Although amiodarone may cause neurotoxicity that can affect patient outcomes when used during cardiopulmonary resuscitation (CPR), it has been commonly prescribed during CPR. This study investigated the possible neurotoxic effects of amiodarone in a rat model of transient forebrain ischemia.
DesignA prospective laboratory animal study was carried out.
SettingAnimal laboratory.
MaterialsMale Sprague-Dawley rats.
InterventionEight minutes of forebrain ischemia was induced in rats by bilateral carotid occlusion and hypotension (mean arterial pressure=35mmHg) under isoflurane (1.5%) anesthesia. Amiodarone (0, 50, 100 and 150mg/kg) with saline was injected intraperitoneally 10min after ischemia. Rats given 0mg/kg of amiodarone were used as saline-treated controls. Sham operated rats received no treatment.
Variables of interestAnimals were evaluated neurologically on postoperative days 4–7, and histologically after a one-week recovery period.
ResultsThe greatest improvement in water maze test performance corresponded to the sham operated group (p=0.015 vs. saline-treated controls). No differences in performance were seen in amiodarone-treated rats compared with saline-treated controls. In the control group, 45% of the CA1 hippocampal neurons survived, compared with 78% in the sham operated group (p=0.009). Neuron survival after ischemia in the amiodarone treatment groups (50, 100 and 150mg/kg) (58%, 40% and 36%, respectively) and in the control rats did not differ significantly.
ConclusionsThe administration of amiodarone immediately after transient forebrain ischemia did not worsen spatial cognitive function or neuronal survival in the hippocampal CA1 region in rats. The current results must be applied with caution in humans. However, they indicate that the potential neurotoxicity induced by amiodarone during resuscitation after cardiac arrest may be negligible.
La amiodarona puede causar neurotoxicidad que afecte a los desenlaces de los pacientes si se usa durante la reanimación cardiopulmonar (RCP), si bien este fármaco se ha prescrito habitualmente a pacientes durante la RCP. Este estudio ha investigado los posibles efectos neurotóxicos de la amiodarona en un modelo de isquemia transitoria del prosencéfalo en ratas.
DiseñoEstudio prospectivo con animales de laboratorio.
ÁmbitoLaboratorio de animales.
MaterialesRatas Sprague-Dawley macho.
IntervenciónSe produjo isquemia del prosencéfalo en ratas durante ocho minutos mediante oclusión bilateral de la carótida e hipotensión (mediana de la presión arterial=35 mmHg) bajo anestesia con isoflurano (1,5%). Se inyectó intraperitonealmente amiodarona (0, 50, 100, 150 mg/kg) con solución salina 10 minutos después de la isquemia. Se administraron 0 mg/kg de amiodarona a las ratas empleadas como controles tratados con solución salina. No se administró ningún producto a las ratas del grupo quirúrgico de referencia.
Variables de interésLos animales fueron evaluados neurológicamente durante los días 4-7 tras la intervención, e histológicamente tras un período de recuperación de una semana.
ResultadosLa mayor mejora del rendimiento en la prueba del laberinto acuático se observó en el grupo quirúrgico de referencia (p=0,015 frente a los controles tratados con solución salina). No se observaron diferencias en el rendimiento de las ratas tratadas con amiodarona respecto a los controles que recibieron solución salina. En el grupo control sobrevivió el 45% de las neuronas del hipocampo CA1, frente al 78% en el grupo quirúrgico de referencia (p=0,009). No se observaron diferencias significativas en cuanto a la supervivencia neuronal tras la isquemia entre los grupos tratados con amiodarona (50, 100 y 150 mg/kg, 58, 40 y 36% respectivamente) y las ratas del grupo control.
ConclusionesLa administración de amiodarona inmediatamente después de la isquemia transitoria del prosencéfalo no empeoró la función cognitiva espacial ni la supervivencia neuronal en la región del hipocampo CA1 en ratas. Se debe tener precaución al aplicar los resultados actuales a los humanos. Sin embargo, dichos resultados señalan que la posible neurotoxicidad inducida por la amiodarona durante la reanimación tras parada cardíaca puede ser insignificante.
Cerebral and cardiac dysfunction may occur in patients following return of spontaneous circulation (ROSC) after cardiac arrest accompanied by prolonged whole-body ischemia. Post cardiac arrest syndrome, comprises anoxic brain injury, myocardial dysfunction, systemic ischemia/reperfusion injury, and persistent precipitating pathology.1,2 Post cardiac arrest brain injury is a major cause of morbidity, and is responsible for approximately two-thirds of post cardiac arrest period deaths.3 Cerebral and cardiopulmonary resuscitation (CPR) before, but not after, ROSC can improve outcomes. The 2015 update of Adult Cardiac Arrest Algorithm recommends amiodarone as the first-line drug for refractory ventricular fibrillation (VF) or pulseless VT.4 However, there are concerns of potential amiodarone neurotoxicity.5 Indeed, in the field of toxicology, amiodarone has recently been used as one of representative drugs inducing neurotoxicity to evaluate the in vitro drug-induced neurotoxicity models.6–8 Amiodarone during CPR may thus be associated with unfavorable neurological outcomes in patients with refractory VF/pulseless VT during CPR. Current clinical practice for resuscitation after cardiac arrest has been implemented although this concern has not been solved yet. This study investigated whether amiodarone was associated with neurotoxic effects in a rat model of transient forebrain ischemia.
MethodsSurgical preparationAll experimental protocols were approved by the Animal Care and Use Committee of Nara Medical University. These protocols were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Male Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) weighing 270–300g were fasted for 12h. Anesthesia was induced with 5% isoflurane in oxygen. Following intubation, the isoflurane concentration was reduced to 1.5–2.0%, and the rats were mechanically ventilated with oxygen and air at a fraction of inspired oxygen of 0.3. A needle thermometer was inserted between the temporal muscle and the skull, and the pericranial temperature was maintained at 37.5±0.5°C by surface heating or cooling. Needle electroencephalographic electrodes were inserted in a biparietal configuration, and the electroencephalogram was monitored continuously (EEG-4217, Nihon Kohden, Tokyo, Japan). A cannula was inserted into the tail artery using PE-50 tubing, and the mean arterial blood pressure (MAP) was monitored continuously. A second cannula for withdrawal of blood was inserted into the external jugular vein using PE-60 tubing.
Induction of forebrain ischemia by bilateral carotid artery occlusion (BCAO)With the animal in the supine position, the isoflurane concentration was adjusted to 1.5%, and the level was allowed to equilibrate for 10–15min. Blood samples were obtained for determination of preischemic hematocrit (Hct), glucose, pH, PaCO2, and PaO2. Ventilation was adjusted to achieve a PCO2 of 35–40mm Hg and a PaO2 ≥100mm Hg. MAP and heart rate were monitored throughout. Heparin (100U/kg) was administered intravenously, and hypotension was induced 5min later, by withdrawal of blood from the superior vena cava catheter into a prewarmed syringe, which reduced perfusion through the vertebral arteries to a point where blood flow was lost or greatly reduced.9 When the MAP decreased to 35mm Hg, both carotid arteries were occluded with vascular clamps. Ischemia was confirmed an isoelectric electroencephalogram, and was maintained for 8min. During occlusion, MAP was maintained at 35mm Hg by withdrawal or reinfusion of blood. After the period of ischemia, reperfusion of the brain was established by removal of the vascular clamps and reinfusion of the withdrawn blood. The vascular catheters were removed, and the wounds were closed. Isoflurane was discontinued when wound closure was completed. Heparin was reversed by intravenous administration of protamine (0.3mg). Ventilation was continued with 100% O2 until the animals recovered from anesthesia. Rectal temperature was recorded hourly and maintained at 37.5±1.0°C by surface heating or cooling, and control of the of the recovery chamber temperature. When spontaneous ventilation resumed, usually immediately after discontinuation of isoflurane, animals were transferred to a prewarmed oxygen-rich humidified recovery chamber. The trachea was extubated after observation of spontaneous movement, which was usually performed within 30–40min after discontinuation of isoflurane. Sham operations were performed without BCAO and hypotension. A period of three days from day 1 to day 3 after surgery was taken for facilitating animals’ physical recovery from surgical injury.
Experimental protocolsPrepared 1mL aliquots of saline, amiodarone 50mg/kg+saline, amiodarone 100mg/kg+saline, or amiodarone 150mg/kg+saline were randomly administered using a 26 gauge needle intraperitoneally 10min after BCAO. Rats given 0mg/kg of amiodarone with saline was used as saline-treated controls. Sham operated rats received nothing but intraperitoneal needle puncture. The investigators were blinded to drug preparation. Each experimental group included 10 rats that were alive 7 days after ischemia.
Motor activity assessmentsMotor activity was assessed as previously described by an investigator blinded to the group assignments on four consecutive days beginning on day 4 after surgery.10 Briefly, the rats were placed on a 30×30cm screen (grid size 1.0×1.0cm) that could be rotated from 0° (horizontal) to 90° (vertical). The animal was placed on a horizontally oriented screen, which was then rotated to the vertical plane. The time that animal was able to hold onto the vertical screen was recorded up to a maximum of 15s. The animal was then placed at the center of a horizontal 2.5-cm diameter wooden rod. The time that the animal was able to remain balanced on the rod was recorded up to a maximum of 30s. Finally, a prehensile traction test determined the time that the animal was able to cling to a horizontal rope up to a maximum of 5s. All tests were scored using the same 3-point system (could not at all=1, failed midway through the test=2, and completed the test=3). The total daily motor score of up to nine points was determined by summing the three individual test scores. The four daily scores were added to determine the total motor score.
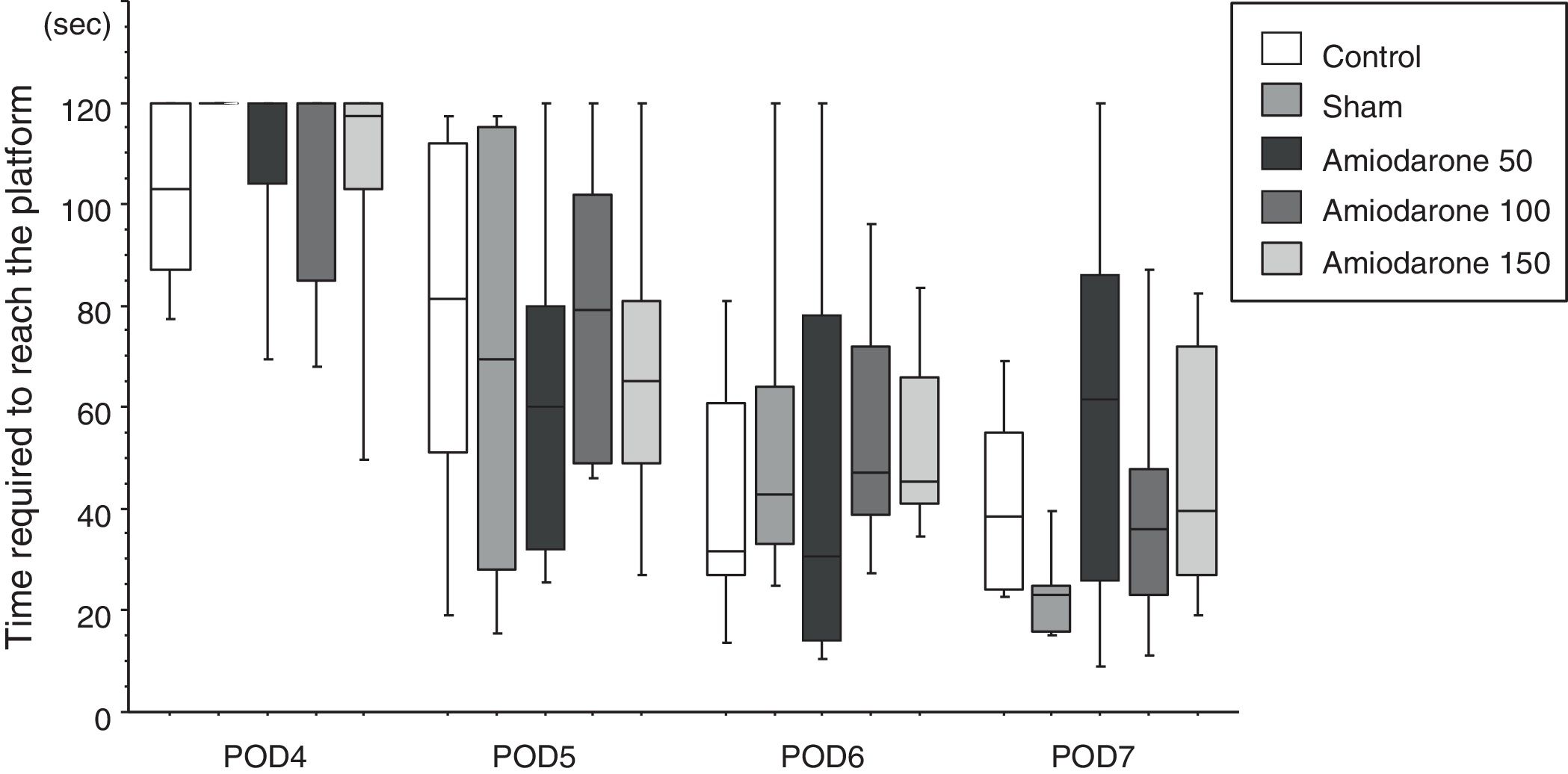
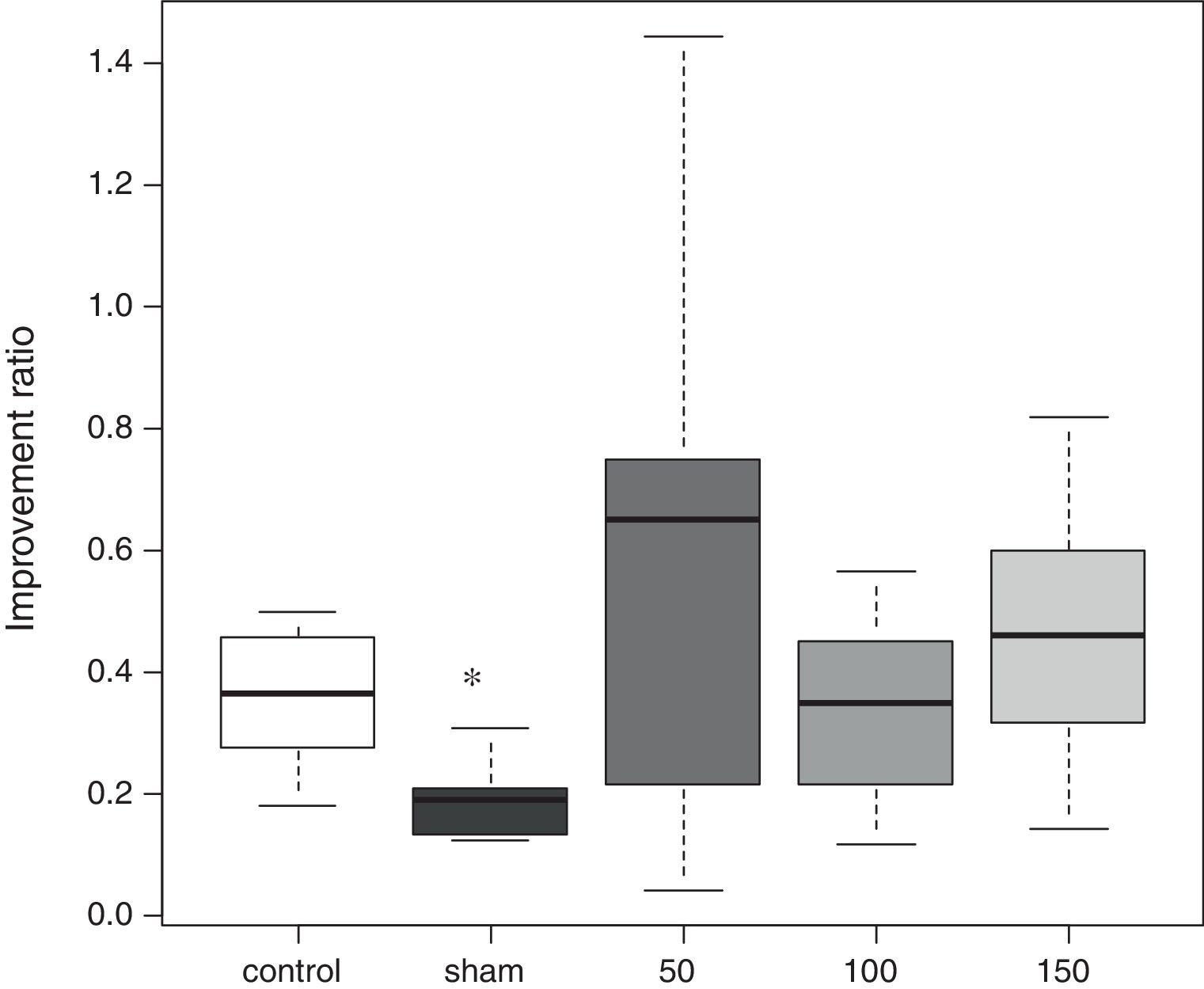
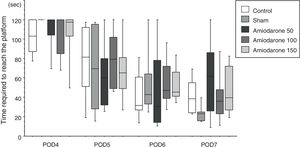
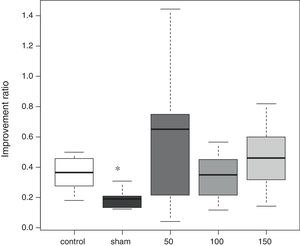
Morris water mazeThe water maze consisted of a circular vinyl-coated polyester mesh pool 1.7m in diameter and 70cm high. A translucent 15cm diameter acrylic platform was placed inside the pool 30cm from the wall and 2cm below the water surface when the pool was filled. The pool was filled with tap water made opaque by nontoxic white paint and maintained at 26±1.0°C. The rats recovered for 3 days following induction of ischemia before the water maze test. Beginning on day 4, the rats were tested for four consecutive days, i.e., days 4–7. The platform remained in the same location during testing, maze was divided into four quadrants, and the starting quadrant was randomly chosen daily. The rats were tested in the same daily order. Rats were released into the maze head-up facing the wall. Latency was recorded on reaching the platform, and the rats were permitted to remain on the platform for an additional 30s. If an animal failed to find the platform in 120s, it was then placed on the platform for 10s. Each session consisted of three trials, and the daily score was the average of the three results. Rats were rested for a minimum of 5min between trials. The improvement in maze test performance was the latency on day 7/the latency on day 4, with a lower score indicating greater improvement.
Harvesting and evaluation of brain tissueAfter evaluation of weight loss compared with the preischemic state, animals were anesthetized using 2.5–3% isoflurane through a mask, and given an intraperitoneal injection of sodium thiopental (2–3mg/kg). After confirmation of loss of consciousness and the absence of a pain response, transcardial perfusion was performed with 150mL saline at 40mL/min followed by 200mL of 4% paraformaldehyde in 0.1M pH 7.4 phosphate buffer at 20mL/min. The brain was removed and stored in 4% paraformaldehyde in 0.1M phosphate buffer for later dissection. Brains were prepared for histological analysis by dehydration in graded concentrations of ethanol and butanol followed by embedding in paraffin. Coronal sections (5μm) were prepared and stained with hematoxylin and eosin. An investigator blinded to the group assignment evaluated the CA1 region of the hippocampus in a coronal plane 3300μm posterior to the bregma. The regions were identified by referring to the rat brain atlas of Palkovits and Brownstein.11 Viable and nonviable neurons were counted manually. The percentage of viable neurons in a 0.5mm×0.5mm counting frame at a magnification ×200 was used to quantify neuronal damage. To standardize the position of the counting frame across animals, the cells in the center of each hippocampal field were counted. Nonviable neurons were identified by cytoplasmic eosinophilia with loss of Nissl substance and by the presence of pyknotic homogeneous nuclei. Values from the hemisphere with the worst damage were used in the final analysis.12
Statistical analysisThe study population size was determined by a power analysis of an effect size ranging from 0.5 to 0.75 based on a previous investigation in our laboratory that approximately 30% of neurons would survive after ischemia in the control group.12 The sample size calculation for F-testing was used even though it was assumed that the data would not be normally distributed. Assuming a type I error of 0.05 and a power of 0.9, 7–14 rats in each of the five experimental groups were required. Consequently, a sample size of ten animals/group was deemed necessary for an adequate study power. Comparisons among groups were performed using the Kruskal–Wallis test. If significant differences were found, the Steel test was used for post hoc comparisons with the saline group as a reference. Results were reported as medians and 25th to 75th percentiles. Statistical analysis was performed with R (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant.
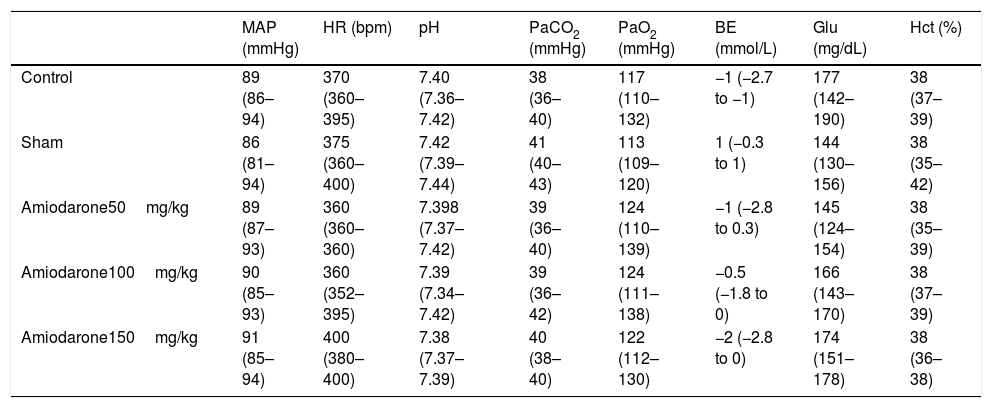
ResultsPreischemic physiological variables, including pH, PaCO2, PaO2, Base excess, Hct, glucose, arterial pressure, and heart rate in the experimental groups were similar (Table 1). Weight loss on day 7 after ischemia was negligible in all groups (data not shown). Several rats died during the 7-day recovery period. Some died in the early recovery period with apparent upper airway obstruction or seizure. The causes of the other deaths were not identified because they were not witnessed. To obtain ten living rats 7 days after ischemia 11–12 were needed in each group, indicating a mortality rate of 9–17%. Two rats in saline control group, 2 in 50 amiodarone group, 2 in 100 amiodarone group, and 1 in 150 amiodarone group died during the 7 days period. One animal in the sham surgery group died during the recovery period.
Preischemic physiological variables.
| MAP (mmHg) | HR (bpm) | pH | PaCO2 (mmHg) | PaO2 (mmHg) | BE (mmol/L) | Glu (mg/dL) | Hct (%) | |
|---|---|---|---|---|---|---|---|---|
| Control | 89 (86–94) | 370 (360–395) | 7.40 (7.36–7.42) | 38 (36–40) | 117 (110–132) | −1 (−2.7 to −1) | 177 (142–190) | 38 (37–39) |
| Sham | 86 (81–94) | 375 (360–400) | 7.42 (7.39–7.44) | 41 (40–43) | 113 (109–120) | 1 (−0.3 to 1) | 144 (130–156) | 38 (35–42) |
| Amiodarone50mg/kg | 89 (87–93) | 360 (360–360) | 7.398 (7.37–7.42) | 39 (36–40) | 124 (110–139) | −1 (−2.8 to 0.3) | 145 (124–154) | 38 (35–39) |
| Amiodarone100mg/kg | 90 (85–93) | 360 (352–395) | 7.39 (7.34–7.42) | 39 (36–42) | 124 (111–138) | −0.5 (−1.8 to 0) | 166 (143–170) | 38 (37–39) |
| Amiodarone150mg/kg | 91 (85–94) | 400 (380–400) | 7.38 (7.37–7.39) | 40 (38–40) | 122 (112–130) | −2 (−2.8 to 0) | 174 (151–178) | 38 (36–38) |
Results were reported as medians and 25th to 75th percentiles.
MBP, mean arterial blood pressure; HR, heart rate; BE, base excess; Glu, glucose.
There were no significant differences in the motor activity assessment scores in the saline control [36 (35–36)] and the sham [36 (36–36), p=0.339] groups or the control and the 50 amiodarone [35.5 (35–36), p=0.952], 100 amiodarone [35.5 (33.5–36), p=0.819], or 150 amiodarone [36 (36–36), p=0.842] groups.
In the water maze, the rats in all groups showed a tendency to shorten the time to reach the platform over the 4 days of testing (Fig. 1). The best improvement was observed in the sham group, but the latency ratio was significantly smaller than that in the control group (p=0.015). There were no significant differences in the performance of any of the amiodarone groups and the control group (50 amiodarone, p=0.774; 100 amiodarone, p=0.902; 150 amiodarone, p=0.865) (Fig. 2).
Improvement in water maze test performance. Improvement was reported as the latency on day 7/latency on day 4. A lower score indicated greater improvement
Control rats received saline. Sham rats received no drug treatment. Saline or amiodarone treatment was given 10min after ischemia. *p<0.05 vs. control.
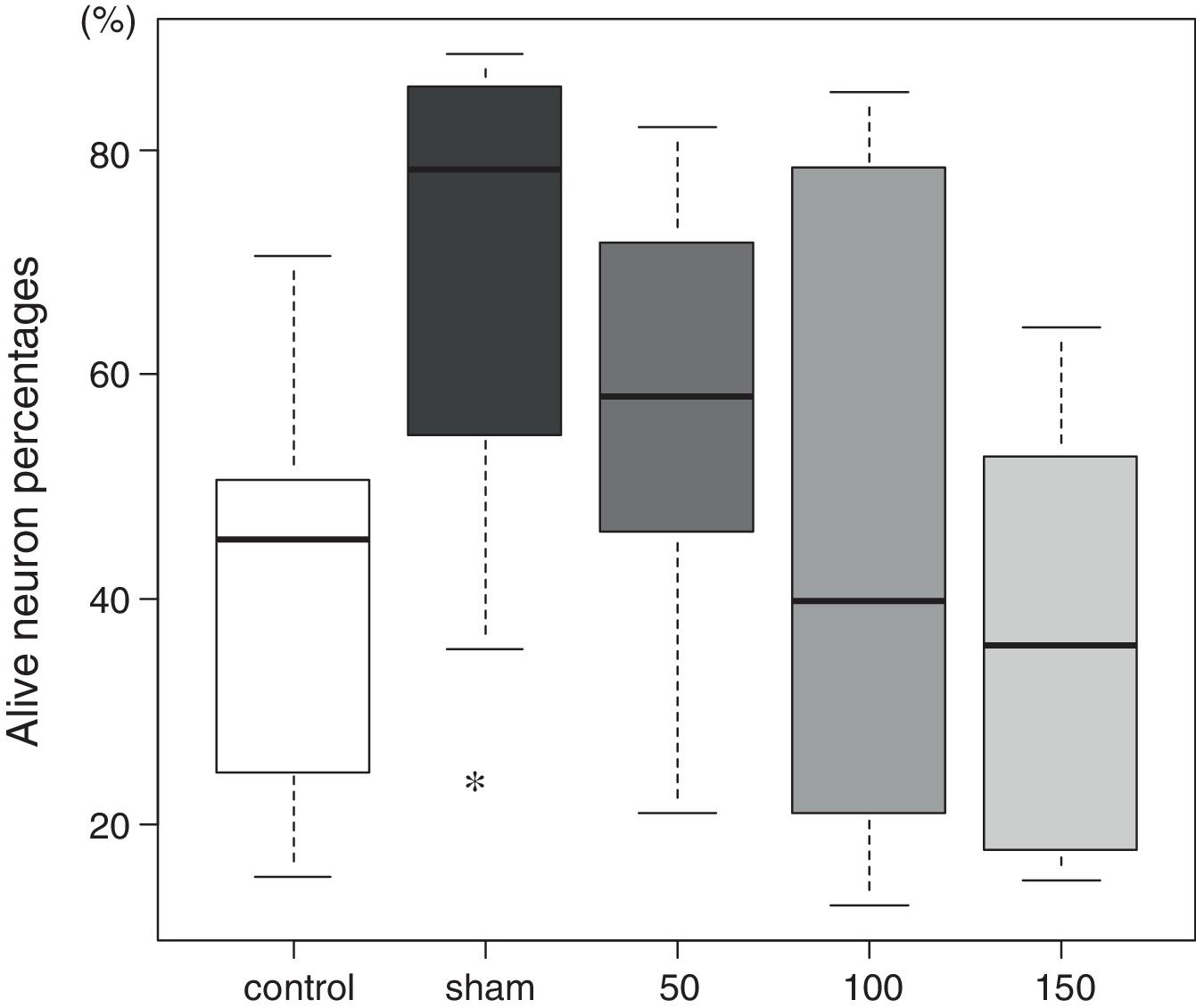
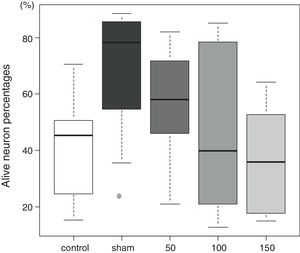
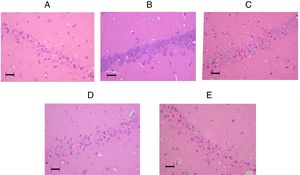
The tissue histology results are shown in Fig. 3. In the control group, 45 (27–51)% of the neurons in the CA1 sector survived. In the sham group, 78 (59–84)% of the neurons survived (p=0.009). The percentages of living neurons in all three amiodarone groups were not significantly different from that observed in the control group [58 (46–69)]% in 50 amiodarone, p=0.278; [40 (22–73)]% in 100 amiodarone, p=0.994; [36 (19–64)]% in 150 amiodarone, p=0.957). Representative histological pictures for each group are presented in Fig. 4.
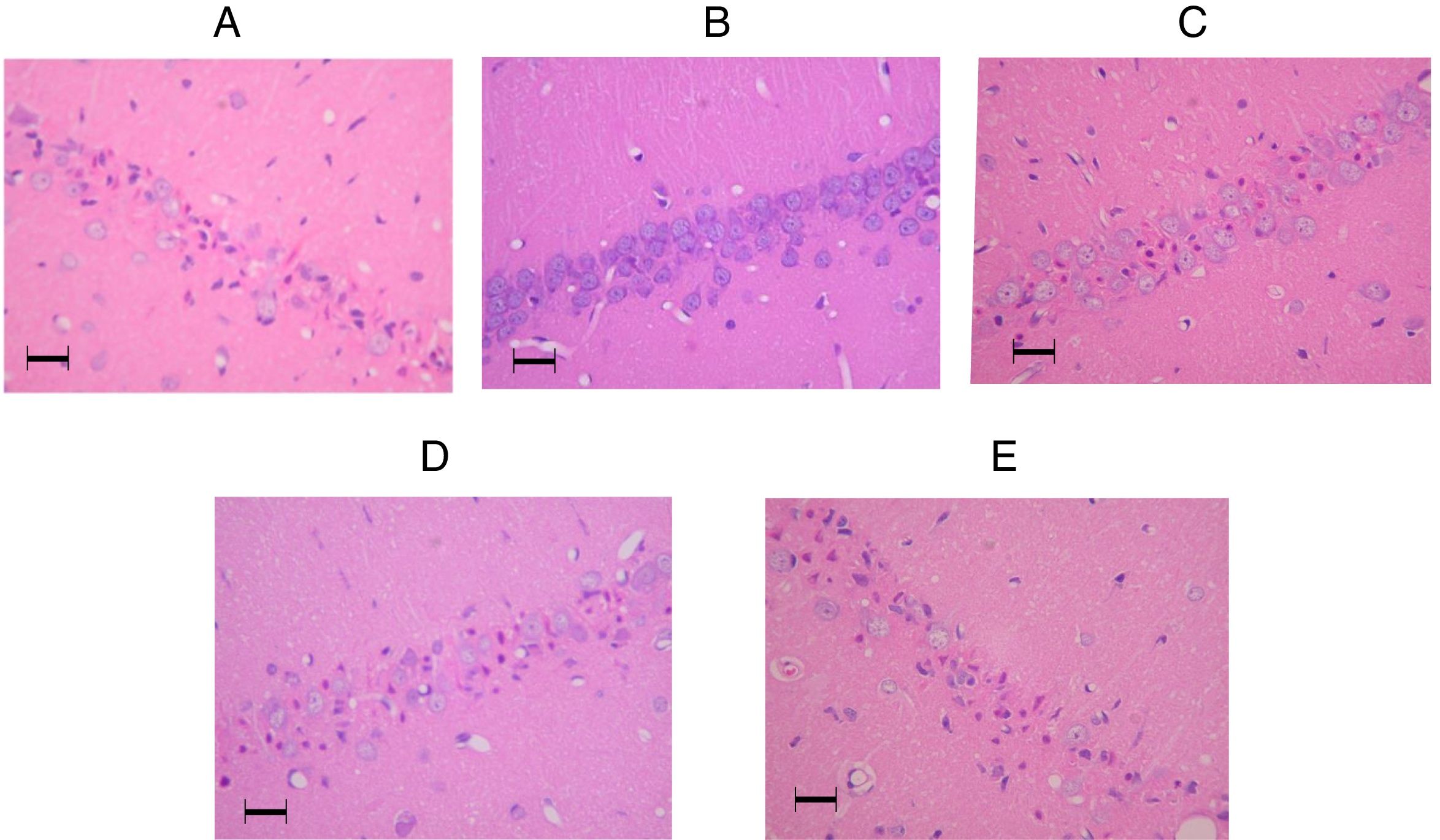
Representative pictures of the hippocampal CA1 regions in the amiodarone-treated animals and a sham-operated animal 7days after surgery. (A) 0mg/kg amodarone-treated animal; (B) Sham-operated animal; (C) 50mg/kg amodarone-treated animal; (D) 100mg/kg amodarone-treated animal; (E) 150mg/kg amodarone-treated animal. Images at magnification ×200. Bar=50μm.
Significant neuronal damage was produced by the transient forebrain ischemia produced in this study. Only around 40% of neurons survived in the hippocampal CA1 region, and the impairment of spatial cognition induced by the ischemia was significant compared with the sham group. There were no significant differences the motor activity assessment scores in the control, sham, or any of the amiodarone groups. This implies that the effect on spatial cognition induced by ischemia did not result from motor activity impairment. As there were no significant differences in the water maze test performance and the histological assessments in any of the amiodarone groups compared with the control group, amiodarone did not contribute to any neuronal damage in addition to that caused by forebrain ischemia.
The potential neurotoxicity of amiodarone has been described,5 but the mechanism is not clear. Amiodarone crosses the blood-brain barrier, and significant concentrations of the drug and its major oxidative metabolite, mono-N-desethylamiodarone, have been detected in the central nervous system.6,13 Increases in iodine content and lysosomal inclusion bodies have been observed in muscle and nerve biopsies.14 In an in vitro model, a concentration-dependent increase of the development of neuronal insult and reactive gliosis was observed after repeated exposure to amiodarone.6 However, it is not clear whether the toxicity is a direct effect of amiodarone on neurons or a result of altered lysosomal function.15 The risk of neurotoxic effects may increase with the duration of amiodarone treatment.5,6 Exposure limited to an early recovery period from ischemia might not deteriorate ischemic neuronal injury as much as expected.
On the contrary, neuroprotective effects of amiodarone have also been reported in animal models.16–18 These include reduced depolarization-evoked glutamate release from hippocampal synaptosomes,16 a shift in the ratio of neuroactive amino acids toward inhibitory transmitters in the medulla oblongata,17 and attenuation of pentylenetetrazole- and caffeine-induced seizures.18 Ischemia–reperfusion injury in the brain involves a complex cascade of events. In this study, the potential neurologic toxic effects of amiodarone might have been offset by its neuroprotective effects. The results suggest that it may not be necessary to change our routine use of amiodarone therapy for refractory VF or pulseless VT in the Adult Cardiac Arrest Algorithm.
The forebrain ischemia in this study was expected to result in moderate neuronal damage in the CA1 region, with survival of 30–40% of neurons.12 This rat model was chosen to provide a bidirectional margin to demonstrate improvement or deterioration by amiodarone. More severe ischemia could have salvaged only 10–20% of the neurons, which would have made it difficult to evaluate further deterioration resulting from amiodarone neurotoxic effects. The amiodarone dose selection was based on a previous study in which no rats used in a pentylenetetrazole-induced seizure model died after receiving 150mg/kg of amiodarone. All rats used in a pentobarbital-induced sleep model died after receiving 200mg/kg of amiodarone. Therefore, we used 50–150mg of amiodarone in this forebrain ischemia model. As another concern, BCAO instead of a real cardiac arrest model was used in this study. Several common models of cardiac arrest are currently available, for example electrically induced ventricular fibrillation, asphyxia or the use of high dose of potassium.19 These models would have mimicked more the effect of amiodarone in the cardiac arrest scenario. They are clinically relevant, however unpredictable resuscitation times can increase variability and may make data analysis difficult to interpret. Additionally, this model is associated with a high mortality rate, further increasing animal number necessary to test a hypothesis.20 Besides, other drugs used during resuscitation could affect neurological outcome19,21 and liver and renal function during cardiac arrest and resuscitation could increase the potential damage of different drugs. Therefore, it is not unreasonable to suppose that BCAO is suitable for evaluating individual effects of each drug on ischemic brain while considering these concerns.
ConclusionsIn this experimental rat model, amiodarone did not worsen spatial cognitive function or neuronal survival in the hippocampal CA1 region when given immediately after establishing frontal ischemia. The results should be applied in humans with caution. However, they indicate that the potential neurotoxicity induced by amiodarone during resuscitation after cardiac arrest may be neglectable.
FundingThis work was supported by a Grant-in-Aid for Scientific Research C (26462763) from the Japan Society for Promotion of Science (to S.I.).
Authors’ contributionsKoutaro Yamanaka conducted all experiments and analyzed the data.
Satoki Inoue design of the study, conducted all experiments and analyzed the data, interpretation of results, and wrote the manuscript.
Yusuke Naito helped to conduct all experiments and analyzed the data.
Masahiko Kawaguchi reviewed the study and modified the concept of the study.
All authors have read and approved the final manuscript.
Conflict of interestsThe authors declare that they have no competing interests.