Acute kidney injury (AKI) in the ICU frequently requires costly supportive therapies, has high morbidity, and its long-term prognosis is not as good as it has been presumed so far. Consequently, AKI generates a significant burden for the healthcare system. The problem is that AKI lacks an effective treatment and the best approach relies on early secondary prevention. Therefore, to facilitate early diagnosis, a broader definition of AKI should be established, and a marker with more sensitivity and early-detection capacity than serum creatinine – the most common marker of AKI – should be identified. Fortunately, new classification systems (RIFLE, AKIN or KDIGO) have been developed to solve these problems, and the discovery of new biomarkers for kidney injury will hopefully change the way we approach renal patients. As a first step, the concept of renal failure has changed from being a “static” disease to being a “dynamic process” that requires continuous evaluation of kidney function adapted to the reality of the ICU patient.
El tratamiento de lesiones renales agudas (LRA) en la UCI requiere habitualmente procedimientos complementarios costosos, se asocia a una elevada morbilidad y su pronóstico a largo plazo no es tan bueno como se creía hasta ahora. En consecuencia, las LRA ocasionan una importante carga para el sistema sanitario. El problema es que no existe un tratamiento eficaz para las LRA y el mejor enfoque se basa en la prevención secundaria precoz. Por consiguiente, para facilitar el diagnóstico precoz, es necesario establecer una definición más amplia de la LRA así como identificar un marcador con mayor sensibilidad y capacidad de diagnóstico precoz que la creatinina sérica (el marcador más habitual de LRA en la actualidad). Afortunadamente, se han desarrollado nuevos sistemas de clasificación (RIFLE, AKIN o KDIGO) para solucionar este problema y se espera que el descubrimiento de nuevos biomarcadores de lesión renal cambie la forma en que abordamos el tratamiento de los pacientes con nefropatía. Como primer paso, el concepto de insuficiencia renal ha pasado de considerarse una enfermedad «estática» a un «proceso dinámico» que requiere una evaluación continua de la función renal adaptada a la realidad del paciente en la UCI.
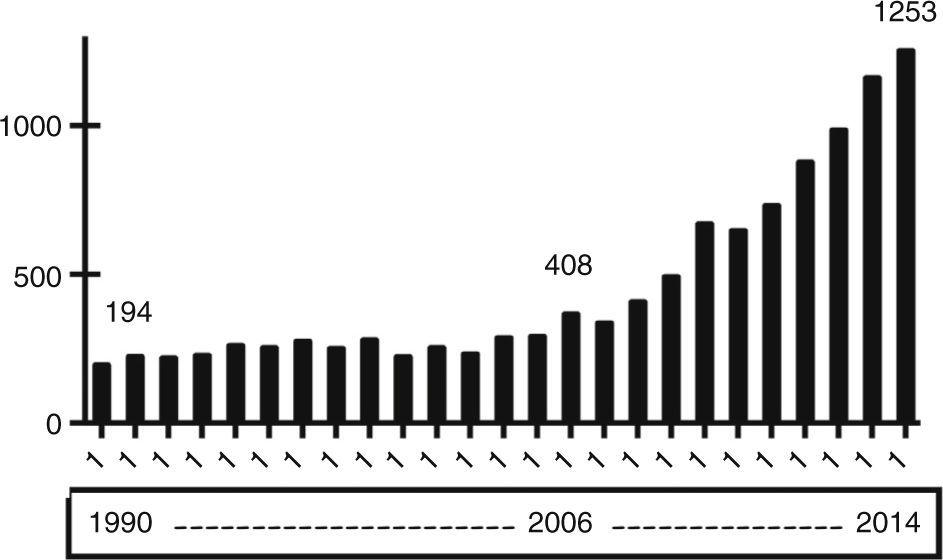
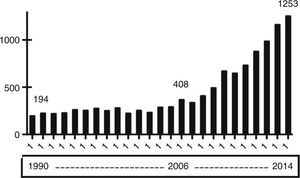
Acute kidney injury (AKI) has been long recognized as a common complication in intensive care unit (ICU) patients. However, it has not been until recently that intensivists have shown a growing interest in and awareness of its real impact on costs and outcomes, as evidenced by the dramatic increase in the number of articles published on this topic in dedicated journals (Fig. 1). At present, it is widely accepted that AKI is a common clinical syndrome in ICUs1–3 and has a central role as a systemic disease causing multiple systemic sequels and lesions in extra-renal organs. Also, AKI exerts a negative effect on the course and short- and long-term prognosis of the disease4 not only for the patient but also for the kidney. These findings have led to a change in the definition of AKI,5 with special emphasis on early detection and prevention. Considering these facts and the emerging role that extracorporeal depuration therapies have in intensive care units, our workgroup resolved to develop a comprehensive up-to-date review of all these topics, which will be presented in this journal in a series of six reviews, being this paper the one that starts the series.
Pathophysiology of AKIThe main causes of AKI are ischemia, hypoxia and nephrotoxicity. The mechanisms involved in kidney injury and repair are complex. The kidney is particularly susceptible to ischemia and toxins, resulting in vasoconstriction, endothelial damage, and activation of inflammatory processes. This susceptibility arises in part from the vascular-tubular relationships in the outer medulla of the kidney, where the partial pressure of oxygen is low, even at baseline, making them more vulnerable to a decreased renal blood flow.6 In the presence of a decreased glomerular filtration rate (GFR) secondary to hypoperfusion,7 the normal response of the kidney is to maximally concentrate urine and reabsorb sodium avidly in an effort to maintain/increase intravascular volume and normalize renal perfusion. However, a prolonged decrease in renal perfusion can result in irreversible ischemic damage, leading to ischemic AKI or acute tubular necrosis (ATN), which it is the most severe form of AKI. ATN is characterized by sub-lethal and lethal injury to the tubular cells, mainly in distal regions of the proximal tubule and thick ascending limb of Henle's loop.7
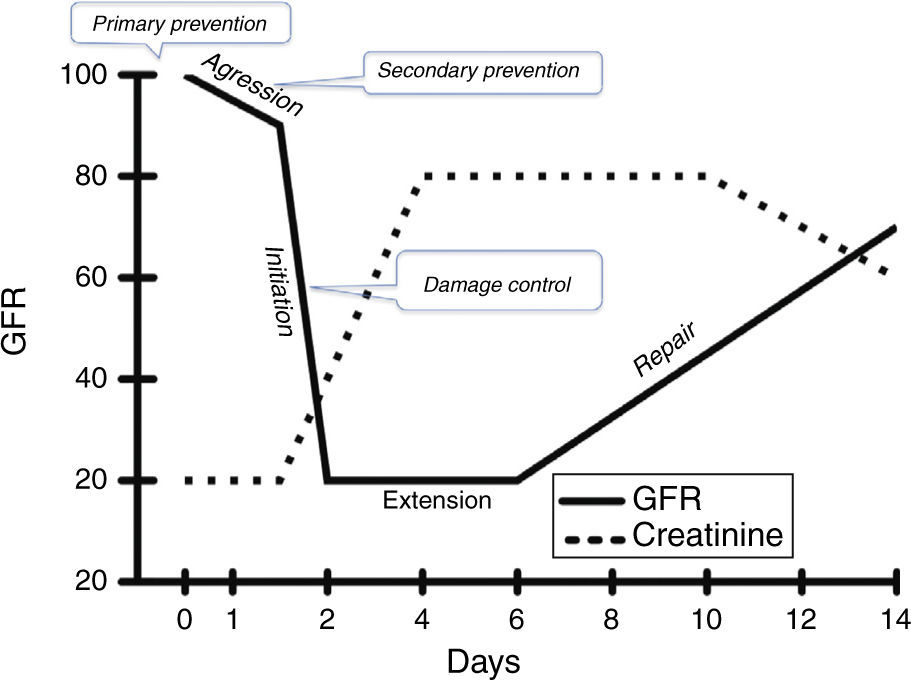
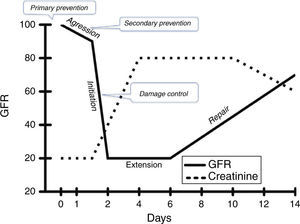
Historically, AKI has been divided into well-characterized and sequential phases, namely, initiation, maintenance and recovery8 and, more recently, Sutton et al.9 added a prerenal and an extension phase, establishing five pathophysiological stages during ischemic ATN7–10 (Fig. 2).
- (1)
Prerenal: continuous with the next stage, occurs when renal blood flow (RBF) decreases but cellular integrity is yet maintained.
- (2)
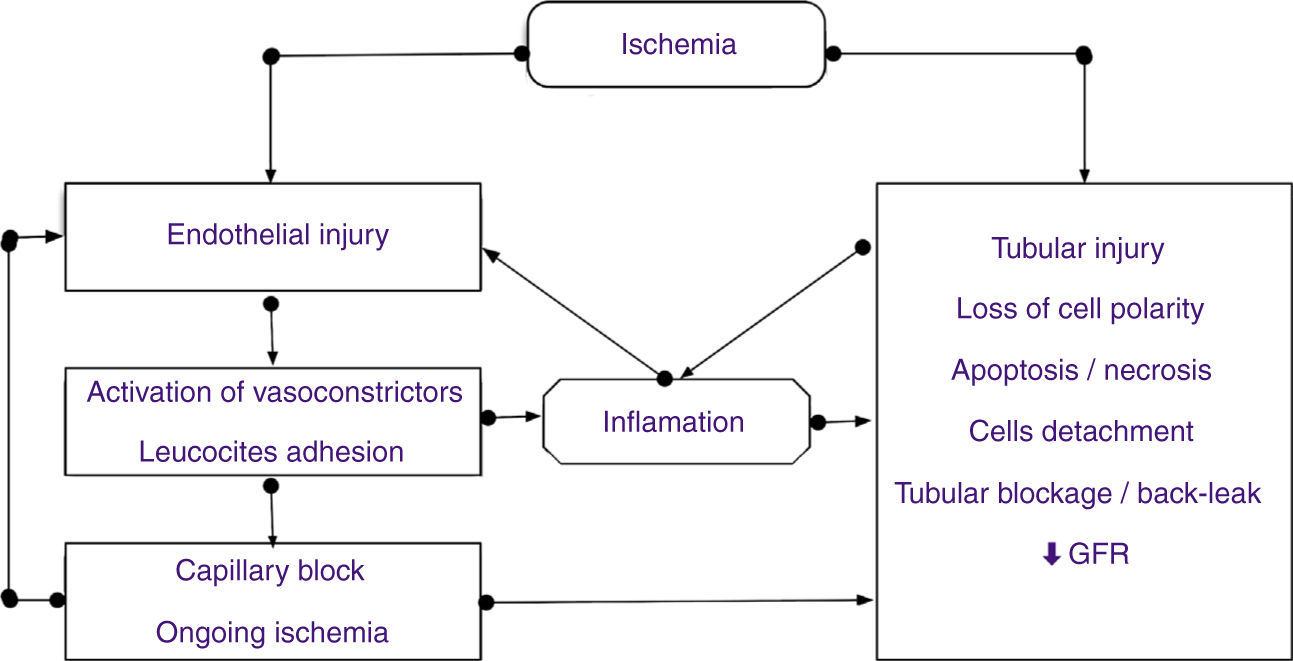
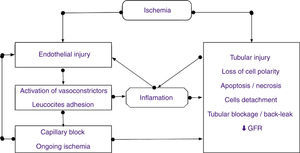
Initiation: characterized by a decrease in GFR due to a decrease in net ultrafiltration pressure. Ischemic injury is higher in the S3 segment of proximal tubule and thick ascending limb due to the high consumption of ATP in these areas, located in the outer medulla where partial pressure of oxygen is lower. Ischemia causes ATP depletion, inhibition of active sodium transport, formation of reactive oxygen species, alterations in the cytoskeletal structure and loss of cell polarity (relocalization of Na/K ATPase), tight junctions between cells (E-cadherin) and attachment of cells to the basement membrane (integrins). The accumulation of detached cells and necrotic debris in the lumen of the tubule contribute to occlusion and back-leak of glomerular filtration (Fig. 2). This damage can be repaired if blood flow restores early.
- (3)
Extension: morphological and functional changes appear in vascular endothelial cells and renal tubular epithelium, resulting in the recruitment of circulating inflammatory cells such as neutrophils, lymphocytes and macrophages, and the expression of adhesion molecules and chemokines. Cells of the S3 segment produce interferon regulatory factor 1 (IRF-1), which activates transcription of proinflammatory genes. Proximal tubule cells produce cytokines (TNF-α, TGF-β, interleukins) and in addition IL-18 and IL-6 are also released into the tubular lumen and can be used as early biomarkers of kidney damage. Therefore, this injury induces the production of inflammatory mediators by endothelial and tubular cells, contributing to the recruitment of leukocytes. We can say that inflammation plays an important role in both the initiation and the extension of kidney damage (Fig. 2).
- (4)
Maintenance: lasts 1 or 2 weeks and during this phase GFR is stabilized at its lowest level and now oliguria and uremic complications can occur. GFR is kept low by dysregulation of possible release of vasoactive mediators from endothelial cells, the congestion of medullary blood vessels and damage by reactive oxygen species and inflammatory mediators produced by leukocytes and renal cells after reperfusion. During this clinical phase, cells undergo repair, migration, apoptosis and proliferation in an attempt to reestablish and maintain cellular and tubule integrity.
- (5)
Recovery: characterized by the repair and regeneration of tubular epithelium, and the gradual return of GFR. During this phase, differentiation continues, epithelial polarity is reestablished and normal cellular and organ function returns. Surviving cells are quiescent and undergo a process of de-differentiation and migration to enter the cell cycle and repopulate the basement membrane, regenerating damaged epithelium. For this to occur successfully, there must be a parallel process to clear the accumulation of tubular cells. The successful recovery from AKI depends on the degree to which these repair processes ensue and may be compromised in elderly or chronic kidney disease (CKD) patients.7–9 Recovery takes 1–2 weeks after normalization of renal perfusion, requiring repair and regeneration of tubular cells.8 This phase may be complicated by a diuretic phase due to lack of functionality of the cells of the proximal tubule to reabsorb water and solutes.
The pathologic features of ischemic ATN are focal lesions of necrotic tubular epithelium, with detachment of cells from the basement membrane and occlusion of the tubular lumen by composite cylinders (casts), protein Tamm–Horsfall and pigments. Although accumulation of leukocytes is frequently observed in the vasa recta, the morphological characteristics of the glomeruli and vasculature are normal. Necrosis is most serious in the S3 segment of the proximal tubule, but it also affects the ascending limb of Henle. After exposure to nephrotoxic agents, morphological changes tend to be more prominent in the proximal tubules and cell necrosis is less pronounced for ischemia8 (Fig. 3).
Anyway, the understanding of the physiopathology of AKI during sepsis is still limited, mainly because scarcity of histological studies and an inability to measure renal microcirculatory flow. As a fact, even when early stages of AKI during sepsis are associated with hypoperfusion and a drop in oxygen transport, once a hyper-dynamic state ensues, RBF is found to be normal or even increased, with no significant histological evidence of tubular necrosis. Thus, factors other than ischemia may participate in the genesis of AKI in sepsis, including apoptosis, glomerular and medullary microcirculatory disorders, cell changes in response to the characteristic pro-inflammatory cascade of sepsis, oxidative stress, mitochondrial dysfunction and damage induced by mechanical ventilation, among others.11
Evaluating kidney function and damageA way to evaluate renal function is studying its capability to maintain GFR, a measure of the amount of blood filtered (but not necessarily of damage) per unit of time. A direct relationship between renal mass and GFR is only present late when the kidneys have lost their capability to compensate changes in the load of solutes (renal reserve) (Fig. 2).12
Serum creatinine (SCr) is produced and eliminated at a constant rate and it is exclusively cleared by the kidneys, and it is the parameter universally adopted for the diagnosis of kidney failure. However, when evaluating SCr, it should be kept in mind that SCr level reflects functionality but not necessarily actual damage, and changes in SCr do not have a linear relationship with changes in GFR until the renal reserve is lost (i.e. until damage is extensive).12,13 Moreover, GFR can be better estimated by the rate of clearance of creatinine or creatinine clearance (CrCl), which is measured by 24-h urine collection. However, this test has been shown to have low operability in the ICU setting. In contrast, different investigators have demonstrated the validity of CrCl measured in samples of urine collected in shorter time intervals. This method makes repeated measures more feasible, facilitates the handling of urine samples from several patients, and – the most critical aspect – results are obtained without delay.14,15 In addition, these studies have shown that up to 25% of patients with SCr within normal or near normal levels (below 1.5mg/dL) had significantly low CrCl levels.14,15
Different equations have been developed to estimate GFR based on isolated samples of SCr without needing to collect urine. Yet, all equations have been shown to have a poor performance in critical care patients, and there is general agreement against its use in this scenario.14–16 Nevertheless, these equations are still a useful guide for drug dosing in renal dysfunction in ICU patients.17
The identification of new biomarkers of renal damage for early diagnosis and stratification of the disease is becoming an urgent need.18 Despite recent advances in this field,19–21 a marker that – as in myocardial injury – allows early diagnosis and risk stratification of AKI in a safe, reliable and specific way has not been found yet. To the discovery of new markers for differential diagnosis of AKI, as well as for predicting the need for renal replacement would be also of great interest. Among these biomarkers, Cystatin-C – used in determinations of blood isolates or incorporated to new equations for estimating CrCl levels22,23 – has been proposed as a simple method for early detection of AKI. Apart from Cystatin-C – a functional marker –, other biomarkers for the stratification of kidney lesions such as NGAL (neutrophil gelatinase-associated lipocalin), KIM-1 (kidney injury molecule-1) or interleukins (i.e. IL-6 and IL-18) are being developed (Table 1), with uncertain results.19 The results of early studies were promising, especially in the pediatric population and in patients where the timing of renal damage is well known (e.g. heart surgery). Yet, the sensitivity of these biomarkers in other populations has not been proven to be as high as in these patients, especially in adult subjects with pre-existing renal disease or other accompanying comorbidities (such as diabetes mellitus or vascular disease). More recently, cycle arrest biomarkers (IGFBP7 and TIMP-2) have shown to be superior for risk stratification of AKI, and they have been proven to be reliable in the identification of patients at high risk of developing moderate to severe AKI within the first 12h of evolution.24,25 Interestingly, these markers are not increased in patients with sepsis, chronic kidney disease and diabetes mellitus who do not develop AKI. The combined use of these two biomarkers improves their performance,24 and it has been the base in the development of a commercial test (NephroCheck®) that measures urinary levels of TIMP-2 and IGFBP7 by immunofluorescence.26 The cut-off level of [TIMP-2.IGFBP7] used for the diagnosis of AKI is 0.3 [ng/ml] 2/1000 (with and adjusted PPV of 69%, adjusted NPV of 96%, and AUC 0.82 (95% CI 0.76–0.88) with a sensitivity of 92% (95% CI 85–98%).27
Early biomarkers for AKI detection.
| Serum markers | |
| Cystatin-C | Early diagnosis AKI |
| Lipocalin (NGAL) | Early diagnosis AKI |
| Urinary markers | |
| Low molecular weight proteins | |
| Cystatin C | Early diagnosis AKI Prognosis (RRT) |
| Enzymes released by renal tubule | |
| α-Glutathione S-transferase (α-GST) | Early diagnosis AKI |
| π-Glutathione S-transferase (π-GST) | Early diagnosis AKI |
| N-acetyl-β-d-glucosaminidase (NAG) | Established AKI diagnosis Prognosis (RRT and mortality) |
| Proteins produced by renal tubule | |
| Neutrophil gelatinase-associated lipocalin (NGAL) | Early diagnosis AKI Differential diagnosis Prognosis (RRT) |
| Kidney injury molecule-1 (KIM-1) | Early diagnosis AKI Established AKI diagnosis Prognosis (RRT and mortality) |
| Liver-type fatty acid binding protein (L-FABP) | Early diagnosis Prognosis (RRT) |
| Inflammatory urinary markers | |
| Interleukin 18 (Il-18) | Early diagnosis AKI Established AKI diagnosis Differential diagnosis Prognosis (RRT and mortality) |
| Cell cycle arrest proteins | |
| Insuline-like growth factor-binding protein 7 (IGFBP7) | Early diagnosis AKI |
| Tissue inhibitor of metalloproteinase-2 (TIMP-2) | Early diagnosis AKI |
RRT: renal replacement therapy.
To increase the predictive value of existing biomarkers, a new and promising approach is the use of panels of several combined biomarkers covering different phases of damage and periods of time. In a recent study, Basu et al.28 demonstrated that the combined use of serum Cystatin-C and urinary NGAL improves their capacity to detect severe AKI, with a high specificity for the diagnosis of transient AKI in patients undergoing cardiac surgery. It has also been proposed that the use of a functional biomarker (Cystatin-C) plus various tubular damage signalers (Il-18, Cystatin-C and KIM-1 or Il-18, NGAL and π-GST)20 could allow earlier diagnosis, which is essential to initiate the implementation of secondary prevention measures and the administration of the appropriate treatment. The ADQI panel of experts, in an effort to diagnose subclinical AKI, has suggested the combined use of serum and urinary NGAL, KIM-1, interleukin 18 and L-FABP.29 Finally, the use of a panel combining urinary NGAL, KIM-1 and Il-18 has been proposed for the evaluation of AKI severity.20
The identification of hospitalized patients with an increased risk for AKI is of great interest. It seems logical that in patients with multiple risk factors for AKI, minor changes in SCr and urine output should be enough to raise suspicion about the presence of AKI. In contrast, in patients with fewer risk factors, a greater change in SCr and urine output would be necessary to create the same level of clinical suspicion. In this context, the term renal angina was coined by Goldstein and Chawla30,31 – paralleling angina in acute coronary syndrome– to predict early stage AKI. Renal angina includes a combination of risk factors for AKI (susceptibility plus exposure), subtle changes in SCr/diuresis and volume overload in the absence of specific renal clinical symptoms. In a study conducted in a pediatric ICU and another in adult patients, renal angina was shown to have a high sensitivity (>90%) and a very high negative predictive value (>95%) for AKI. In addition– paralleling the role of troponine in coronary syndromes– the use of early biomarkers for renal injury could further increase the sensitivity of renal angina to predict the development of AKI.30
Definition and stagingAcute kidney injury is defined as a failure of the kidneys to eliminate waste products and maintain homeostasis of water and electrolytes. However, no definite and measurable parameters have been established for the diagnosis of AKI, and there is no general agreement on what AKI definitely is among clinicians and investigators. In light of the wide range of definitions available (almost every study published provides a different definition of AKI), it is very difficult to compare experiences and determine the exact incidence and impact of AKI in the critical care setting.32 When a renal aggression occurs and until the kidneys show any alterations, a compensation mechanism based on decreasing GFR levels is activated.33 Yet, such decrease in GFR is not detected early because of the lack of a method that is sensitive enough.34 As a result, AKI is only detected in later stages. This problem is worsened by the fact that a standard definition of AKI has not been established yet. A clear framework – or “Standardized Criteria” – should be established for an objective clinical diagnosis of AKI. These standardized criteria should have sufficient sensitivity and specificity, and they should serve to diagnose and categorize the severity of AKI. Standardization is not only critical to clinical practice, but it is also essential for future research, epidemiological studies (maximizing sensitivity) and clinical trials (maximizing specificity).35,36 A clear definition of AKI that makes it objectively evaluable would help identify the presence of the disease, assess its severity and determine a prognosis.37
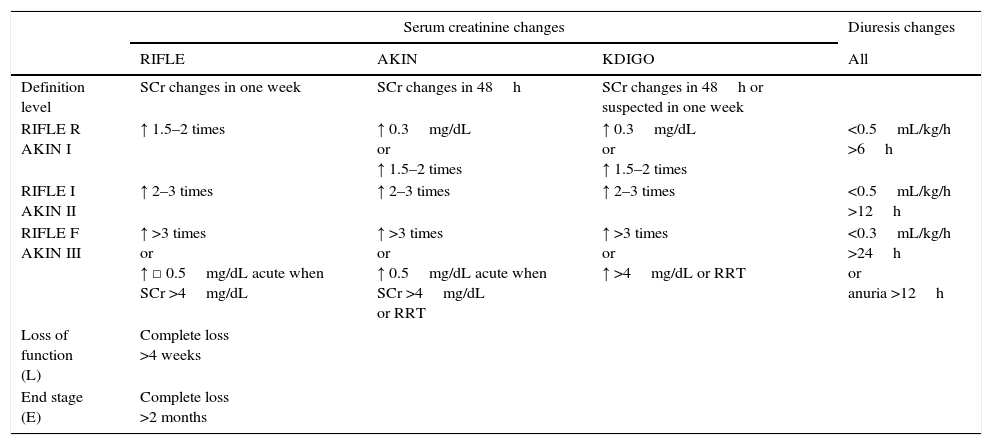
The diagnosis of AKI is mainly based on SCr but, after a serious renal insult, changes depend largely on the baseline level of serum creatinine. For this reason, it is now advocated that a definition of AKI should be based upon changes in SCr levels for a given period of time.38 In this regard, the RIFLE (an acronym for Risk, Injury Failure, Loss, and End-stage) criteria4 should be considered a significant step forward, not only because they provide a consensus definition, but also because AKI is considered a dynamic process. In 2004, the group “acute dialysis quality initiative” (ADQI) was the first to establish a consensus definition of AKI and developed the RIFLE criteria – and its adjusted version for pediatric patients (pRIFLE).39 The definition of AKI by this group is essentially based on the increase of SCr over 7 days. According to the RIFLE criteria, AKI is defined as a SCr increase ≥50% over baseline and/or a decrease renal glomerular filtration rate (GFR) ≥25% and/or a diuresis ≤0.5ml/kg/h ≥6h. However, emerging evidence suggests that slight changes in SCr (i.e. 0.3–0.4mg/dL) are associated with increased in-hospital mortality.40–42 This evidence made it necessary to redefine the RIFLE criteria, and the AKI Network developed the AKIN criteria.43 Thus, RIFLE stages (Risk, Injury and Failure) were renamed as stage I, II and III; L (Loss) and E (End-Stage) categories were entirely eliminated, and the need for renal replacement therapy (RRT) was classified as stage III.
A problem with the RIFLE classification is that it relies on changes in CRs or CrCl, although they are not necessarily equivalent.44,45 According to the kinetics of renal damage, an initial renal insult is followed by a period during which SCr does not increase, whereas renal damage continues worsening.46 In some way, later revisions of RIFLE have tried to solve this problem by searching slight changes in SCR in a shorter time-window and eliminating CrCl as a parameter definitely.47
More recently, the Current International Kidney Disease Improving Global Outcomes initiative has developed the KDIGO guidelines for AKI,48 where RIFLE and AKIN criteria have been merged. The main difference concerning these guidelines is that AKIN criteria recommend adequate resuscitation and exclusion of urinary obstruction before making a diagnosis, whereas this is not recommended in KDIGO guidelines (even when it seems reasonable to consider them when working out an AKI diagnosis). In addition, KDIGO guidelines modify stage III of chronic kidney disease (CKD). Thus, patients with an increase of ≥0.3mg/dl when baseline SCr was ≥4mg/dl will be staged as III instead of I. Diuresis – as some essential staging criteria of these classification systems – is prone to error and should be corrected for ideal weight in order to avoid over- (obesity false positives) and under-diagnosis (false negatives in cachexia) (Table 2).
Diagnostic criteria and main differences between RIFLE, AKIN and KDIGO systems.
| Serum creatinine changes | Diuresis changes | |||
|---|---|---|---|---|
| RIFLE | AKIN | KDIGO | All | |
| Definition level | SCr changes in one week | SCr changes in 48h | SCr changes in 48h or suspected in one week | |
| RIFLE R AKIN I | ↑ 1.5–2 times | ↑ 0.3mg/dL or ↑ 1.5–2 times | ↑ 0.3mg/dL or ↑ 1.5–2 times | <0.5mL/kg/h >6h |
| RIFLE I AKIN II | ↑ 2–3 times | ↑ 2–3 times | ↑ 2–3 times | <0.5mL/kg/h >12h |
| RIFLE F AKIN III | ↑ >3 times or ↑ □ 0.5mg/dL acute when SCr >4mg/dL | ↑ >3 times or ↑ 0.5mg/dL acute when SCr >4mg/dL or RRT | ↑ >3 times or ↑ >4mg/dL or RRT | <0.3mL/kg/h >24h or anuria >12h |
| Loss of function (L) | Complete loss >4 weeks | |||
| End stage (E) | Complete loss >2 months | |||
SCr: serum creatinine; RRT: renal replacement therapy.
In a recent systematic review (the NICE study)49 comparing the ability of pRIFLE, RIFLE, AKIN and KDIGO criteria to diagnose and stratify AKI and establish a prognosis, acceptable agreement was observed between the RIFLE and AKIN criteria for adults, and a study also found a good correlation between AKIN and KDIGO.36
Regarding outcomes, a multivariate analysis controlling for confounding factors found that AKIN, RIFLE and KDIGO were independent prognostic factors with comparable power to predict mortality. There was no evidence that the combination of SCr and diuresis could improve outcome prediction, as compared to SCr alone.
The studies published comparing these criteria have some drawbacks that have not been resolved yet. Data were almost exclusively collected from ICU patients and cannot be extrapolated to other populations. There is significant variability in the criteria employed in the definition of AKI, as some groups only used SCr, whereas others used SCr plus dieresis, and there is no definitive consensus on the criteria to be employed to define baseline renal function.
Whatever the criteria used, increased AKI severity is associated with increased mortality,45,50–52 and this association persists after controlling for covariates that could influence mortality (mortality according to KDIGO increases steeply with odds ratio of 2.09 [CI 1.19–3.67] for stage I; 2.94 [CI 1.38–6.27] for stage II and odds ratio 6.88 [CI 3.87–12.22] for stage III).53 It also seems clear that ICU and hospital stay increase with increased AKI severity, and prospects of kidney recovery diminish.
These and other controversies have been raised by different authors and societies54–56 but ultimately, KDIGO guidelines represent the best consensus available today, are based on solid evidence and expert opinions, and provide a solid basis for current practice, ongoing research, and health resource allocation. However, further research is needed to determine whether these classification systems improve patient outcomes.
Impact of AKIThe incidence of AKI in intensive care units (ICUs) shows wide variability, depending on the population analyzed and the criteria employed in its definition. The incidence of AKI has traditionally been considered to be low. Liaño et al. reported in 1996 an incidence of 209 cases per million population.57 The incidence of AKI is greater in hospitalized patients53,58–60; and it could raise up to 50%53,60 in ICU patients with septic shock, whereas mortality ranges from 40 to over 70%.59,60 Since investigations have shifted to new stratification systems such as RIFLE47 or AKIN43 for the diagnosis of AKI, figures have risen on the order of 2–10 times and more than 30% of ICU patients (up to 70% in some studies) develop some degree of AKI.50,51,53,61–66 Mortality rate increases with an increasing degree of renal dysfunction.2,45,50 There are some additional problems when trying to characterize the epidemiology of AKI. Most research published so far does not consider that some patients may experience multiple events or the fact that over 25% of patients not fulfilling AKI criteria actually have a moderate-severe dysfunction.2 This means that the incidence of AKI might be underestimated.2,67,68 Nowadays the scenario has changed dramatically and we must admit that AKI in ICU has a high incidence69 and a relatively prolonged course, compared to the length of stay,70–72 with a serious impact on mortality, with over 2 million people expected to die annually of acute kidney injury.73,74
Our perception of the prognosis is also changing substantially. Until recently it was widely accepted that chances for recovery after an AKI episode were excellent, but recent reports estimate that the incidence of chronic kidney disease (CKD) after an episode of AKI is as high as 7.8 per 100 patients per year.75 Even mild episodes of AKI have an impact on long-term mortality.76 It is time to shift to a new paradigm and consider different episodes of AKI and CKD as different stages or episodes of the same disease. Chawla et al. recently proposed a new model integrating AKI and chronic kidney disease and consider a reduced GFR as a clinical entity that has differential initiation and expression in time,73 which is referred by them as renal disease.
From acute renal failure to acute kidney injuryAKI poses a serious problem to ICU physicians, since it causes high morbidity among ICU patients. Also, long-term prognosis is not as good as it has been presumed so far, even in patients that seem to have recovered complete functionality. AKI is a permanent variable in almost all severity scores used by intensivists in clinical practice, and it is always closely related to mortality, whatever the kind of patients treated. Moreover, the development of AKI frequently involves the initiation of a costly maintenance treatment (i.e. RRT) that eventually generates a significant burden to the health system.
Given that there is not an effective treatment for AKI, diagnosis should be ideally made in the first stages of the disease to allow for the implementation of preventive measures aimed at minimizing renal damage and shortening the episode. However, early diagnosis can only be possible if a clear definition is established of what AKI is. Such standard definition should allow the assessment of disease severity, have prognostic capability and, most importantly, be easy to understand and implement.
Serum creatinine is the ideal marker because of its universality, but it clearly lacks the sensitivity and precocity that is required for AKI testing. These problems have been significantly (but not definitely) solved by the use of new SCr-based classification systems (RIFLE, AKIN or KDIGO) that are widely used nowadays. The characterization of new biomarkers for kidney injury will hopefully change the way we approach and manage renal disease in the near future. For the moment, these classification systems have allowed a conceptual change in the definition of renal failure from being a “static” disease to being a “dynamic process” that requires continuous monitoring and evaluation of kidney function, which is better adapted to the continuous changes that characterize the evolution of ICU patients.
ContributionAll authors have actively participated in the preparation of the article. GSP has made the final editing of the manuscript. MEHG has made the traducciónque was subsequently reviewed by a professional service. All authors have approved the final version of the article.
Funding sourceNone.
Conflict of interestsThe authors declare no conflict of interest.