Central venous pressure (CVP) is still the most frequent hemodynamic variable for deciding when to administer fluids.1 This is interesting regarding numerous trials demonstrating that CVP is not a reliable index for predicting fluid responsiveness,2 and most of the clinical guidelines in which CVP is no longer recommended for such a purpose.3
Despite its current discredit, should we exclude CVP from our usual hemodynamic evaluation? Or does it still bring relevant information for the patient assessment? Following, we will describe some principles (Table 1) that might be useful for a correct interpretation of CVP measurements from its physiological meaning to its clinical use.
Central venous pressure (CVP): use and misuse.
| When not to use CVP |
| To evaluate preload |
| To evaluate volemia |
| To predict fluid responsiveness (preload dependence) |
| Why to measure CVP |
| Because it is related with the venous return |
| Because it affects tissue blood flow |
| Because a high CVP value is always pathologic, regardless of the cause |
| To establish a “limit” for fluid administration |
| CVP, when assessed with the cardiac output, provides information about changes in venous return and cardiac function |
Preload is the myocardial tension at the end of diastole. CVP is used as a measure of preload due to the directly proportional relationship between pressure and tension. However, CVP is an intracavitary pressure and preload is defined not only by the intravascular pressure, but also by the pressure surrounding the heart. As pericardial pressure is almost identical to CVP except in pathological states,4 this external pressure is represented mostly by the pleural pressure (Ppl). Subtracting Ppl from CVP results in the transmural pressure (Ptm). Transmural pressure is related with the force that distends cardiac cavities and that actually defines the cardiac preload. This is the source of frequent mistakes when measuring intravascular pressures and why CVP should be measured at the end of expiration. In normal conditions, Ppl is close to zero at end-expiration and the effect of surrounding pressure can be then neglected, so the CVP is closest to the right atrial Ptm. However, in pathological situations, Ppl can be significantly increased, making such approach unreliable. This circumstance is particularly evident during pathological conditions such as intra-abdominal hypertension or in presence of pulmonary hyperinsuflation. In these situations, Ppl is increased and transmitted to the cardiac cavities raising CVP. However, the Ptm and cardiac preload could be reduced.
Another factor to consider when using CVP for estimating preload is that end-diastolic ventricular volume (EDV) is not related with pressure in a linear nor unique way. This can be explained by the fact that atrial compliance decreases as EDV increases. So, CVP increases more as EDV increases. Moreover, in some pathological situations, such as myocardial ischemia or septic shock, this relationship is frequently altered. In other words, the same CVP may correspond with different EDVs according with the actual atrial compliance.5
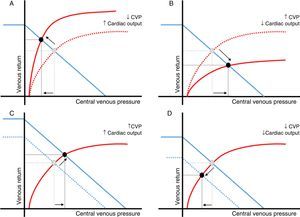
Finally, CVP results from the interaction between the right ventricular (RV) function and the venous return (Fig. 1). Consequently, a single CVP value may involve numerous cardiac function and venous return states.6 Therefore, CVP changes may be the consequence of variations in cardiac function, venous return, or both.6
Relationship between changes in central venous pressure and cardiac output. Central venous pressure (CVP) is defined by the relationship between the right ventricular function (red) and venous return curves (blue). Intersection of both curves (black dot) determines a unique value of CVP and cardiac output. Changes in cardiac output and CVP in the same direction mainly reflect variations in the venous return (peripheral function). Changes in cardiac output and CVP in opposite directions are usually the result of a variation in cardiac function (central function). A: cardiac function improvement; B*: cardiac function worsening; C: venous return increase; D: venous return decrease. *In this particular scenario, an increase in extravascular pressure should be also considered (air trapping, intraabdominal hypertension, etc.). In these circumstances, transmural pressure and cardiac preload could be reduced.
Regardless of its limited value as a preload index, CVP is also unable to predict whether the cardiac output (CO) will increase after fluid administration.2 Since CO variations do not depend only on preload changes but also on the ventricular function, an isolated preload value, as estimated by CVP, will not reliably predict the CO increase after a fluid challenge.2 It is noteworthy that this is purely a physiological but not technological limitation, so it does not rely on the accuracy of the preload estimation, either measured by the CVP or by any other preload variable.
Why should we still measure CVP?Because CVP is a determinant of the venous returnThe venous return is determined by the gradient created between the average pressure in the venous system or mean filling pressure (MFP), and the CVP. Venous resistances oppose to this gradient resulting in a simple relationship that describes venous return as (MFP–CVP)/venous resistances. Keeping constant other factors, any increase in CVP will reduce the MFP-CVP gradient and hence the venous return.
When evaluating the influence of CVP on venous return, the intravascular pressure, not the Ptm, must be considered.7 Since venous return is usually simplify as a continuous flow returning to heart, the mean value of CVP throughout the entire respiratory cycle has to be used.
Because CVP influence the blood capillary flowCapillary blood flow depends on the gradient between the mean arterial pressure (MAP) and the CVP. Although, under normal conditions, autoregulatory mechanisms allow to maintain a stable MAP despite highly variable CO,8 CVP increases can affect significantly tissue blood flow, particularly during low MAP states. Therefore, a high CVP value could decrease the MAP-CVP gradient, which in turn, could also result in a reduced capillary and organ blood flow.
Because a high CVP value is always pathologicIn a healthy person, CVP is close to zero,6 and for an optimal performance, the heart will always try to keep the CVP as low as possible.9 Therefore, in normal conditions, changes in venous return or CO are not usually followed by significant changes in CVP.9 This is explained because CVP results from the interaction between venous return and RV function. As long as RV function is preserved, CVP will be kept as low as possible. In other words: the heart regulates CO modulating the CVP.6 Therefore, CVP should be interpreted as a coupling index between RV function and venous return, rather than as a preload variable.
Regardless of its cause, a high CVP will always have a negative impact on venous return and capillary blood flow. This could explain why high CVP values have been associated with increased mortality and higher renal failure incidence.10 Accordingly, a high CVP value should be considered an alarm signal and should trigger an urgent diagnostic assessment aimed to determine the underlying cause (Table 2). However, it is important to remember that a high CVP may be the result of several pathological conditions and can be associated with different preload states. Therefore, the therapeutic approach could be quite different according to the situation. An adequate echocardiographic evaluation may be helpful to find out the main mechanism involved.
Causes for a high central venous pressure.
| Intravascular causes (increased transmural pressure and preload) |
| Heart failure |
| Hypervolemia |
| Pulmonary hypertension |
| Pulmonary embolism |
| Extravascular causes (decreased transmural pressure and preload) |
| Pericardial effusion/Cardiac tamponade/Constrictive pericarditis |
| Air trapping/High PEEP |
| Valsalva maneuver |
| Pneumothorax |
| Intra-abdominal hypertension |
Fluid administration aimed to achieve an arbitrary CVP value lacks of physiological rationale. Pursuing a fixed value of CVP, such as 12cmH2O, can be deleterious in a patient with ventricular dysfunction, whereas for a patient with intra-abdominal hypertension, this CVP could be associated with a decreased preload.
However, since a healthy heart is associated with low CVP values, a significant CVP raise after fluid administration should be interpreted as an early sign of RV dysfunction. Giving more fluids beyond this point could worsen cardiac function and impair venous return and capillary blood flow. Therefore, the role of CVP for guiding fluid therapy is not for defining how much, but rather when to stop giving fluids.5
Because, when analyzed together, cardiac output and CVP changes can provide information about changes in venous return and cardiac functionIt has been explained that an isolated CVP value is difficult to interpret. However, assessing CVP and CO together could provide a valuable information about what is happening with the cardiac function and/or the venous return.
As CVP is defined by the interaction between RV function and the venous return, CVP and CO changes are determined by a unique peripheral (venous return) and central (cardiac function) relationship. Consequently, when CO and CVP change in the same direction, they mainly reflect a change in the venous return (either by an increase in the MFP-CVP gradient or by a decrease in venous resistances). On the other hand, when changes in CO and CVP are in opposite directions, they usually result from a variation in cardiac function (Fig. 1).6
ConclusionAn adequate use of CVP measurements requires a solid knowledge of its physiological basis and limitations. In this regard, we strongly believe that, understanding these physiological boundaries, CVP measurement may still have a role in the hemodynamic assessment.
Authors contributionBoth authors contributed to the original idea and writing of this manuscript.
FundingA. Santos is M+Vision COFUND Advanced Fellow and has received funding from Consejería de Educación, Juventud y Deporte of the Comunidad de Madrid and the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under Research Executive Agency grant agreement n¿ 291820.
Conflict of interestThe authors declare no conflict of interest regarding this paper.