinaria para todos los pacientes sometidos a ventilación mecánica; por lo tanto, dada la posibilidad de ocasionar daños, sería mejor seguir el principio de no maleficencia hasta que se disponga de más estudios.
Ventilator-Associated Pneumonia (VAP) is the most common infection in the intensive care unit.1,2 According to the World Health Organization, it has a prevalence of 15–45% and a higher incidence in countries with limited resources, being associated with high hospital mortality, duration of mechanical ventilation and costs.2,3
During critical illness, the stomach is often colonized by Gram-negative bacteria, Streptococcus spp. and Candida albicans, which, by gastrointestinal reflux, colonize the oral cavity. In cases of VAP, the germs isolated in oral secretion and sputum are the same. In addition, pre-existing dental disease is associated with both community-acquired pneumonia and hospital-acquired pneumonia.4 Thus, in VAP prevention bundles, oral hygiene is an important strategy to minimize the chance of tracheobronchitis and pneumonia.5
The Brazilian intensive care institutions and societies recommendation, including the Brazilian Association of Intensive Care Medicine and the National Health Surveillance Agency, is that oral care in critically ill mechanically ventilated patients should be performed with 0.2% chlorhexidine digluconate, due to its possible benefits.6,7 However, recent meta-analyses of several studies have presented contradictory conclusions.8 Some of them even found benefits in the use of oral chlorhexidine, but limited to cardiac surgical patients and in high concentrations, that is, at 2%.8,9 Despite that, this agent was widely used in oral hygiene of mechanically ventilated patients to prevent infections and was recommended by intensive care societies in several countries.6,10–13
Klompas warn about the risk of increased mortality and ventilator-associated events when chlorhexidine is used in the oral care of mechanically ventilated patients to prevent VAP13 and reports speculations that the potential damage of chlorhexidine may be due to a possible mechanism of direct pulmonary toxicity after its aspiration, causing lung injury and acute respiratory distress syndrome (ARDS).14 According to Ricard and Lisboa,5 given the uncertainty regarding the effectiveness of chlorhexidine in the decontamination of the oropharynx, reduction of VAP, changes in the susceptibility of pathogens and potential damage related to its exposure, as reported by Deschepper et al.,15 more data is urgently needed to guide preventive VAP strategies.5 Klompas, Price, Lisboa and other researchers consider that the use of oral chlorhexidine in these patients should be evaluated in further studies.5,14,16
Therefore, should we expose patients to a substance whose effectiveness is uncertain and maybe can increase mortality risk?17 Should intensivists reconsider oral chlorhexidine for VAP prevention?18 Could this discussion prompt the search for alternative strategies that provide safer practice in critical care?19
Considering that oral chlorhexidine is indiscriminately used in hospitals,12 as one of VAP prevention resources, the aim of this review is to verify whether the adverse effects of this substance outweigh its benefits and assess the need for alternatives strategies.
Chlorhexidine digluconateIn 1946 chlorhexidine was created by scientists Rose and Swain, who were looking for an agent to cure malaria, but failed in their goal due to the inefficiency of the drug for this purpose20 and, since 1950, is used as an antiseptic.21 Chlorhexidine is a biguanide,19 a cationic compound and a strong base; poorly soluble in water, and therefore used in the form of salt: digluconate, diacetate and dihydrochloride.22 From these salts, chlorhexidine digluconate is the most soluble in water and alcohols, so is the one used to perform oral hygiene of the patients.22 Although chlorhexidine is a base, chlorhexidine digluconate, whose pH ranges from 5.5 to 6.0, is an acid.21
Chlorhexidine action and microbial resistanceChlorhexidine's action is related to the electrostatic bond between the cationic molecules of chlorhexidine to the negative charge of the bacteria cell wall, which causes changes in the osmotic balance and loss of intracellular components.21 It is active against gram-positive bacteria, mainly gram-negative bacteria, some fungi (when at 2% concentration) and enveloped viruses (respiratory syncytial virus, influenza, HIV, herpes simplex and cytomegalovirus) and it is not active against acid-alcohol resistant bacteria or spores.22
The variable effect of chlorhexidine appears to be related to its limited microbiological effect, while reducing oropharyngeal carriage with S. aureus leaves Gram-negative colonization largely unaffected.23 And also to the local concentrations of chlorhexidine, as demonstrated in a trial, in which administration of 2% chlorhexidine, a high concentration solution, was associated with a reduced rates of VAP, but 10% of patients in the test group developed irritation of the oral mucosa.23
Possibly, the effectiveness of chlorhexidine against Gram-positive bacteria in laboratory experiments may be causing an overestimation of the clinical usefulness of this antimicrobial agent.24 According to Alvarez et al., albeit there seems to be no risk of inducing cross-resistance to antibiotics, chlorhexidine-resistant strains of MRSA (Methicillin-resistant Staphylococcus aureus) can replace susceptible strains soon after routine chlorhexidine application begins23; and up to 63% of European strains actually express plasmid-borne qacA/B genes that code for multi-drug efflux pumps, which confer chlorhexidine resistance in MRSA.23
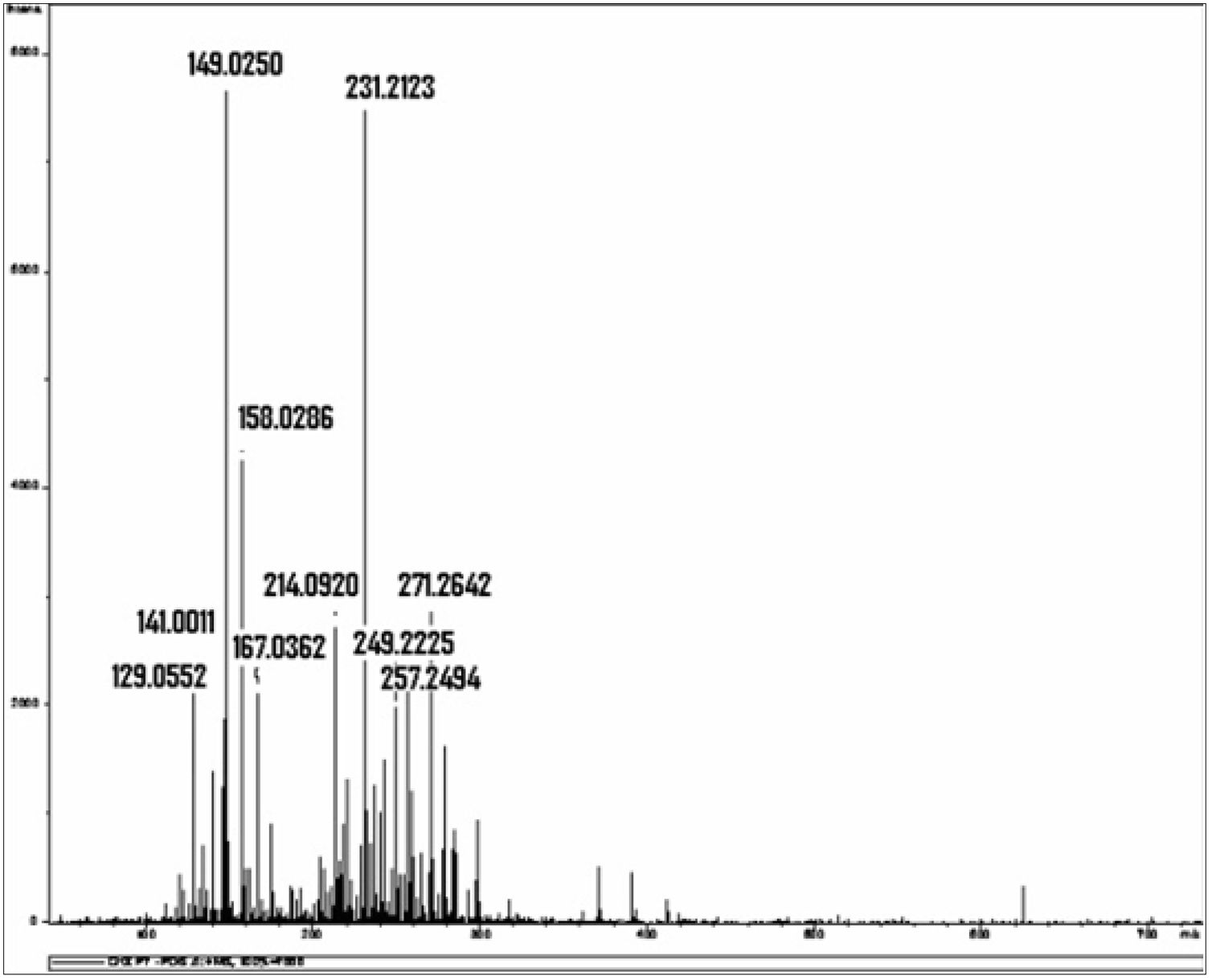
Chlorhexidine decompositionIn a study that chemically investigated chlorhexidine using mass spectrophotometry and liquid chromatography, isolated chlorhexidine digluconate stored at a temperature of 36.5°C was decomposed over a period of 14 days, with formation of a large number of different by-products (Fig. 1).21 The author of this study reported that chlorhexidine and its by-products can act as free radicals or ROS “Reactive Oxygen Species”, directly affecting protein synthesis and structure, DNA synthesis and cell repair mechanisms, increasing TNF-α, TGF-β and MCP-1, which are toxic to the cell21; or due to the proper action of the molecular identity of each by-product, such as, for example, the para-Chloroaniline,21 that according to the International Agency for Research on Cancer (IARC) is a possible carcinogenic agent for humans.25–27
Mass spectrum obtained from the isolated 0.2% chlorhexidine digluconate solution examined after 14 days of storage at a constant temperature of 36.5°C. The horizontal axis represents the relationship between mass and charge (m/z) of the ionized substances present in the sample. The vertical represents the relative abundance of these substances.24 *Source (with authorization): Barbin EL. Análise química da clorexidina misturada ou não ao hidróxido de cálcio. Universidade de São Paulo: Dissertation; 2008.
Chlorhexidine can lead to oral ulcerations and other local damage28–30 and, the higher the concentration, the lower the tolerance.30,31 It can be directly toxic or cause hypersensitivity reactions,28,29,32,33 contributing to erosive mucosal injury5,30 and predisposing infections.10
Yeung et al. reported the fact that chromosomal aberrations, due to DNA breakdown and fragmentation, were observed in lymphocytes and epithelial cells in the mouth of patients who used 0.2% chlorhexidine digluconate mouthwash for a period of 18 days.34 Bonacorsi et al. demonstrated that chlorhexidine is toxic to a variety of eukaryotic cells and, depending on the concentration and time of exposure, can interact or damage membrane receptors, interfering with the binding of the receptors to lipopolysaccharides35; it can have an immunosuppressive effect on exposed macrophages, since the production of nitric oxide induced by lipopolysaccharides is reduced; and it is toxic to macrophages at concentrations 100 times lower than those used in clinical practice, that is, usually 0.2 to 0.5%.35
Chlorhexidine aspirationHirata and Kurokawa described a case of an 80-year-old woman who ingested chlorhexidine and, 12h later, presented ARDS; and they concluded that, when aspiration occurs, chlorhexidine has the potential for fatal ARDS.36 Orito et al. reported the case of a patient who died of ARDS after inhaling chlorhexidine and suggested that the mechanism was direct lung injury damage.37 The toxicity of chlorhexidine was then evaluated in a study with rats, exposed to 1%, 0.1% and 0.01% concentrations of this substance, with severe congestion of alveoli and capillaries, perivascular and interalveolar hemorrhage and infiltration of the collagen fibers by inflammatory cells observed within 28 to 84 days of exposure to chlorhexidine, especially in concentrations above 0.1%.38
ARDS is characterized by acute diffuse inflammatory lung injury, which occurs in response to a pulmonary or systemic insult and invariably leads to abnormalities in gas exchange and pulmonary mechanics.39,40 Diffuse alveolar damage is considered the main histological feature of the disease acute phase.41 Considering the fact that acid substances have potential to cause pulmonary epithelial damage, by oxidative mechanisms, as in the case of chlorine ingestion42 and gastric fluids aspiration,43 would chlorhexidine digluconate, which is also acid, have this same potential?
Oral chlorhexidine for ventilator-associated pneumonia prevention and its impact on mortalityA summary of meta-analyses that investigated the association between oral chlorhexidine to prevent VAP and mortality can be seen in Tables 1 and 2. Pileggi et al. found a reduction of VAP and an increase in mortality with oral chlorhexidine; and reported that, unlike antiseptics, the use of topical antibiotics seemed to be effective in preventing all ICU-acquired infections.44 Li et al., in another systematic review and meta-analysis of randomized controlled trials, observed a reduction of VAP with the use of oral chlorhexidine and an increase in mortality.12 In a subsequent meta-analysis, by Price et al., the use of oral chlorhexidine was associated with increased mortality in patients in general intensive care units and both selective digestive decontamination and selective oropharyngeal decontamination were superior to chlorhexidine.16
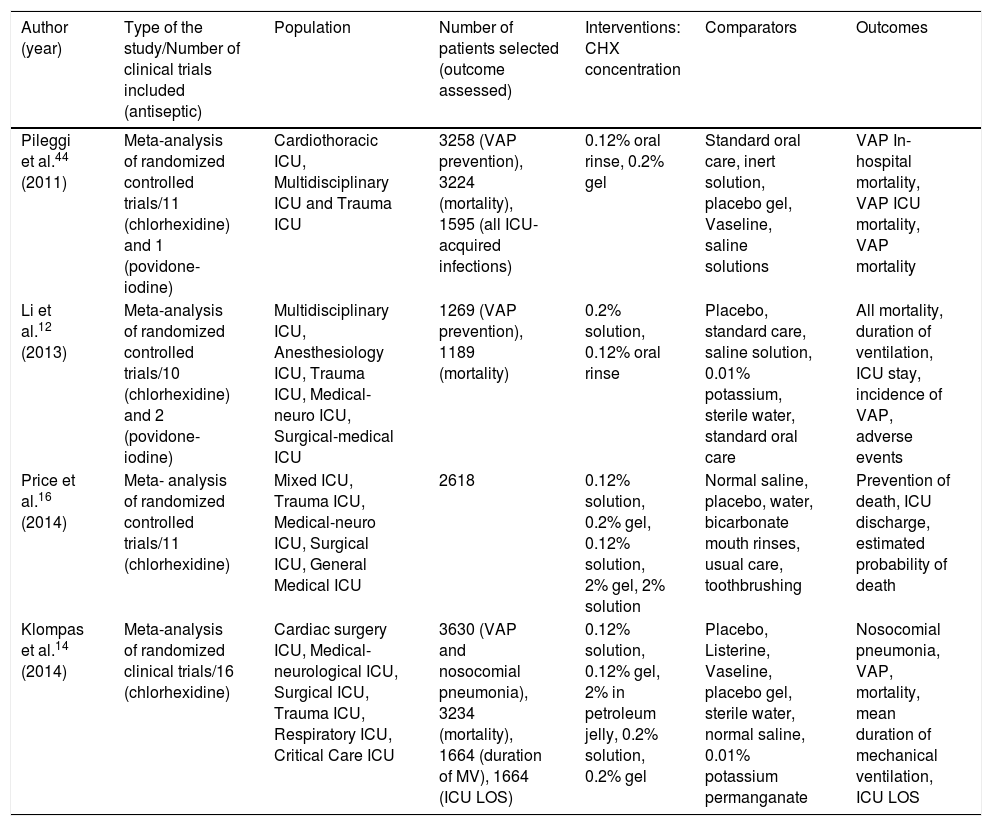
The PICO strategy of meta-analyses that assessed the association between the use of oral chlorhexidine for the prevention of ventilator-associated pneumonia and its impact on mortality.
| Author (year) | Type of the study/Number of clinical trials included (antiseptic) | Population | Number of patients selected (outcome assessed) | Interventions: CHX concentration | Comparators | Outcomes |
|---|---|---|---|---|---|---|
| Pileggi et al.44 (2011) | Meta-analysis of randomized controlled trials/11 (chlorhexidine) and 1 (povidone-iodine) | Cardiothoracic ICU, Multidisciplinary ICU and Trauma ICU | 3258 (VAP prevention), 3224 (mortality), 1595 (all ICU-acquired infections) | 0.12% oral rinse, 0.2% gel | Standard oral care, inert solution, placebo gel, Vaseline, saline solutions | VAP In-hospital mortality, VAP ICU mortality, VAP mortality |
| Li et al.12 (2013) | Meta-analysis of randomized controlled trials/10 (chlorhexidine) and 2 (povidone-iodine) | Multidisciplinary ICU, Anesthesiology ICU, Trauma ICU, Medical-neuro ICU, Surgical-medical ICU | 1269 (VAP prevention), 1189 (mortality) | 0.2% solution, 0.12% oral rinse | Placebo, standard care, saline solution, 0.01% potassium, sterile water, standard oral care | All mortality, duration of ventilation, ICU stay, incidence of VAP, adverse events |
| Price et al.16 (2014) | Meta- analysis of randomized controlled trials/11 (chlorhexidine) | Mixed ICU, Trauma ICU, Medical-neuro ICU, Surgical ICU, General Medical ICU | 2618 | 0.12% solution, 0.2% gel, 0.12% solution, 2% gel, 2% solution | Normal saline, placebo, water, bicarbonate mouth rinses, usual care, toothbrushing | Prevention of death, ICU discharge, estimated probability of death |
| Klompas et al.14 (2014) | Meta-analysis of randomized clinical trials/16 (chlorhexidine) | Cardiac surgery ICU, Medical-neurological ICU, Surgical ICU, Trauma ICU, Respiratory ICU, Critical Care ICU | 3630 (VAP and nosocomial pneumonia), 3234 (mortality), 1664 (duration of MV), 1664 (ICU LOS) | 0.12% solution, 0.12% gel, 2% in petroleum jelly, 0.2% solution, 0.2% gel | Placebo, Listerine, Vaseline, placebo gel, sterile water, normal saline, 0.01% potassium permanganate | Nosocomial pneumonia, VAP, mortality, mean duration of mechanical ventilation, ICU LOS |
PICO: population, intervention, comparator, outcome; VAP: ventilator-associated pneumonia; ICU: Intensive Care Unit; CHX: Chlorhexidine; LOS: length of stay; MV: mechanical ventilation; VAE: ventilator-associated pneumonia; IVAC: infection-related ventilator-associated complications.
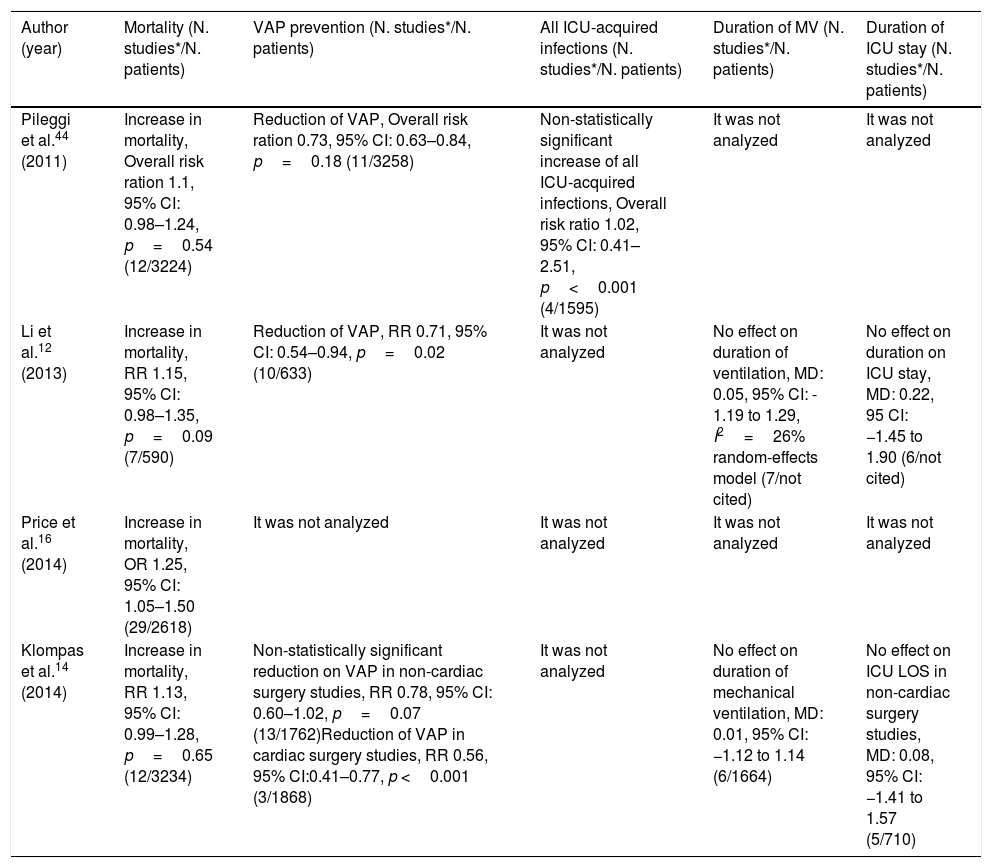
Summary of the results of metanalyses that assessed the association between oral chlorhexidine use for ventilator-associated pneumonia prevention and its impact on mortality.
| Author (year) | Mortality (N. studies*/N. patients) | VAP prevention (N. studies*/N. patients) | All ICU-acquired infections (N. studies*/N. patients) | Duration of MV (N. studies*/N. patients) | Duration of ICU stay (N. studies*/N. patients) |
|---|---|---|---|---|---|
| Pileggi et al.44 (2011) | Increase in mortality, Overall risk ration 1.1, 95% CI: 0.98–1.24, p=0.54 (12/3224) | Reduction of VAP, Overall risk ration 0.73, 95% CI: 0.63–0.84, p=0.18 (11/3258) | Non-statistically significant increase of all ICU-acquired infections, Overall risk ratio 1.02, 95% CI: 0.41–2.51, p<0.001 (4/1595) | It was not analyzed | It was not analyzed |
| Li et al.12 (2013) | Increase in mortality, RR 1.15, 95% CI: 0.98–1.35, p=0.09 (7/590) | Reduction of VAP, RR 0.71, 95% CI: 0.54–0.94, p=0.02 (10/633) | It was not analyzed | No effect on duration of ventilation, MD: 0.05, 95% CI: - 1.19 to 1.29, I2=26% random-effects model (7/not cited) | No effect on duration on ICU stay, MD: 0.22, 95 CI: −1.45 to 1.90 (6/not cited) |
| Price et al.16 (2014) | Increase in mortality, OR 1.25, 95% CI: 1.05–1.50 (29/2618) | It was not analyzed | It was not analyzed | It was not analyzed | It was not analyzed |
| Klompas et al.14 (2014) | Increase in mortality, RR 1.13, 95% CI: 0.99–1.28, p=0.65 (12/3234) | Non-statistically significant reduction on VAP in non-cardiac surgery studies, RR 0.78, 95% CI: 0.60–1.02, p=0.07 (13/1762)Reduction of VAP in cardiac surgery studies, RR 0.56, 95% CI:0.41–0.77, p <0.001 (3/1868) | It was not analyzed | No effect on duration of mechanical ventilation, MD: 0.01, 95% CI: −1.12 to 1.14 (6/1664) | No effect on ICU LOS in non-cardiac surgery studies, MD: 0.08, 95% CI: −1.41 to 1.57 (5/710) |
VAP: Ventilator-associated pneumonia; MD: mean difference; RR: risk ratio; OR: odds ratio; CI: credible interval ICU: Intensive Care Unit; N: number of; MV: mechanical ventilation. *Number of clinical trials included in the meta-analyses that assessed the outcome of interest, that is, only studies referring to each outcome that is placed in each column of this table were quantified.
Klompas et al., in a more recent meta-analysis, compared oral chlorhexidine and placebo in adults receiving mechanical ventilation and found that routine care with chlorhexidine prevents nosocomial pneumonia in cardiac surgery patients but not decrease ventilator-associated pneumonia risk in non-cardiac surgery patients. Besides that, they found association between chlorhexidine use and increased mortality in non-cardiac surgery studies (RR: 1.13), statistically nonsignificant, but with proximity of the lower limit of the confidence interval to 1 (95% CI: 0.99–1.29).14 They observed a stepwise increase in mortality RR point estimates with increasing concentrations of chlorhexidine and reported that the point estimated for mortality was higher in double-blind studies (RR: 1.15, 95% CI 0.99–1.29)14 than in open-label studies (RR 1.06, 95% CI 0.80–1.41).14 To confirm the findings, they reanalyzed this data using random-effects model with odds ratios rather than RRs, because RR methods can assign disproportionate weights to small studies with high event rates, and the point estimate for mortality remained elevated (OR 1.20, 95% CI 0.95–1.50, I2=0%).14 Finally, to go further in this analysis, they excluded all studies with possible methodological concerns in the meta-analysis, and this finding remained evident.14
In a recent observational study, investigating the association between ventilator bundle components and outcomes, was found a non-statistically significant reduction of VAP (RR 0.55, 95% CI: 0.27–1.14, p=0.11)45; however, it was observed an association between oral chlorhexidine use to prevent VAP and statistically increase in ventilator-associated mortality (RR, 1.63; 95% CI, 1.15–2.31; p=0.06) and in hospital mortality (RR 1.01, 95% CI: 0.98–1.05, p=0.44).45
Deschepper et al., in a further observational cohort study,15 performed in a hospital-wide population, evaluate the effects of chlorhexidine gluconate oral care on hospital mortality. The authors enrolled a total of 82,274 patients, of which more than 11,000 (14%) received oral care with chlorhexidine, and found that a low exposure (<300mg) was related to statistically significant increase in mortality (OR 2.92, 95% CI: 2.32–3.26).15 From all patients analyzed, 2847 had been on mechanical ventilation for less than 96h, and in this group there was no increase in mortality, neither in those exposed to low dose of chlorhexidine (< 300mg), OR 0.58, 95% CI: 0.39–0.87, p=0.008, nor in those exposed to high dose of chlorhexidine (>300mg), OR 0.51, 95% CI: 0.34–0.79, p=0.003.15 In the 903 patients who were on mechanical ventilation for more than 96h, an increase in mortality was observed both in those exposed to low dose of chlorhexidine (<300mg), OR 1.47, 95% CI: 0.75–2.91, p=0.26, as in those exposed to high dose of chlorhexidine (>300mg), OR 1.11, 95% CI: 0.59–2.14, p=0.74.15 All the patients, in low or high doses, were treated with a mouth rinse containing low concentrations of chlorhexidine (0.05% or 0.12%), and yet, the use of oral chlorhexidine was significantly associated with increased mortality in patients mechanically ventilated for more than 4 days and also in the whole population analyzed in the study.15
A clinical trial, whose results were presented during the 30° European Society of Intensive Care Medicine (ESICM) Annual Congress in 2017, enrolled 438 patients in an ICU who had been on mechanical ventilation for at least 4 days and underwent oral care with chlorhexidine gluconate.46 An association between oral care with chlorhexidine and a statistically significant increase in hospital mortality (OR 9.09, 95% CI: 1.11–74.25, p=0.03) was reported.46 Although the study was not fully published, considering the magnitude of the finding, we believe that this data should be considered.
In a recently published observation analysis between VAP prevention strategies and outcomes, of all individual components of the ventilator bundle, compliance with oral chlorhexidine was the only component associated with ventilator-associated events (VAE) risk.50 Compliance with oral chlorhexidine care was associated with an increased risk of VAE using cumulative compliance 3 days prior to VAE (OR 1.45, p=0.007).50 The authors, therefore, performed a similar analysis but extended the period of interest to include up to 7 days before the event, and compliance with oral chlorhexidine care remained a risk factor for VAE (OR 1.42, p=0.03).50 Finally, the authors performed a multivariable analysis including age, gender and compliance with chlorhexidine for 3 days prior to the event, and the association persisted (OR 1.45, p=0.008).50
Do the adverse effects of oral chlorhexidine outweigh its benefits?Concerning the possible benefits of oral chlorhexidine in mechanically ventilated patients, as shown in Table 2, previous meta-analyses12,44 reported significant reduction in VAP, however these studies have not been performed of double-blind randomized trials.10,14,47 In addition, recent meta-analyses14,16 observed reduction of VAP statistically significant in cardiac surgery studies (RR 0.56; 95% CI: 0.41–0.77), but not in non-cardiac ones (RR 0.88; 95% CI: 0.66–1.16).14 In one of this recent meta-analysis, Klompas et al. declared that, in most individual trials, there was no sign of VAP prevention with oral chlorhexidine.14
In a recent observational analysis between VAP prevention strategies and outcomes, although there was reported reduction in ventilator-associated events (RR 0.8; 95% CI: 0.61–1.23; p=0.42), infection-related ventilator-associated complications (RR 0.60; 95% CI: 0.36–1.00; p=0.05) and VAP (RR 0.55; 95% CI: 0.27–1.14; p=0.11), this data was non-statistically significant.45
Hence, considering that there was no sign of VAP prevention with oral chlorhexidine in recent meta-analyses of double-blind studies,14 challenging our beliefs, perhaps the use of this agent does not have such a benefit.13,14,17 A variety of mechanisms have been proposed to explain why chlorhexidine may fail to prevent VAP, such as possible reduction in bacterial susceptibility to chlorhexidine24,48 and local and systemic damage.14,16 Further studies are needed49 to verify if its widespread use is related with bacterial and antimicrobial resistance acquisition.24,48
As to the risks, oral care with chlorhexidine was associated with increased mortality,14–16,45,46 ICU associated infections44 and risk of VAE.50 This was evidenced in four meta-analyses of randomized clinical trials,12,14,16,44 in two recent observational analysis between VAP prevention strategies and outcomes45,50 and in one in-hospital observational analysis.15 The increase in mortality was statistically significant in one of the recent meta-analysis16 and, in all other meta-analyses12,14,44 the lower limit of the confidence interval presented a proximity to 1, as can be seen in Table 1, what merit careful evaluation.
In these studies, we noticed that, in cardiac surgical patients14 and patients on mechanical ventilation for less than 96h,15 there was no increase in mortality in the patients submitted to oral chlorhexidine. As most cardiac surgery patients are extubated in less than 1 day,14 this finding could be explained by the shorter time both these groups are exposed to oral chlorhexidine.
The mechanism that could explain this increase in mortality remains unclear.45,47 Klompas et al.45 speculated that ARDS development, after chlorhexidine aspiration, is the most likely mechanism and other authors suggested a direct pulmonary toxicity.36,45,51 However, there is no study analyzing the association between oral chlorhexidine care and lung injury or ARDS, so we have no specific data to confirm or refute this hypothesis.14 Thereby, for now, maybe some aspects of chlorhexidine metabolism, potential toxicity and possible pathophysiological mechanisms could explain these authors’ speculations to justify the observed increase in mortality and assist in the design of future studies.
In order to decide whether the adverse effects of oral chlorhexidine outweigh its benefits and recommend this practice to all critically ill patients on mechanical ventilation, some aforementioned information can be taken into account. The perception that oral chlorhexidine prevents VAP can be biased, since this data is present only in meta-analyses of “open-label” studies and not in double-blind ones18,52 and is speculated that its widespread use may be related with bacterial and antimicrobial resistance acquisition.24,48 Besides that, oral care with chlorhexidine for VAP prevention was associated with increase in ventilator associated events50 and mortality.14–16,18,45 To attempt to explain these findings, once bronchial aspiration with subsequent ARDS development is the most likely mechanism,14,51,53 maybe we can consider some chemical aspects of chlorhexidine. Chlorhexidine digluconate is an acid substance,21 has adverse effects on oral mucosa18,28–30,32,33; some patients may experience allergic reactions, including anaphylaxis28,32,33; spectrophotometric studies have shown that chlorhexidine can be decomposed into by-products21 and, one of them, is para-Chloroaniline, which according to the IARC is a possible carcinogen25–27; chlorhexidine can be involved in free radicals production and cytotoxicity mechanisms34,35,45; and, even in low concentrations, such as 0.2%, reduces the production of nitric oxide, an important healing process cellular mediator.35
Worldwide ventilator-associated pneumonia prevention bundlesThe SHEA (Society of Healthcare Epidemiology of America) and IDSA (Infectious Diseases Society of America) guidelines8,54 state that the benefits of oral care with chlorhexidine appear to be more pronounced in preventing postoperative respiratory tract infections in cardiac-surgery patients and data for non-cardiac-surgery patients are more equivocal,8 therefore, routine oral care without chlorhexidine may be indicated for reasons other than VAP prevention and in children and newborns is not recommended due to inadequate risk data.8,54
Recently, the European Respiratory Society, European Society of Intensive Care Medicine, European Society of Clinical Microbiology and Infectious Diseases and Asociación Latinoamericana del Tórax have decided not to issue a recommendation on the use of oral chlorhexidine in their VAP prevention international guidelines until more safety data becomes available.55,56
In Spain, in collaboration with the Spanish Society of Intensive and Critical Medicine and Coronary Units (SEMICYUC) and the Spanish Society of Intensive Nursing and Coronary Units (SEEIUC), in June 2018, the Ministry of Health's Advisory Council on the Critical Patient Safety Project made an addendum57 to the Pneumonia Zero Project. In the document, the Council issued that, based on recent systematic reviews and meta-analyses assessing the effectiveness of oral chlorhexidine in preventing VAP and its impact on mortality and, on the coincidence in most assessments of the need for studies that analyze the safety of this procedure, the board decided to modify the recommendation of oral hygiene with chlorhexidine in ventilated patients from mandatory to not mandatory.57
Barriers and limitationsThis review shows that recent studies have also called into question the effectiveness and safety of oral chlorhexidine,14 but currently there is no proven better alternative oral antiseptic. Therefore, further studies are needed,52 since finding surrogate strategies can be challenging.
Perspectives and future researchDale et al. assert that, with evidence of a lack of benefit in VAP prevention, as well as possible harm, immediate discontinuation of oral chlorhexidine in the ICU is a possible solution.10 Garnacho-Montero et al., considering that chlorhexidine is not a harmless compound and cases of severe acute respiratory distress syndrome have been reported in case of ingestion and accidental inhalation,36–38,42,51 recommend its use but with extreme caution during application in order to avoid aspiration of the antiseptic.51 Pileggi et al., in a meta-analysis of randomized controlled trials in intensive care units that evaluate oropharyngeal decontamination using chlorhexidine and others antiseptics, declares that, actually, unlike antiseptics, the use of topical antibiotics seems to be effective in preventing all ICU-acquired infections and the effectiveness on mortality needs to be investigated in further studies.44 Bouadma and Klompas argue that, for the moment, it is better to remove chlorhexidine from oral care regimes13,18,58,59 and the latter suggests that brushing and oral care with sterile water may be the most prudent choice.59
A recent publication of a large cluster randomized clinical trial failed to assess the safety of oral chlorhexidine.59 It compared different oral decontamination strategies and observed an adjusted 28-day mortality hazard ratio of 1.13, 95% CI: 0.68–1.88, after 6 months of routine care with 2% chlorhexidine; nonetheless, it provided a comparison between high and low-concentration chlorhexidine, but not on low-concentration chlorhexidine versus placebo.59 In Canada, a cluster randomized de-adoption study, which is currently underway in intensive care units in Toronto, adopted a protocol for the gradual withdrawal of chlorhexidine from the oral care of critically ill patients and will assess the impact on mortality, ventilator-associated infections, ventilator-associated complications and oral health status.10 In Brazil, a double-blind randomized trial (ICTRP: RBR-7p6568)60 is in progress and will evaluate the association between oral care, in critically ill ventilated patients, with different antiseptics and VAP rates, ARDS and mortality. Perhaps the results of these studies fill gaps in knowledge.
ConclusionNowadays, many intensive care institutions and societies still recommend the use of chlorhexidine in the oral hygiene of critically ill patients on mechanical ventilation as one of the strategies to prevent VAP. Although it could be useful in specific scenarios, its routine application in all patients of a general ICU requires further evaluation since recent meta-analyses have reported an increase in mortality and no association between lower VAP rates and oral chlorhexidine was found on meta-analyses of double-blind randomized trials.
FundingNo financial support.
Conflict of interestsThe authors declare that they have no conflict of interest.