To determine the viral etiology of severe lower respiratory tract infections (LRTIs), their clinical significance and prognosis among critically ill children.
DesignA prospective descriptive study was carried out.
SettingPediatric Intensive Care Unit (PICU) of Istanbul Medeniyet University, Goztepe Training and Research Hospital, Istanbul, Turkey.
PatientsA total of 115 patients hospitalized in the PICU were evaluated for inclusion in the study. Children with underlying comorbidities and those who did not require mechanical ventilation were excluded.
Main variables of interestDemographic, clinical, laboratory test and radiographic data were recorded.
ResultsA total of 63 patients were eligible for the study. The most common diagnosis was bronchiolitis (57.1%). Respiratory syncytial virus (RSV) was the most frequent causal virus (36.5%). The most common complication was acute respiratory distress syndrome (ARDS) (28.6%). Multiple viral infection was identified in 20.6% of the patients, the most common in this subgroup being rhinovirus. Patients with bocavirus infection had a higher likelihood of needing invasive mechanical ventilation (IMV) at presentation. Children who died were likely to be <12 months old, have ARDS, hepatitis, pneumomediastinum, multiple viral infection, and required IMV support with an increased duration of MV. Additionally, they were found to have a high Pediatric Risk of Mortality III score, Predicted Death Rate and increased need for inotropic support at admission.
ConclusionsOur study showed critically ill children with LRTI without known risk factors to have high mortality when aged <12 months, in the presence of multiple agents and when certain complications (ARDS, hepatitis) and X-ray findings were identified.
Determinar la etiología vírica de las infecciones graves de las vías respiratorias bajas (IGVRB), su importancia clínica y su pronóstico en niños críticamente enfermos.
DiseñoEstudio descriptivo prospectivo.
ÁmbitoUnidad de cuidados intensivos pediátricos (UCIP) del Hospital Universitario y de Investigación Goztepe, Universidad Medeniyet de Estambul, Turquía.
Pacientes y participantesSe evaluó a un total de 115 pacientes ingresados en la UCIP para su posible inclusión en el estudio. Se excluyó a los niños con comorbilidades subyacentes y a aquellos que no requerían ventilación mecánica.
Variables de interés principalesSe registraron los datos demográficos, clínicos, de laboratorio y radiológicos de los pacientes.
ResultadosUn total de 63 pacientes fueron elegidos para participar en el estudio. El diagnóstico más habitual era bronquiolitis (57,1%). El virus sincitial respiratorio era el más común de los virus (36,5%). La complicación más habitual era el síndrome de dificultad respiratoria aguda (SDRA) (28,6%). Se identificaron múltiples infecciones víricas en el 20,6% de los pacientes, siendo la infección por el rinovirus la más común en este subgrupo. Los pacientes con infección por bocavirus presentaban una mayor probabilidad de necesitar ventilación mecánica invasiva (VMI) en el momento de la presentación. Los niños que murieron tenían una mayor probabilidad de presentar: edad <12 meses, SDRA, hepatitis, neumomediastino, infección multiviral y requerir MVI, con una mayor duración de la VM. Además, se observó que presentaban unos valores más altos de la escala PRISM III (Pediatric Risk of Mortality III) y tasa de mortalidad prevista, además de necesidad apoyo inotrópico en el momento del ingreso.
ConclusionesNuestro estudio demostró que los niños críticamente enfermos con LRTI sin factores de riesgo conocidos tienen una alta mortalidad cuando tienen menos de 12 meses, en presencia de múltiples agentes y cuando se identifican ciertas complicaciones (SDRA, hepatitis) y hallazgos de rayos X.
Acute Lower Respiratory Tract Infection (LRTI) is considered to be one of the most common causes of hospitalization and is an important cause of morbidity and mortality in infants and young children, especially in severe cases.1,2 Most investigators utilize the World Health Organization (WHO) definition for severe LRTI in which children are defined as critically ill when they have warning signs such as, difficulty breathing with increased respiratory rate for age, chest wall retractions and/or stridor.3 WHO's definition of severe LRTI does not define the need for intensive care, but the need for hospitalization. Severe LRTI cases requiring intensive care treatment do not demonstrate homogeneous features due to variations in intensive care hospitalization criteria and severe LRTI definitions.4,5 In the current study, severe LRTI was defined as the presence of warning signs, need for Pediatric Intensive Care Unit (PICU) admission, and also having a requirement for invasive mechanical ventilation (IMV) or non-invasive mechanical ventilation (NIV) at the time of PICU admittance.
The agents which cause LRTI are most commonly viruses, among which the respiratory syncytial virus (RSV) accounts for up 50–90% of cases. However, the etiology of LRTIs is not always limited to a single viral agent; in hospitalized children, 10–20% of cases has been shown to involve two or more viral agents. Furthermore, viral agents may also coexist with bacterial agents.1,2,6,7 Despite being a widespread disease that has received much attention, there are conflicting results about viral infectious etiology, disease severity and outcomes.8–10
Infants are more vulnerable to LRTIs and may often present with bronchiolitis and pneumonia. Although bronchiolitis is usually a benign self-limited disease that is managed on an outpatient basis, many children are admitted to the PICU due to respiratory failure caused by severe form of acute LRTI, with variable length of stay (LOS) at the PICU. Studies have shown that 2–3% of <1-year-old children with RSV are hospitalized in PICUs.11–13
Despite differences between each agent, viral respiratory viruses have the potential to cause severe LRTIs, particularly in younger children. However, the number of studies on the etiology and outcomes of severe LRTIs requiring critical care in the PICU is limited. Therefore, it is important to analyze the characteristics of cases with severe pediatric LRTI in patients with respiratory failure who need mechanical ventilation (MV) support. The primary objective of this study was to explore viral etiologies of severe LRTI in children who require PICU management with MV support. The secondary objective was to define associations between specific viral pathogens and disease severity, progression or outcome.
Material and methodsStudy designThis prospective, single centered, descriptive study was conducted at the PICU of Istanbul Medeniyet University, Goztepe Training and Research Hospital in Istanbul, Turkey, for 2 consecutive years: September 2016–August 2018. Informed consent was obtained from parents or legal guardians of children and the study was approved by the ethics board of Medeniyet University Goztepe Training and Research Hospital.
Study populationPatients with LRTI that met one of the following criteria were admitted to the PICU: (I) necessity for invasive mechanical ventilation (IMV) or non-invasive mechanical ventilation (NIV) due to acute respiratory failure, (II) presence of comorbid factors (such as congenital heart disease, metabolic diseases, prematurity, low birth weight and immunodeficiency) in patients with LRTI irrespective of clinical severity, (III) persisting need for high-flow nasal oxygen despite initial medical treatment. Among these patients admitted to the PICU, those who met all of the following criteria were included in the study: (I) patients with ‘severe LRTI’, (II) being previously healthy and not having any known diseases or comorbidities. Severe LRTI was defined as requiring IMV or NIV at the time of admittance to the PICU in addition to the need for intensive care.
The exclusion of children was performed when they met one of the following criteria: (I) lack of indication for IMV or NIV (therefore, not meeting the severe LRTI definition of our study), (II) undetermined viral etiology, (III) presence of co-morbidities or clinical conditions that may have caused confounding [such as congenital heart diseases, chronic lung diseases, neurodevelopmental disorders, metabolic diseases, prematurity (less than 37th week of gestation), low birth weight (<2.5kg) and suspected or diagnosed immunodeficiency].
Presence of apnea and superficial respiration, hypoventilation, loss of protective respiratory reflexes, severe refractory hypoxemia (PO2<50mmHg) despite oxygen support, and severe acidosis (pH<7.20 or PaCO2>60mmHg) were criteria for IMV. Patients who had signs of moderate respiratory effort, such as retractions, tachypnea, paradoxical respiration and the use of accessory muscles of respiration were firstly followed on NIV; however, they were transitioned to IMV if and when worsening of ventilation/perfusion ratio, hypercapnia, hypoxemia, respiratory distress and progression of disease were found.
Medical records were reviewed for demographic, clinical, laboratory data and all the necessary data including; sex, age, initial diagnosis of severe LRTI, white blood cell (WBC) count, C-reactive Protein (CRP) levels, the need for inotropic support at admission, PRISM III score (Pediatric Risk of Mortality) with predicted death rate (PDR%), sample type received for viral etiology, time between onset of symptoms to hospital admission, LOS at hospital, LOS at PICU, MV type and duration, time period between PICU admission to initiation of IMV, radiological findings such pleural effusion, pneumothorax or pneumomediastinum, complications associated with the course of the disease (ARDS, hepatitis, myocarditis, myositis and encephalitis), and survival were recorded during hospitalization. Clinical diagnosis of ARDS was based on the modified Berlin Criteria.14 The PRISM III score was calculated using the formula available in the original article.15
Patients with severe LRTI were classified according their clinical and chest X-ray findings. Acute wheezing was considered to be bronchiolitis on the basis of clinical signs and symptoms, such as wheezing, dyspnea and possible X-ray findings of hyperinflation of the lungs, peribronchial thickening and collapsed segment of lung, and increased interstitial markings when occurring for the first time in children aged less than 2 years. Patients with focal infiltrates along with consolidation in chest X-rays were classified as pneumonia in the absence of wheezing. Asthma crisis was diagnosed on the basis of The Global Initiative for Asthma guidelines.16 All other episodes of acute wheezing were considered to be recurrent wheezing.
Nasopharyngeal or tracheal aspiration material collectionTracheal and nasopharyngeal aspiration materials were collected within the first day of PICU admission. Nasopharyngeal aspirate was collected from patients who were on NIV support by inserting a swab (Virocult Medical Wire Equipment CO, UK) in both nostrils to the nasopharynx, and rotating 360°. Tracheal aspiration materials were collected by a commercial kit (Bicakcilar trakeal suction kit, Turkey) from patients that were put on IMV support.
Nucleic acid extractionEZ1 Virus Mini Kit V 2.0 (QIAGEN, Germany) was used according to the manufacturer's instructions for nucleic acid extraction.
Multiplex PCR for respiratory virusesMultiplex, real time, polymerase chain reaction (RT-PCR) using FTD Respiratory pathogens 21 kit (Fast-track Diagnostics, Junglinster, Luxembourg) was used for the detection of viral respiratory pathogens on instrument Rotor Gene 3000 (QIAGEN, Germany). The kit detects the following respiratory pathogens: influenza A (flu A), influenza A/H1N1 (flu A/H1N1), influenza B (flu B), human parainfluenza viruses (PIV 1–4), human respiratory syncytial viruses (RSV A/B), human adenovirus (AdV), human metapneumovirus (hMPV), human rhinovirus (hRV), human bocavirus (hBoV), human coronaviruses (hCoV), human parechoviruses (hPeV), enteroviruses (hEV) and mycoplasma pneumonia (Mpp).
Statistical analysisData analysis was performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL) version 15.0 software. Simple descriptive statistics were used to calculate the mean, median and standard deviation for quantitative data, while proportions and count were used for qualitative data. Variables such as age, LOS at PICU were analyzed via the Student's t-test or Mann Whitney U test for parametric and nonparametric data, respectively. Categorical variables were evaluated by Chi-squared test. Monte Carlo simulation was applied when there was uncertainty. All p-values less than 0.05 were considered statistically significant.
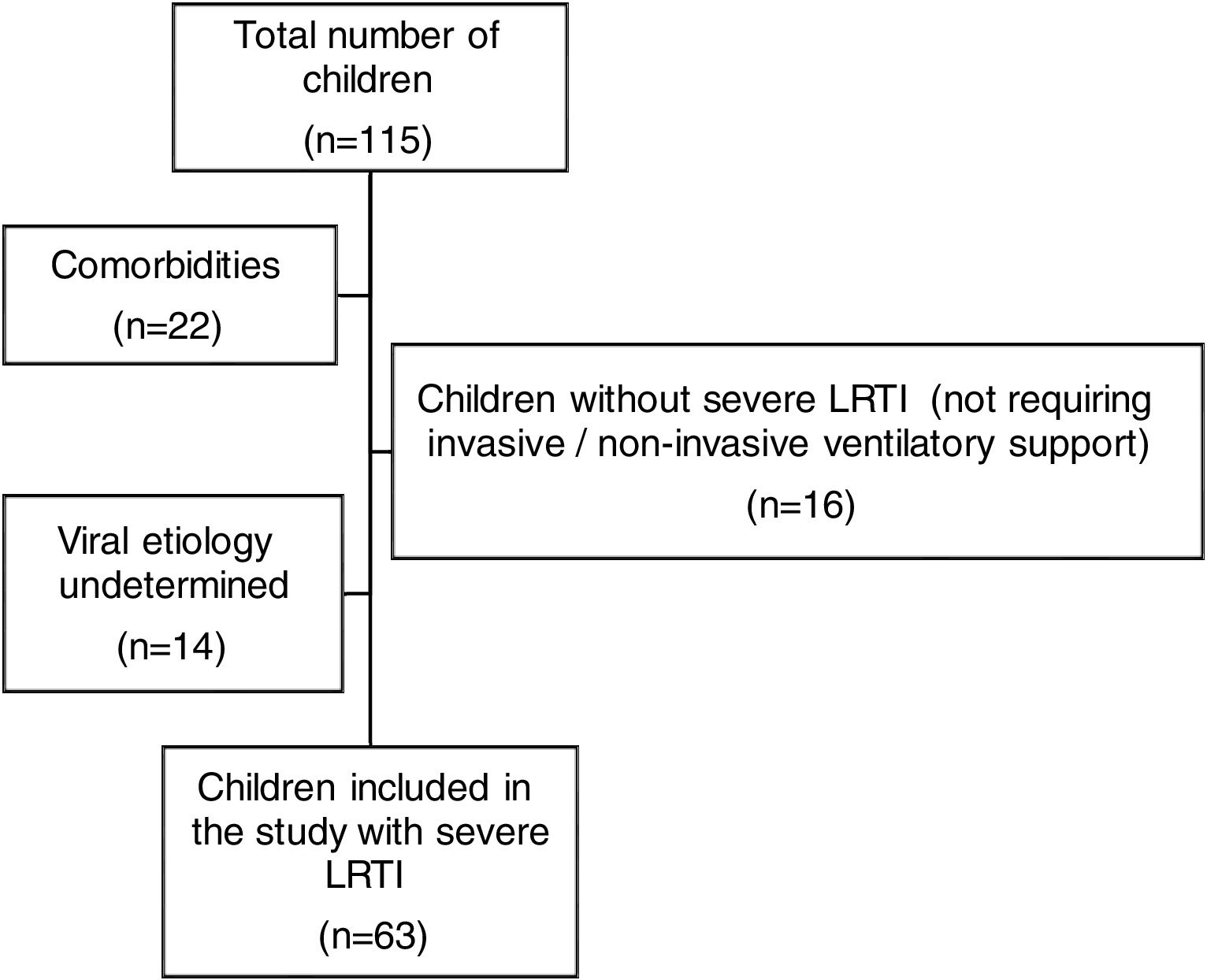
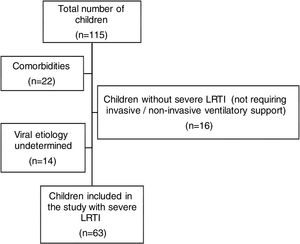
ResultsWe analyzed 115 pediatric patients aged 1 month to 16 years who were admitted to our PICU with a diagnosis of LRTI. Among these, 63 children with severe LRTI were included in this study. The exclusion of 52 children was performed due to following reasons: not meeting the definition of severe LRTI (n=16), undetermined viral etiology (n=14), having co-morbidities such as congenital heart diseases (n=4), chronic lung diseases (such as BPD) (n=4), neurodevelopmental disorders (n=3), metabolic diseases (n=2), prematurity (less than 37th week of gestation) (n=4), low birth weight (<2.5kg) (n=3) and suspected or diagnosed immunodeficiency (n=2) (Fig. 1).
Detailed demographic, clinical, laboratory characteristics and outcome of patients included in the study are described in Table 1. Out of the 63 patients, 33 (52.4%) were male. Mean age of patients was 17.4±26.8 months, and the majority of patients were younger than 6 months of age (n=27, 42.9%). Most of the patients were diagnosed with acute bronchiolitis (57.1%), while 31.7% had pneumonia and 11.1% had asthma crisis or recurrent wheezing. The mean time between onset of symptoms to hospital admission was 4.4±2.8 days, and LOS at hospital was 20.4±15.1 days. The great majority of patients (90.5%) stayed at the PICU for more than 3 days.
Demographic, clinical and laboratory characteristics of children with critical LRTI.
| Variables | Values |
|---|---|
| Sex, n (%) | |
| Female | 30 (47.6) |
| Male | 33 (52.4) |
| Age (month), mean±SD (min–max) | 17.4±26.8 (1–178) |
| Age ranges, n (%) | |
| 0–6 months | 27 (42.9) |
| 7–12 months | 10 (15.9) |
| 13–24 months | 15 (23.8) |
| >24 months | 11 (17.4) |
| Initial diagnosis, n (%) | |
| Bronchiolitis | 36 (57.1) |
| Pneumonia | 20 (31.7) |
| Asthma crisis, or recurrent wheezing | 7 (11.1) |
| WBC count at admission (cells/mm3), mean±SD (min-max) | 13,155±11,061 (2900–79,400) |
| CRP level at presentation (mg/dL), mean±SD (min-max) | 4.1±5.4 (0–24) |
| Inotropic support at admission, n (%) | 31 (49.2) |
| PRISM III score, mean±SD (min-max) | 12.9±6.5 (2–24) |
| Predicted death rate (%), mean±SD (min-max) | 16.5±16.0 (1.1–54.4) |
| Sample type, n (%) | |
| Nasopharyngeal aspirate | 20 (31.7) |
| Tracheal aspirate | 43 (68.3) |
| TOSHA (days), mean±SD (min–max) | 4.4±2.8 (1–14) |
| Total LOS at hospital (days), mean±SD (min–max) | 20.4±15.1 (1–99) |
| Day interval, n (%) | |
| ≤3 days | 1 (1.6) |
| 3–7 days | 10 (15.9) |
| 8–14 days | 15 (23.8) |
| 15–21 days | 13 (20.6) |
| ≥21 days | 24 (38.1) |
| Length of stay at PICU (days), mean±SD (min-max) | 15.6±15.3 (1–99) |
| ≤3 days, n (%) | 6 (9.5) |
| >3 days, n (%) | 57 (90.5) |
| Mechanical ventilation types, n (%) | |
| IMV at onset of admission | 30 (47.6) |
| NIV at onset of admission | 33 (52.4) |
| Need of transition from NIV to IMV support | 16 (25.4) |
| Time period between PICU admission to start of IMV (hours), mean±SD (min-max) | 28.8±38.3 (0–144) |
| Hour interval, n (%) | |
| 0–48h | 37 (80.4) |
| >48h | 9 (19.6) |
| Duration of mechanical ventilation (days), mean±SD (min-max) | 10.3±14.7 (1–99) |
| Hour interval, n (%) | |
| 0–48h | 19 (30.2) |
| 48–96h | 9 (14.3) |
| ≥96h | 35 (55.5) |
| Organ involvement/complications, n (%) | |
| ARDS | 18 (28.6) |
| Hepatitis | 13 (20.6) |
| Myositis | 13 (20.6) |
| Encephalitis | 6 (9.5) |
| Myocarditis | 2 (3.2) |
| Radiographic findings, n (%) | |
| Atelectasis | 32 (50.8) |
| Pneumomediastinum | 13 (20.6) |
| Pneumothorax | 10 (15.9) |
| Pleural effusion | 8 (12.7) |
| Survival | |
| Death | 8 (12.7) |
| Recovery | 55 (87.3) |
LRTI: Lower respiratory tract infection, WBC: White blood cell, CRP: C-reactive protein, PRISM: Pediatric risk of mortality, TOSHA: Time between onset of symptoms to hospital admission, LOS: Length of stay, PICU: Pediatric intensive care unit, IMV: Invasive mechanical ventilation, NIV: Non-invasive mechanical ventilation, ARDS: Acute respiratory distress syndrome.
Most of the children were managed successfully by NIV support (52.4%). Thirty patients (47.6%) required IMV support at admission. Sixteen patients (25.4%) required transition from NIV to IMV support. Mean duration of MV was 10.3±14.7 days. The mean duration from PICU admission to initiation of IMV was 28.8±38.3h.
The most common complication was ARDS (28.6%) followed by hepatitis (20.6%) and myositis (20.6%). The most common radiography finding was atelectasis (50.8%), followed by pneumomediastinum (20.6%), pneumothorax (15.9%) and pleural effusion (12.7%).
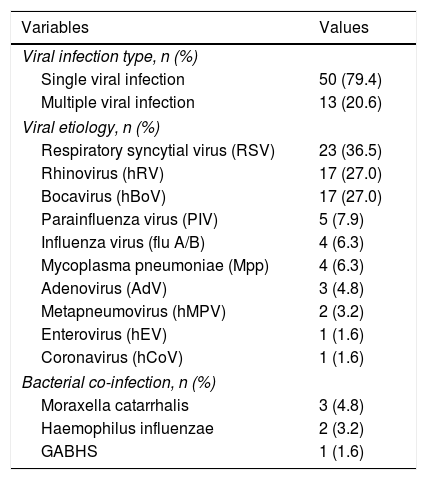
A single viral pathogen was identified in 50 (79.4%) children and at least two viruses were detected in 13 (20.6%) children. RSV was the most common virus (36.5%) followed by hRV and hBoV sharing equal frequency (27%). All specimens were negative for flu B virus. All samples were tested for bacterial co-infections and 6 children had positive results for Moraxella Catarrhalis, Haemophilus Influenzae and Group A beta Haemolytic Streptococcus (GABHS) infection (Table 2).
Frequency of respiratory viruses in LRTI.
| Variables | Values |
|---|---|
| Viral infection type, n (%) | |
| Single viral infection | 50 (79.4) |
| Multiple viral infection | 13 (20.6) |
| Viral etiology, n (%) | |
| Respiratory syncytial virus (RSV) | 23 (36.5) |
| Rhinovirus (hRV) | 17 (27.0) |
| Bocavirus (hBoV) | 17 (27.0) |
| Parainfluenza virus (PIV) | 5 (7.9) |
| Influenza virus (flu A/B) | 4 (6.3) |
| Mycoplasma pneumoniae (Mpp) | 4 (6.3) |
| Adenovirus (AdV) | 3 (4.8) |
| Metapneumovirus (hMPV) | 2 (3.2) |
| Enterovirus (hEV) | 1 (1.6) |
| Coronavirus (hCoV) | 1 (1.6) |
| Bacterial co-infection, n (%) | |
| Moraxella catarrhalis | 3 (4.8) |
| Haemophilus influenzae | 2 (3.2) |
| GABHS | 1 (1.6) |
LRTI: Lower respiratory tract infection, GABHS: Group A beta-haemolytic streptococcus.
LRTI due to multiple viral infection was identified in 13 (20.6%) patients, among which the most common pathogen encountered in multiple viral infection was hRV (35.2% of all hRV infections) which were mostly observed to be with viral pathogens other than RSV (hRV+non-RSV), followed by hBoV (23.5% of all hBoV infections) and RSV (13% of all RSV infections). It was also noteworthy that one patient had triple multiple infection with hRV, hMPV and hEV.
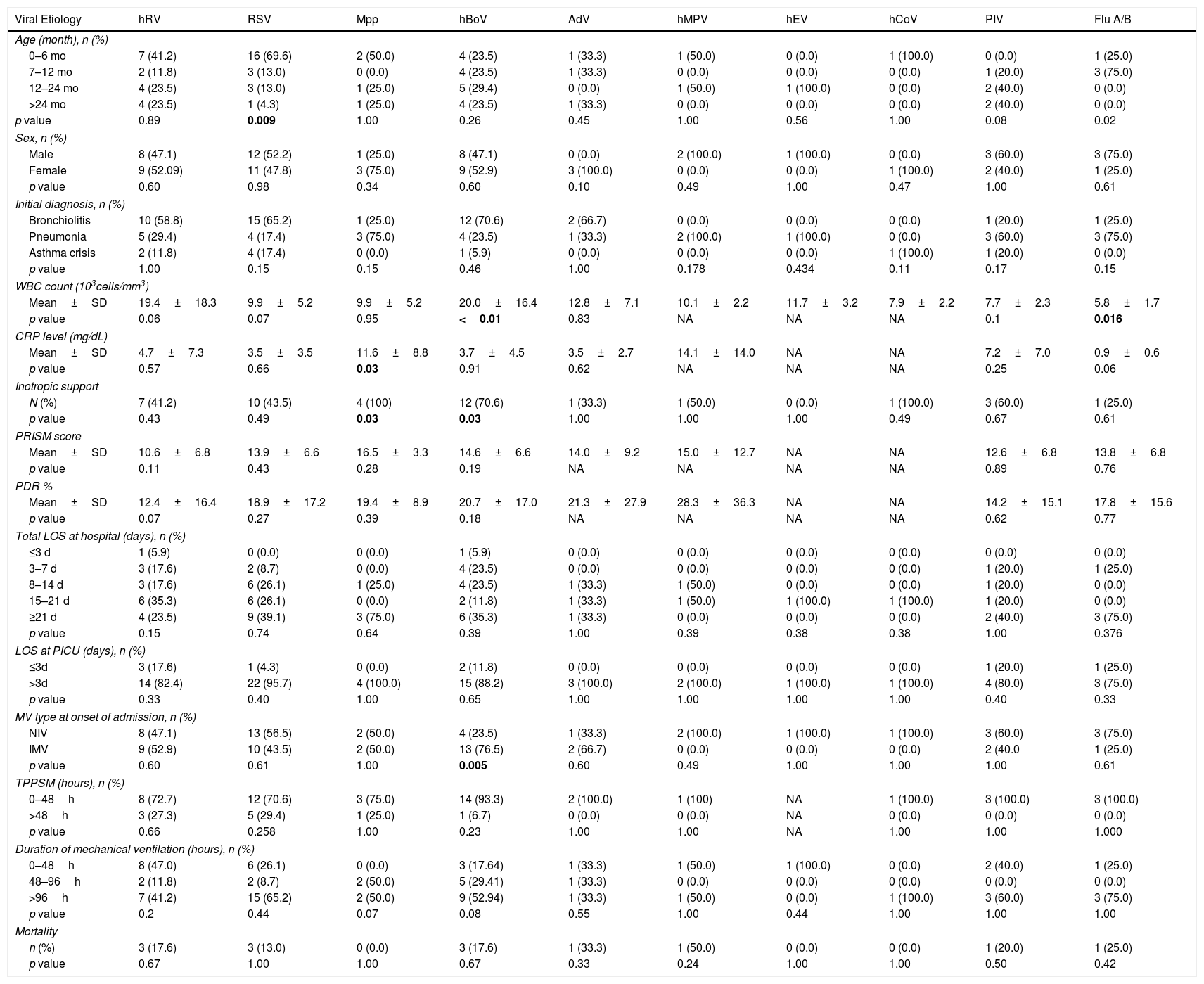
Comparison for demographic, clinical and laboratory variables with each specific viral infection are shown in Table 3. RSV was detected extensively (69.6%) in children aged 0–6 months (p=0.009) and flu A in children aged 7–12 months (p=0.02). All flu A patients were H1N1 positive and other subtypes were not seen. There were no differences in gender and the initial diagnoses of patients did not show any significant differences with regard to virus types. In terms of laboratory parameters, patients with hBoV had elevated WBC count; whereas children with flu A had low WBC count (p<0.001 and p=0.016, respectively). CRP levels were found to be elevated in Mpp positive children compared to other viruses (p=0.03). Patients with hBoV and Mpp infection required inotropic support significantly more frequently (p=0.039 and p=0.036, respectively). PRISM III and PDR (%) were found to be non-significant for viral etiologies. Total LOS at hospital and LOS at PICU did not differ between specific viral etiologies (p>0.05). Patients with hBoV infection had a higher frequency for IMV support requirement at presentation (76.5%) (p=0.005). Analysis of the time period between PICU admission to the initiation of IMV did not yield any significant relationships. Total LOS at hospital and LOS at PICU did not differ between specific viral etiologies (p>0.05).
Comparison of demographic, clinical and laboratory characteristics with regard to specific respiratory viruses.
| Viral Etiology | hRV | RSV | Mpp | hBoV | AdV | hMPV | hEV | hCoV | PIV | Flu A/B |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (month), n (%) | ||||||||||
| 0–6 mo | 7 (41.2) | 16 (69.6) | 2 (50.0) | 4 (23.5) | 1 (33.3) | 1 (50.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (25.0) |
| 7–12 mo | 2 (11.8) | 3 (13.0) | 0 (0.0) | 4 (23.5) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 3 (75.0) |
| 12–24 mo | 4 (23.5) | 3 (13.0) | 1 (25.0) | 5 (29.4) | 0 (0.0) | 1 (50.0) | 1 (100.0) | 0 (0.0) | 2 (40.0) | 0 (0.0) |
| >24 mo | 4 (23.5) | 1 (4.3) | 1 (25.0) | 4 (23.5) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (40.0) | 0 (0.0) |
| p value | 0.89 | 0.009 | 1.00 | 0.26 | 0.45 | 1.00 | 0.56 | 1.00 | 0.08 | 0.02 |
| Sex, n (%) | ||||||||||
| Male | 8 (47.1) | 12 (52.2) | 1 (25.0) | 8 (47.1) | 0 (0.0) | 2 (100.0) | 1 (100.0) | 0 (0.0) | 3 (60.0) | 3 (75.0) |
| Female | 9 (52.09) | 11 (47.8) | 3 (75.0) | 9 (52.9) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 2 (40.0) | 1 (25.0) |
| p value | 0.60 | 0.98 | 0.34 | 0.60 | 0.10 | 0.49 | 1.00 | 0.47 | 1.00 | 0.61 |
| Initial diagnosis, n (%) | ||||||||||
| Bronchiolitis | 10 (58.8) | 15 (65.2) | 1 (25.0) | 12 (70.6) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (25.0) |
| Pneumonia | 5 (29.4) | 4 (17.4) | 3 (75.0) | 4 (23.5) | 1 (33.3) | 2 (100.0) | 1 (100.0) | 0 (0.0) | 3 (60.0) | 3 (75.0) |
| Asthma crisis | 2 (11.8) | 4 (17.4) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (20.0) | 0 (0.0) |
| p value | 1.00 | 0.15 | 0.15 | 0.46 | 1.00 | 0.178 | 0.434 | 0.11 | 0.17 | 0.15 |
| WBC count (103cells/mm3) | ||||||||||
| Mean±SD | 19.4±18.3 | 9.9±5.2 | 9.9±5.2 | 20.0±16.4 | 12.8±7.1 | 10.1±2.2 | 11.7±3.2 | 7.9±2.2 | 7.7±2.3 | 5.8±1.7 |
| p value | 0.06 | 0.07 | 0.95 | <0.01 | 0.83 | NA | NA | NA | 0.1 | 0.016 |
| CRP level (mg/dL) | ||||||||||
| Mean±SD | 4.7±7.3 | 3.5±3.5 | 11.6±8.8 | 3.7±4.5 | 3.5±2.7 | 14.1±14.0 | NA | NA | 7.2±7.0 | 0.9±0.6 |
| p value | 0.57 | 0.66 | 0.03 | 0.91 | 0.62 | NA | NA | NA | 0.25 | 0.06 |
| Inotropic support | ||||||||||
| N (%) | 7 (41.2) | 10 (43.5) | 4 (100) | 12 (70.6) | 1 (33.3) | 1 (50.0) | 0 (0.0) | 1 (100.0) | 3 (60.0) | 1 (25.0) |
| p value | 0.43 | 0.49 | 0.03 | 0.03 | 1.00 | 1.00 | 1.00 | 0.49 | 0.67 | 0.61 |
| PRISM score | ||||||||||
| Mean±SD | 10.6±6.8 | 13.9±6.6 | 16.5±3.3 | 14.6±6.6 | 14.0±9.2 | 15.0±12.7 | NA | NA | 12.6±6.8 | 13.8±6.8 |
| p value | 0.11 | 0.43 | 0.28 | 0.19 | NA | NA | NA | NA | 0.89 | 0.76 |
| PDR % | ||||||||||
| Mean±SD | 12.4±16.4 | 18.9±17.2 | 19.4±8.9 | 20.7±17.0 | 21.3±27.9 | 28.3±36.3 | NA | NA | 14.2±15.1 | 17.8±15.6 |
| p value | 0.07 | 0.27 | 0.39 | 0.18 | NA | NA | NA | NA | 0.62 | 0.77 |
| Total LOS at hospital (days), n (%) | ||||||||||
| ≤3 d | 1 (5.9) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3–7 d | 3 (17.6) | 2 (8.7) | 0 (0.0) | 4 (23.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (25.0) |
| 8–14 d | 3 (17.6) | 6 (26.1) | 1 (25.0) | 4 (23.5) | 1 (33.3) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) |
| 15–21 d | 6 (35.3) | 6 (26.1) | 0 (0.0) | 2 (11.8) | 1 (33.3) | 1 (50.0) | 1 (100.0) | 1 (100.0) | 1 (20.0) | 0 (0.0) |
| ≥21 d | 4 (23.5) | 9 (39.1) | 3 (75.0) | 6 (35.3) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (40.0) | 3 (75.0) |
| p value | 0.15 | 0.74 | 0.64 | 0.39 | 1.00 | 0.39 | 0.38 | 0.38 | 1.00 | 0.376 |
| LOS at PICU (days), n (%) | ||||||||||
| ≤3d | 3 (17.6) | 1 (4.3) | 0 (0.0) | 2 (11.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (25.0) |
| >3d | 14 (82.4) | 22 (95.7) | 4 (100.0) | 15 (88.2) | 3 (100.0) | 2 (100.0) | 1 (100.0) | 1 (100.0) | 4 (80.0) | 3 (75.0) |
| p value | 0.33 | 0.40 | 1.00 | 0.65 | 1.00 | 1.00 | 1.00 | 1.00 | 0.40 | 0.33 |
| MV type at onset of admission, n (%) | ||||||||||
| NIV | 8 (47.1) | 13 (56.5) | 2 (50.0) | 4 (23.5) | 1 (33.3) | 2 (100.0) | 1 (100.0) | 1 (100.0) | 3 (60.0) | 3 (75.0) |
| IMV | 9 (52.9) | 10 (43.5) | 2 (50.0) | 13 (76.5) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (40.0 | 1 (25.0) |
| p value | 0.60 | 0.61 | 1.00 | 0.005 | 0.60 | 0.49 | 1.00 | 1.00 | 1.00 | 0.61 |
| TPPSM (hours), n (%) | ||||||||||
| 0–48h | 8 (72.7) | 12 (70.6) | 3 (75.0) | 14 (93.3) | 2 (100.0) | 1 (100) | NA | 1 (100.0) | 3 (100.0) | 3 (100.0) |
| >48h | 3 (27.3) | 5 (29.4) | 1 (25.0) | 1 (6.7) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| p value | 0.66 | 0.258 | 1.00 | 0.23 | 1.00 | 1.00 | NA | 1.00 | 1.00 | 1.000 |
| Duration of mechanical ventilation (hours), n (%) | ||||||||||
| 0–48h | 8 (47.0) | 6 (26.1) | 0 (0.0) | 3 (17.64) | 1 (33.3) | 1 (50.0) | 1 (100.0) | 0 (0.0) | 2 (40.0) | 1 (25.0) |
| 48–96h | 2 (11.8) | 2 (8.7) | 2 (50.0) | 5 (29.41) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| >96h | 7 (41.2) | 15 (65.2) | 2 (50.0) | 9 (52.94) | 1 (33.3) | 1 (50.0) | 0 (0.0) | 1 (100.0) | 3 (60.0) | 3 (75.0) |
| p value | 0.2 | 0.44 | 0.07 | 0.08 | 0.55 | 1.00 | 0.44 | 1.00 | 1.00 | 1.00 |
| Mortality | ||||||||||
| n (%) | 3 (17.6) | 3 (13.0) | 0 (0.0) | 3 (17.6) | 1 (33.3) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (25.0) |
| p value | 0.67 | 1.00 | 1.00 | 0.67 | 0.33 | 0.24 | 1.00 | 1.00 | 0.50 | 0.42 |
hRV: Rhinoviruses, RSV: Respiratory syncytial virus, Mpp: Mycoplasma pneumonia, hBoV: Bocavirus, AdV: Adenovirus, hMPV: Metapneumovirus, hEV: Enterovirus, hCoV: Coronavirus, PIV: Parainfluenza virus, Flu A/B: Influenza virus A/B, WBC: White blood cell, PRISM: Pediatric risk of mortality, PDR: Predicted death rate, LOS: Length of stay, PICU: Pediatric intensive care unit, MV: Mechanical ventilation, NIV: Non-invasive mechanical ventilation; IMV: Invasive mechanical ventilation, TPPSM: Time period between PICU admission to start of IMV, NA: Not available.
Regarding the associations between viral pathogens and various clinical complications (hepatitis, myocarditis, myositis, encephalitis and ARDS), no significant relationships were found (p>0.05). Radiographic complications were also analyzed and pneumothorax, pleural effusion and atelectasis were not found to be correlated with specific viral pathogens (p>0.05); however, pneumomediastinum was found to be more frequent (n=3, 75%) in flu A/H1N1 positive children (p=0.02).
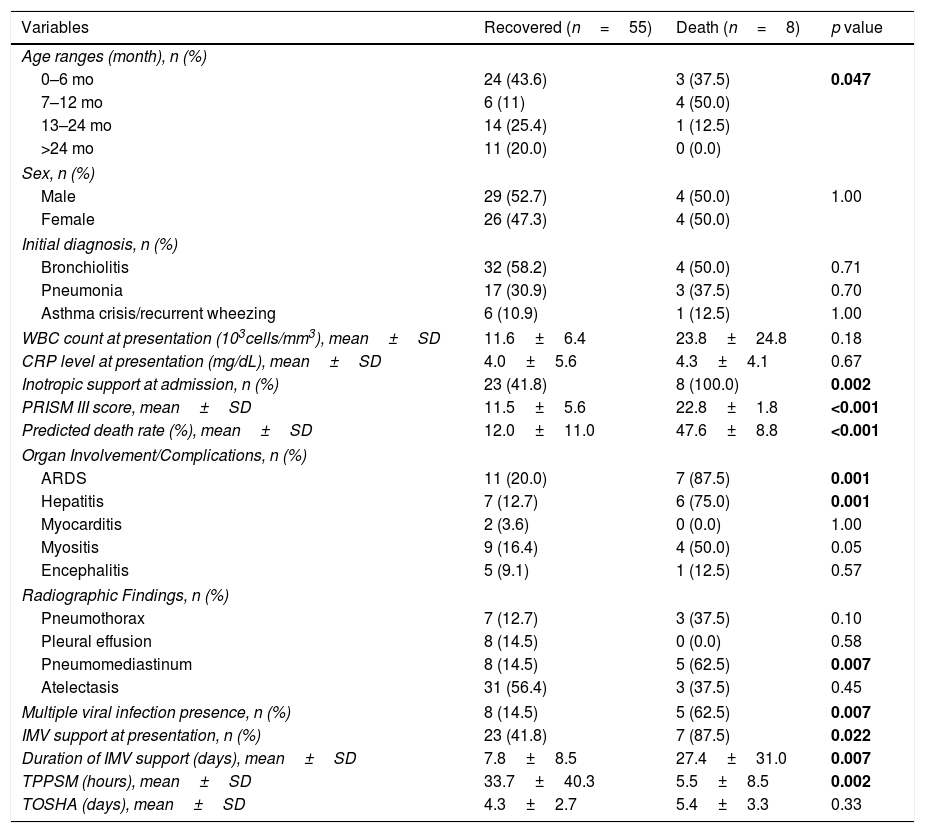
The demographic, clinical and laboratory variables and their relationships with survival are shown in Table 4. Children who had died in the PICU had a higher propensity to be aged 7–12 months and were found to have the following characteristics: short period of time from PICU admission to IMV initiation, high PRISM III score, high PDR%, requirement for ionotropic support usually with complications (ARDS and hepatitis) (p<0.01), radiographic evidence of pneumomediastinum on chest X-ray (p<0.01), multiple viral etiology (p=0.007), and they were also found to require IMV support with increased duration of MV (p<0.05). There was no statistically significant relationship between survival and viral etiology (p>0.05).
Comparison of clinical outcome and effect of demographic, clinical and laboratory characteristics among children with viral LRTI.
| Variables | Recovered (n=55) | Death (n=8) | p value |
|---|---|---|---|
| Age ranges (month), n (%) | |||
| 0–6 mo | 24 (43.6) | 3 (37.5) | 0.047 |
| 7–12 mo | 6 (11) | 4 (50.0) | |
| 13–24 mo | 14 (25.4) | 1 (12.5) | |
| >24 mo | 11 (20.0) | 0 (0.0) | |
| Sex, n (%) | |||
| Male | 29 (52.7) | 4 (50.0) | 1.00 |
| Female | 26 (47.3) | 4 (50.0) | |
| Initial diagnosis, n (%) | |||
| Bronchiolitis | 32 (58.2) | 4 (50.0) | 0.71 |
| Pneumonia | 17 (30.9) | 3 (37.5) | 0.70 |
| Asthma crisis/recurrent wheezing | 6 (10.9) | 1 (12.5) | 1.00 |
| WBC count at presentation (103cells/mm3), mean±SD | 11.6±6.4 | 23.8±24.8 | 0.18 |
| CRP level at presentation (mg/dL), mean±SD | 4.0±5.6 | 4.3±4.1 | 0.67 |
| Inotropic support at admission, n (%) | 23 (41.8) | 8 (100.0) | 0.002 |
| PRISM III score, mean±SD | 11.5±5.6 | 22.8±1.8 | <0.001 |
| Predicted death rate (%), mean±SD | 12.0±11.0 | 47.6±8.8 | <0.001 |
| Organ Involvement/Complications, n (%) | |||
| ARDS | 11 (20.0) | 7 (87.5) | 0.001 |
| Hepatitis | 7 (12.7) | 6 (75.0) | 0.001 |
| Myocarditis | 2 (3.6) | 0 (0.0) | 1.00 |
| Myositis | 9 (16.4) | 4 (50.0) | 0.05 |
| Encephalitis | 5 (9.1) | 1 (12.5) | 0.57 |
| Radiographic Findings, n (%) | |||
| Pneumothorax | 7 (12.7) | 3 (37.5) | 0.10 |
| Pleural effusion | 8 (14.5) | 0 (0.0) | 0.58 |
| Pneumomediastinum | 8 (14.5) | 5 (62.5) | 0.007 |
| Atelectasis | 31 (56.4) | 3 (37.5) | 0.45 |
| Multiple viral infection presence, n (%) | 8 (14.5) | 5 (62.5) | 0.007 |
| IMV support at presentation, n (%) | 23 (41.8) | 7 (87.5) | 0.022 |
| Duration of IMV support (days), mean±SD | 7.8±8.5 | 27.4±31.0 | 0.007 |
| TPPSM (hours), mean±SD | 33.7±40.3 | 5.5±8.5 | 0.002 |
| TOSHA (days), mean±SD | 4.3±2.7 | 5.4±3.3 | 0.33 |
LRTI: Lower respiratory tract infection, WBC: White blood cell, CRP: C-reactive protein, PRISM: Pediatric risk of mortality, ARDS: Acute respiratory distress syndrome, IMV: Invasive mechanical ventilation, TPPSM: Time period between PICU admission to start of IMV, TOSHA: Time between onsets of symptoms to hospital admission.
This study was conducted to examine the viral etiology and characteristics of severe LRTIs in children admitted to the PICU. Most of the studies until now have focused on LRTI usually in children with underlying neonatal history, but the current study focused on otherwise healthy children rather than children with underlying chronic disease. Another discrete feature of our study is the inclusion of only patients who met the previously-determined ‘severe LRTI’ definition. The mortality rate in our study was found to be 12.7%, which is somewhat higher than previous publications. This may very well be associated with the fact that the study was conducted on a population with severe LRTI which was defined as the need for MV in LRTI. Another reason may be the higher PRISM III scores and PDR% values that are seen in patients with severe LRTI. Although there are various studies in the literature which have investigated LRTI in the emergency room or intensive care settings, these patient groups are often heterogeneous.
The mean age of patients with severe LRTI was 17.4 months, with the majority of patients determined to be younger than 6 months of age. In the literature, the majority of LRTI cases requiring hospitalization are infants and patients younger than 6 months of age.4,5,12,13 However, although age ranges are similar in most publications, there are significant differences between studies in terms of viral etiologic factors and initial diagnoses. In our study, no significant relationship was found between viral etiology and initial diagnoses. The only stand-out result on this topic was the fact that we found initial diagnoses to be bronchiolitis in the majority of cases with RSV and hRV, similar to prior reports.17,18 In our study, bronchiolitis was observed more frequently in hBoV infection, while recurrent wheezing was found more frequently in the study conducted by Calvo et al.18
In the current study, RSV (36.5%) was the most commonly detected virus isolated in children with severe LRTI, followed by hRV and hBoV with equal frequency. Although etiology of viral LRTI may differ in different climates and countries, RSV continues to be the most common virus in almost all environments.19–21 However, in severe disease or among critically ill children who require intensive care, there are often a number of other, more virulent viruses. Due to this reason, we emphasize that the frequency of respiratory viruses may differ on the basis of where the samples are collected from, whether they are from the PICU or the pediatric ward.
There are studies showing the clinical importance of hBoV as one of the most virulent respiratory viruses.22–25 This hypothesis is also supported by the result that the second most common virus in children with severe LRTI was hBoV and hRV in this study. The frequency of hBoV was 27% in our study which is the highest among prior studies. Calvo et al. reported an incidence rate of 11.4% for hBoV which was much lower than our result.23 It is becoming increasingly apparent that hBoV and hRV are important respiratory viruses which cause significant disease burden in infants and children with critical LRTI. It is worth to mention that absence of detection of hBoV in asymptomatic children adds some supporting evidence to its pathogenicity.26 Although the pathogenic role of hBoV is under discussion, there are other studies that show the presence of hBoV in symptomatic children.18,27 Considering that we found higher prevalence of hBoV and hRV – rather close to RSV percentage, we believe both viruses may be important in patients with severe LRTI requiring PICU admission. Additionally, we found that children with positive tracheal aspirate for hBoV were significantly more likely to require IMV support at admission, compared to other viruses. No significant relationships were determined between clinical characteristics and various parameters; however, patients positive for hBoV had shorter time until hospital admission and showed rapid progression of disease – many requiring PICU management within 3 days. Also patients infected with hBoV or Mpp were found to need inotropic support at a higher frequency. This adds strong evidence that hBoV is more aggressive than other respiratory pathogens and is associated with more severe LRTI.
On the basis of epidemiological studies, chronological age is the single most important predictor of the likelihood of severe LRTI, given the observation that almost 2/3rd of hospital admissions due to RSV occurs in first 5 months of life.28,29 In the current study, RSV peaked at the age of 0–6 months and flu A at 7–12 months, which are in line with previous studies, suggesting that passive immunity is not effective in preventing viral LRTI at these age groups, increasing disease severity. Calvo et al.18,27 found that older children are more susceptible to hBoV infection as compared to RSV, similar to our findings. They found that bronchiolitis and hypoxia were more common in RSV infected children but our result did not show such a difference. Hypoxia, which was considered as an indicator of disease severity in other studies, was already present in patients included in our study as all of them had acute respiratory failure. The same study showed elevation of WBC and CRP in single hBoV infection as compared to RSV and Mpp. Similarly, our study found high WBC count in hBoV infected children, but CRP was found to be elevated in Mpp infected children.
In regard to bacterial co-infections, we found additional bacterial pathogens in 6 children (9.6%), but no relationships could be determined with any characteristics analyzed in this study. This result shows that LRTI is most commonly caused by viral organisms and highlights the pivotal role of viruses in LRTIs because it is possible that primary infections with viral pathogens could predispose children to subsequent bacterial infections. It was interesting to note that patients with bacterial co-infection had only one respiratory virus in their etiology analysis. None of the samples showed both bacterial co-infection and multiple viral infections. In the literature, bacterial co-infection rates range from 16% to 37%, with higher rates determined in the PICU.30,31 Similar to our study, bacterial co-infection with typical organisms, such as Moraxella Catarrhalis, and Haemophilus Influenzae, have been reported in previous studies focused on patients in the PICU.30,31
In previous studies, multiple viral infection rate is reported to be between 31 and 37%.32,33 Recent studies found hRV, RSV and AdV as the leading viruses in multiple infections.1,32,33 In our study, hRV was often associated with high rates of multiple viral infection and the most common combination was: hRV+non-RSV. A study from Kuwait defined the most common multiple viral infections in LRTI were hRV, AdV and hCoV, with similar results from other studies.33–36 The most frequent combinations detected were hRV+adenovirus, hRV+hCoV and hRV+Flu A. There was no multiple viral infection with RSV and hMPV, similar to our result. Previous studies have conflicting results about clinical characteristics of single versus multiple viral infections; some showed no significant difference between multiple and single viral infection but others showed both hMPV and RSV increase the risk of admission to PICU and use of MV by 10 folds.32 We found that multiple viral infection decreased survival rate and therefore had poor prognosis explaining the theory that multiple viral infections result from the alteration of immune response or host vulnerability.36,37 Manbach et al. reported that RSV+hRV multiple infection increased severity of illness and also reported that hRV+non-RSV combinations have shorter LOS.13 Our findings were conflicting, as we found hRV+non-RSV was more commonly isolated in critically ill children with severe LRTI admitted to PICU – with a 57.1% MV rate and most common combination of hRV+hBoV (42.8%), this result may be attributed to the virulence of hBoV.
We also evaluated clinical and radiographic findings in order to be able to determine associations between disease characteristics/severity and viral etiology. Although we found ARDS as the most frequent complication, we found no significant difference between specific viruses in terms of complications. Pneumomediastinum was significantly more frequent in children infected with flu A compared to other viruses. In previous reports, there were also cases with influenza-associated spontaneous pneumomediastinum.38 Also, with regard to mortality, children who had died were more likely to have pneumomediastinum as a radiographic complication on chest X-rays.
In the current study, children with multiple infections had lower survival than children with single infection. This result may be attributed to viral load and suggests that multiple etiology in viral LRTI may be associated with worse prognosis. We found relatively high mortality (12.7%) in our study population. In previous studies mortality rate due to LRTI varies from 2.3 to 12.6% in PICUs.39,40 This variation in the mortality values may be caused by the differences between populations and the lack of homogeneity in the patient populations. High mortality in our study was presumably associated with the fact that all patients included in the study were critically ill with severe form of LRTI and were in need of mechanical ventilator support. As most of our patients needed IMV during their admission, we believe that beyond the need of IMV, other clinical parameters may have negative impact on the survival of patients who already had severe LRTI; it is therefore feasible to suggest that all of these factors may cumulatively increase the mortality rate. Additive effect on mortality may be related to or can be explained by findings such as: increased duration of IMV, shorter time between PICU admission to IMV initiation, high PRISM III score at admission, increased PDR (%), more frequent need for inotropic support, presence of radiological findings on chest X rays (pneumomediastinum) and presence of multiple viral infections. We believe that these characteristics, even though they cannot be a direct cause of high mortality, can be related to the finding of high mortality rate in the population studied; however, these results have to be supported by other prospective studies and also in large cohorts.
This study has potential limitations. Since the number of patients with complications in each subgroup is very low, there is a need for larger patient populations in order to be able to evaluate the differences in terms of complication rates and etiology. Considering the low number of cases, it is difficult to draw conclusions concerning the mortality rate of specific viruses. Viral respiratory pathogens are known to demonstrate seasonal variations in terms of virulence, so prospective and long term studies are necessary to limit single-season confounding affects. We could not include detailed clinical parameters such as mode and parameters of MV and ventilator-associated pneumonia development. However, these might also have been associated with mortality and complications. Finally, due to the prospective study design, clinical characteristics and mortality analyses of children who did not need MV could not be assessed during the course of this study.
We concluded that RSV was the most common virus in our group of patients, followed by hRV and hBoV. Patients with hBoV infection were more likely to require IMV support at initial presentation. Clinical complications were not associated with etiology, except for the presence of pneumomediastinum which was found to be more frequent in patients with flu A/H1N1. Children between 7 and 12 months old had significantly higher mortality. Patients who died were more likely to have multiple viral infections and radiographic evidence of pneumomediastinum, as well as increased use of IMV ventilation and duration, increased need of inotropic support and high PRISM III score.
Authors’ contributionMD and ZK were involved in the treatment of this case series. Study design, collecting analyzing and interpretation of the patient data were performed by ZK and MD. ZK was the major contributor in writing the manuscript. MD revised the manuscript critically for important intellectual content and approved the final form of the manuscript. All authors read, edited and approved the final manuscript.
FundingThere is no funding received for the current study.
Conflict of interestAll authors declare that there is no conflict of interest.