To analyze the profile, incidence of life support therapy limitation (LSTL) and donation potential in neurocritical patients.
Study designA multicenter prospective study was carried out.
SettingNine hospitals authorized for organ harvesting for transplantation.
PatientsAll patients consecutively admitted to the hospital with GCS<8 during a 6-month period were followed-up on until discharge or day 30 of hospital stay.
Study variablesDemographic data, cause of coma, clinical status upon admission and outcome were analyzed. LSTL, brain death (BD) and organ donation incidence were recorded.
ResultsA total of 549 patients were included, with a mean age of 59.0±14.5 years. The cause of coma was cerebral hemorrhage in 27.0% of the cases.LSTL was applied in 176 patients (32.1%). In 78 cases LSTL consisted of avoiding ICU admission. Age, the presence of contraindications, and specific causes of coma were associated to LSTL.
A total of 58.1% of the patients died (n=319). One hundred and thirty-three developed BD (24.2%), and 56.4% of these became organ donors (n=75). The presence of edema and midline displacement on the CT scan, and transplant coordinator evaluation, were associated to BD. LSTL was associated to a no-BD outcome. Early LSTL (in the first 4 days) was applied in 9 patients under 80 years of age, with no medical contraindications for donation and GCS≤4 who finally died in asystole.
ConclusionsLSTL is a frequent practice in neurocritical patients. In almost one-half of the cases, LSTL consisted of avoiding admission to the ICU, and on several occasions the donation potential was not evaluated by the transplant coordinator.
Analizar el perfil, la incidencia de limitación de tratamiento de soporte vital (LTSV) y la potencialidad de donación de órganos en pacientes neurocríticos.
DiseñoMulticéntrico prospectivo.
ÁmbitoNueve centros autorizados para extracción de órganos para trasplante.
PacientesTodos los pacientes ingresados en el hospital con GCS<8 durante 6 meses fueron seguidos hasta su alta o hasta 30 días de estancia hospitalaria.
Variables de interésDatos demográficos, causa del coma, situación clínica al ingreso y evolución. Incidencia de LTSV, muerte encefálica (ME) y donación de órganos.
ResultadosSe incluyó a 549 pacientes. Edad media 59,0±14,5. El 27,0% de los comas fueron por hemorragias cerebrales.
Se aplicó LTSV en 176 pacientes (32,1%). En 78 casos consistió en no ingreso en la UCI. La edad, presencia de contraindicaciones y determinadas causas del coma se asociaron a LTSV.
Fallecieron 319 pacientes (58,1%); 133 fueron ME (24,2%) y el 56,4% de ellos fueron donantes de órganos (n=75). Edema y desviación de la línea media en la TAC y la evaluación previa por el coordinador de trasplantes se asociaron a ME. La LTSV se asoció a no evolución a ME. Nueve pacientes de menos de 80 años, sin contraindicaciones para donación y con un GCS≤4 fueron limitados en los 4 primeros días y fallecieron en asistolia.
ConclusionesLa aplicación de LTSV es frecuente en el paciente neurocrítico. Casi la mitad de LTSV consistió en el no ingreso en unidades de críticos y, en ocasiones, sin evaluar su potencialidad como donante por la coordinación de trasplantes.
In a serious attempt to homogenize donation programs throughout the world, The third World Health Organization (WHO) Global Consultation on Organ Donation and Transplantation, held in Madrid (Spain)1 called upon governments to advance toward self-sufficiency in transplantation, and thus to cover the needs of patients using all the resources available to increase organ availability. Despite the many initiatives to increase the number of organ donors, further effort is needed in this field, since there is a constant and growing need for organs for transplantation. Although the total deceased solid organ donors reached 1655 in Spain in 2013 (a figure similar to those of previous years),2 the number of patients on the waiting list for organ transplantation has remained stable or has even increased in recent years.
Up until now, most organs for transplantation in Spain have come from patients who die in hospital under conditions of brain death (BD).3 Brain death has been estimated to represent 2.3% of all in-hospital fatalities and 12.4% of all deaths in the Intensive Care Unit (ICU).4 Although these indicators are based on retrospective analyses of deaths occurring in critical care settings, they serve as a reference for calculating donation potential in our centers. The quality assurance program of the Spanish National Transplant Organization (Organización Nacional de Trasplantes, ONT), designed to determine the donation potential of critical care units (based on internal and external audits that evaluate the ultimate circumstances in which patients die in such units), retrospectively identifies the “losses” of potential donors not identified by the transplant coordinators in real time, and helps to define areas for improvement in the audited hospitals. These audits show that the number of donors could be 21.6% greater if all potential donors were identified (i.e., patients with a clinical condition suggesting compliance with BD criteria)5 and all possible losses were avoided.
In order to estimate the BD donation potential, other authors have considered it more appropriate to analyze the number of patients admitted in coma to the ICU,6 and even to hospital,7 since certain decisions referred to diagnosis and treatment in the course of the neurocritical patient care process could influence the final number of possible donors. In this regard, the impact of end of life care decisions upon donation potential has been little studied to date. The Ethicus8 study showed life support therapy limitation (LSTL) to be a common practice in European ICUs (being applied to 10% of the admitted patients and to 73% of the patients that die in such units). Although less information is available regarding the incidence of LSTL in other hospital areas, studies published in the last decade indicate that the proportion of patients receiving LSTL in the emergency services is significant–particularly among elderly subjects with multiple comorbid conditions.9,10
The changing profile of potential donors in Spain, and the new reality of our healthcare system–with more limited resources–make it necessary to optimize the detection of potential donors both within and outside the ICU.11 In this context, the present study was carried out to analyze the profile of neurocritical patients admitted not only to the ICU but also to any other hospital area; the incidence of LSTL in this group of patients; and the BD organ donation potential.
Materials and methodsDesign, data inclusion and participating centersNine centers (7 with a Department of Neurosurgery and 3 with a transplantation program) out of a total of 26 authorized to harvest organs in Catalonia participated in the project. The study was approved by the Clinical Research Ethics Committee of each participating center.
During a 6-month period (from 1 October 2009 to 31 March 2010), all patients admitted to hospital in coma, defined as a Glasgow Coma Score (GCS) of under 8, at any time in the course of their disease process were registered by an investigator in each participating center. Those cases in which the cause of coma precluded the diagnosis of BD were excluded.
The included patients were followed up on until hospital discharge (including patients who died) or until 30 days of stay. The information concerning LSTL was obtained from the case history and an interview of the physician in charge of the patient.
All forms of the cases included according to the established criteria were entered in a web database coordinated by the Catalan Transplant Organization (Organización Catalana de Trasplantes, OCATT). The study coordinators checked the data included in the registry for consistency.
Definition of variablesClinical variables upon admission and during the subsequent clinical course were recorded for each patient enrolled in the study, including: date of admission and discharge, age, gender, comorbidities, Department of initial admission, and cause of coma. The GCS was documented, along with the computed axial tomography (CAT) findings upon admission to hospital. In relation to the hospital admission period, we recorded the Department in which the patient was admitted when the GCS score decreased (GCS<8), with inclusion in the study; the CAT findings; the adopted life support measures; admission to the ICU (or Department with monitoring and life support resources allowing adequate and continuous donor maintenance); and the application of LSTL during admission. Life support therapy limitation was defined as both the non-introduction and suspension of life support therapy, and was recorded as such in the study if registered in the case history or stated by the physician in charge of the patient. According to the recommendations of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias, SEMICYUC),12 cardiopulmonary resuscitation, mechanical ventilation, renal replacement therapy, vasoactive drugs, artificial nutrition and blood products were all taken to represent life support therapies. Although non-admission of a patient to the ICU may be regarded as a form of non-treatment initiation, it is detailed as a specific type of LSTL.
Possible evaluation by the transplant coordination team at some point during the clinical course of the patient was also documented, along with the final outcome, evolution toward BD, organ donation, and cause of non-donation. In the case of the survivors, we recorded performance status at discharge or after 30 days, based on the GOS (Glasgow Outcome Scale).13
The diagnosis of BD was established according to Spanish legislation in force at the time of the study.14 Contraindications were taken to be the evidence of malignant disease, uncontrolled acute infection, or multiorgan dysfunction. Patient age in itself was not regarded as a contraindication.
The effectiveness of the donation process was calculated according to its definition within the quality indicators of the SEMICYUC as the proportion of real donors among the total cases of BD.15 A real donor was defined as a BD donor entering the operating room and from whom at least one organ was harvested for transplantation.4,5,16 The BD potential in turn was defined as the proportion of cases diagnosed with BD among the total patients admitted to hospital with GCS<8.
Statistical analysisAbsolute and relative frequencies were used as classical descriptors for categorical variables, while continuous variables exhibiting a normal distribution as determined by the Kolmogorov–Smirnov test were reported as the mean and standard deviation. In the case of continuous variables without a normal distribution, the median and interquartile range (IQR) were used. The association between two categorical variables was explored with the chi-squared test or Fisher exact test, as applicable.
The comparison of continuous variables exhibiting a normal distribution between two independent groups was carried out using the Student's t-test for independent samples, while the Mann–Whitney U-test was applied in the case of continuous variables with a non-normal distribution. Multivariate analysis of the effect of independent variables upon a dependent variable (LSTL and evolution toward BD) was based on logistic regression analysis. The multivariate analysis included those variables found to be associated to LSTL or to evolution toward BD in the univariate analysis. In all cases, statistical significance was considered for p<0.05. The SPSS version 18 statistical package was used throughout.
ResultsStudy populationA total of 605 patients with GCS<8 were identified during the study period. We excluded 24 cases of acute intoxication, 17 cases of metabolic encephalopathy and 15 cases of status epilepticus, due to the impossibility of establishing a diagnosis of BD in these situations. A final total of 549 patients were thus included in the analysis. Of these patients, 400 corresponded to hospitals with a transplant program. The mean age of the study population was 59.0±14.5 years, and 345 were males (62.8%).
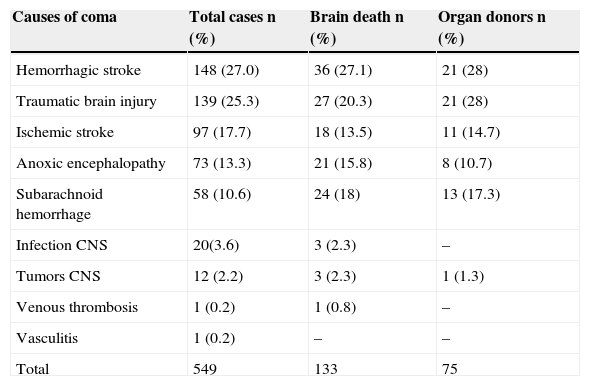
The causes of coma among the patients included in the study are described in Table 1. The most frequent cause was hemorrhagic stroke (n=148; 27.0%), followed by traumatic brain injury (n=139; 25.3%) and ischemic stroke (n=97; 17.7%).
Causes of coma.
| Causes of coma | Total cases n (%) | Brain death n (%) | Organ donors n (%) |
|---|---|---|---|
| Hemorrhagic stroke | 148 (27.0) | 36 (27.1) | 21 (28) |
| Traumatic brain injury | 139 (25.3) | 27 (20.3) | 21 (28) |
| Ischemic stroke | 97 (17.7) | 18 (13.5) | 11 (14.7) |
| Anoxic encephalopathy | 73 (13.3) | 21 (15.8) | 8 (10.7) |
| Subarachnoid hemorrhage | 58 (10.6) | 24 (18) | 13 (17.3) |
| Infection CNS | 20(3.6) | 3 (2.3) | – |
| Tumors CNS | 12 (2.2) | 3 (2.3) | 1 (1.3) |
| Venous thrombosis | 1 (0.2) | 1 (0.8) | – |
| Vasculitis | 1 (0.2) | – | – |
| Total | 549 | 133 | 75 |
CNS, central nervous system.
A total of 55.4% of the patients (n=304) were in coma at the time of hospital admission. In 245 cases the GCS decreased to under 8 during the stay in hospital (44.6%). Most subjects (n=481; 87.6%) were included in the study in the first three days of hospital stay. The median GCS at the time of detection and inclusion in the study was 5 (IQR25–75 3–6).
The transplant coordinator had evaluated the patient as a possible donor at some point during the clinical course in 433 cases (78.9%). A total of 141 of the patients (24.2%) included in the study presented medical contraindications for organ donation.
A total of 68.7% of the patients (n=377) were admitted to the ICU during hospital admission, 448 received mechanical ventilation (81.6%), and 304 were administered vasoactive drugs (55.4%).
Life support therapy limitation (LSTL)Life support therapy limitation was applied in 176 patients (32.1%). The adoption of LSTL occurred three days after admission (median, IQR25–75 1–9). In 78 cases LSTL consisted on non-admission (n=73) or non-readmission (n=5) to the UCI.
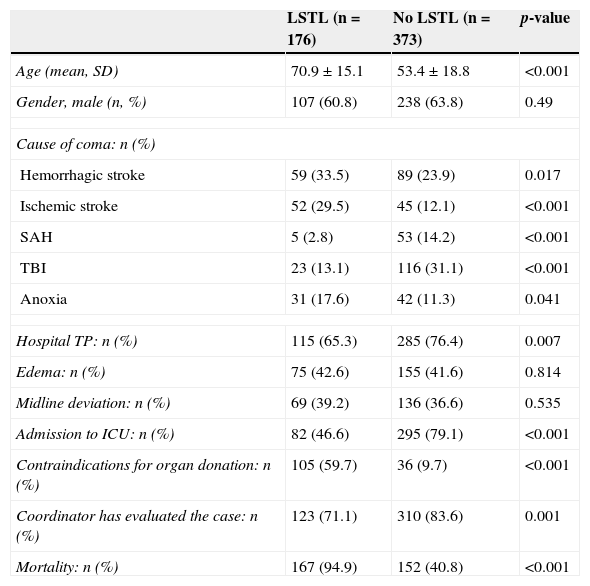
Table 2 shows the characteristics of the patients subjected to some form of LSTL versus those of the patients not subjected to LSTL at any time during their clinical course. The patients in the LSTL group were 17 years older than those in the non-LSTL group. The cause of coma influenced the incidence of LSTL, which was greater in patients with hemorrhagic stroke, ischemic stroke, and anoxic encephalopathy. Of the patients in the non-LSTL group, 78 were not admitted to the ICU: 39 because they died before admission, and the rest because their level of consciousness improved.
Univariate analysis of factors associated to LSTL.
| LSTL (n=176) | No LSTL (n=373) | p-value | |
|---|---|---|---|
| Age (mean, SD) | 70.9±15.1 | 53.4±18.8 | <0.001 |
| Gender, male (n, %) | 107 (60.8) | 238 (63.8) | 0.49 |
| Cause of coma: n (%) | |||
| Hemorrhagic stroke | 59 (33.5) | 89 (23.9) | 0.017 |
| Ischemic stroke | 52 (29.5) | 45 (12.1) | <0.001 |
| SAH | 5 (2.8) | 53 (14.2) | <0.001 |
| TBI | 23 (13.1) | 116 (31.1) | <0.001 |
| Anoxia | 31 (17.6) | 42 (11.3) | 0.041 |
| Hospital TP: n (%) | 115 (65.3) | 285 (76.4) | 0.007 |
| Edema: n (%) | 75 (42.6) | 155 (41.6) | 0.814 |
| Midline deviation: n (%) | 69 (39.2) | 136 (36.6) | 0.535 |
| Admission to ICU: n (%) | 82 (46.6) | 295 (79.1) | <0.001 |
| Contraindications for organ donation: n (%) | 105 (59.7) | 36 (9.7) | <0.001 |
| Coordinator has evaluated the case: n (%) | 123 (71.1) | 310 (83.6) | 0.001 |
| Mortality: n (%) | 167 (94.9) | 152 (40.8) | <0.001 |
Hospital TP, hospital with transplant program; SAH, subarachnoid hemorrhage; LSTL, life support therapy limitation; TBI, traumatic brain injury; ICU, Intensive Care Unit.
There were a larger proportion of patients admitted to the ICU and evaluated by the transplant coordinator in the non-LSTL group. There were no significant differences in the CAT findings, such as midline deviation or the presence of brain edema, between the two groups. However, there was a larger proportion of patients with medical contraindications for donation in the group of patients with LSTL.
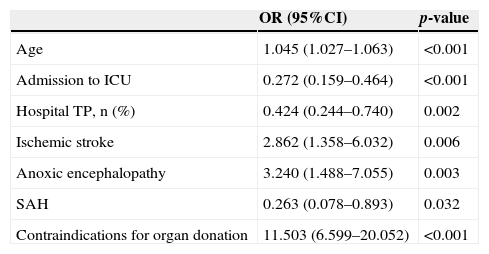
The multivariate analysis found patient age, the presence of medical contraindications for donation, and certain causes of coma (greater LSTL in ischemic stroke and anoxic encephalopathy) to be associated to LSTL. However, subarachnoid hemorrhage as a cause of coma was negatively correlated to LSTL. As expected, admission to the ICU was associated to lesser LSTL. Admission to a transplanting hospital was seen to be a “protective factor” referred to LSTL (Table 3).
Logistic regression analysis. Factors associated to LSTL.
| OR (95%CI) | p-value | |
|---|---|---|
| Age | 1.045 (1.027–1.063) | <0.001 |
| Admission to ICU | 0.272 (0.159–0.464) | <0.001 |
| Hospital TP, n (%) | 0.424 (0.244–0.740) | 0.002 |
| Ischemic stroke | 2.862 (1.358–6.032) | 0.006 |
| Anoxic encephalopathy | 3.240 (1.488–7.055) | 0.003 |
| SAH | 0.263 (0.078–0.893) | 0.032 |
| Contraindications for organ donation | 11.503 (6.599–20.052) | <0.001 |
Hospital TP, hospital with transplant program; SAH, subarachnoid hemorrhage; ICU, Intensive Care Unit.
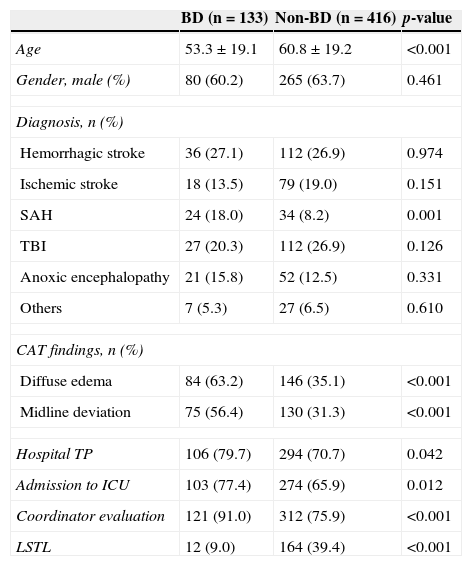
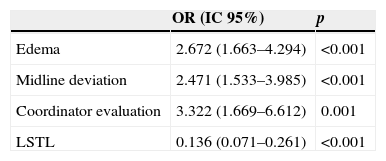
In a total of 319 deceased patients (58.1% of the studied population), 133 died in BD (24.2%). Brain death occurred two days after admission (median, IQR25–75 1–6). Table 4 shows the characteristics of the patients who died in BD versus those of the rest of the patients included in the study. In the multivariate analysis (Table 5), the presence of edema and midline deviation in the CAT study, and the existence of previous evaluation by the transplant coordinator during the patient care process were associated to evolution toward BD, while the application of LSTL was associated to evolution toward non-BD.
Factors associated to evolution toward brain death. Univariate analysis.
| BD (n=133) | Non-BD (n=416) | p-value | |
|---|---|---|---|
| Age | 53.3±19.1 | 60.8±19.2 | <0.001 |
| Gender, male (%) | 80 (60.2) | 265 (63.7) | 0.461 |
| Diagnosis, n (%) | |||
| Hemorrhagic stroke | 36 (27.1) | 112 (26.9) | 0.974 |
| Ischemic stroke | 18 (13.5) | 79 (19.0) | 0.151 |
| SAH | 24 (18.0) | 34 (8.2) | 0.001 |
| TBI | 27 (20.3) | 112 (26.9) | 0.126 |
| Anoxic encephalopathy | 21 (15.8) | 52 (12.5) | 0.331 |
| Others | 7 (5.3) | 27 (6.5) | 0.610 |
| CAT findings, n (%) | |||
| Diffuse edema | 84 (63.2) | 146 (35.1) | <0.001 |
| Midline deviation | 75 (56.4) | 130 (31.3) | <0.001 |
| Hospital TP | 106 (79.7) | 294 (70.7) | 0.042 |
| Admission to ICU | 103 (77.4) | 274 (65.9) | 0.012 |
| Coordinator evaluation | 121 (91.0) | 312 (75.9) | <0.001 |
| LSTL | 12 (9.0) | 164 (39.4) | <0.001 |
Hospital TP, hospital with transplant program; SAH, subarachnoid hemorrhage; BD, brain death; TBI, traumatic brain injury; ICU, Intensive Care Unit.
Logistic regression analysis. Factors associated to brain death.
| OR (IC 95%) | p | |
|---|---|---|
| Edema | 2.672 (1.663–4.294) | <0.001 |
| Midline deviation | 2.471 (1.533–3.985) | <0.001 |
| Coordinator evaluation | 3.322 (1.669–6.612) | 0.001 |
| LSTL | 0.136 (0.071–0.261) | <0.001 |
LSTL, life support therapy limitation; BD, brain death.
A total of 56.4% of the patients (n=75) who died in BD were organ donors. The causes of coma in the patients who died in BD and who were finally donors are shown in columns 2 and 3 of Table 1. The reasons for non-donation in the rest of cases of BD (n=58) were absolute medical contraindications (15.8% of the total; n=21), other contraindications (5.3%; n=7), family rejection (18.1%; n=24), and maintenance problems (2.3%; n=3). In addition, there was one case of legal court rejection of donation, and in two cases the legal diagnosis could not be completed (the clinical diagnosis was not made by a third physician, or asystolic occurred while in wait of the confirmatory complementary test).
Some type of LSTL had been adopted in 30.6% of the patients who died in asystolia (n=57), and there were no medical contraindications for organ donation. Only 35 of these cases (61.4%) had been evaluated by the transplant coordinator. The time from admission to LSTL in these patients was three days (median, IQR25–75 1–7). Nine of them were under 80 years of age, with GCS≤4 upon admission, and LSTL was applied within the first four days of admission.
Survivor outcomeOf the patients who did not die during the study period (n=230), 114 were discharged within 30 days after admission and 116 remained in admission after 30 days (LSTL had been applied in 5 of them). Of the patients discharged from hospital, 13 (11.4%) were discharged in a vegetative state, 19 (16.7%) with severe disabilities, 39 (34.2%) with moderate disabilities, and 43 (37.7%) with good recovery according to the GOS. Of the 114 discharged patients, only four had received some type of LSTL (all 4 were discharged in a vegetative state).
DiscussionThis is the first study to prospectively evaluate the donation potential of BD and the effectiveness of the organ donation process among patients admitted to hospital with coma defined as GCS<8. The main finding of the study is that the death in BD potential is 24.2%. Other relevant findings were the fact that LSTL was applied in 32.1% of the patients and that in 44.3% of these cases LSTL consisted of non-admission to the ICU. Furthermore, LSTL was associated to non-evolution toward BD.
In the 1990s, the efforts to increase the number of organs amenable to transplantation focused on optimization of the donation in BD process. However, such measures were not enough to cover the rising demand for organs, largely because of the decrease in incidence of BD (fewer traffic accidents, prevention of cerebrovascular disease). In the last decade in Spain and from the National Transplant Organization, a project known as Plan 40 was promoted11,17 with the aim of raising the donation rate to 40 per million population (pmp) in the country. In addition to contemplating optimization of the donation in BD process, the mentioned project included increased detection of possible donors outside the critical care areas, expanding the use of all possible dead donors–including donation in asystolia (controlled and uncontrolled), and the implementation of live donation as a complement to dead donor use.
The present study shows the potential and effectiveness of donation in BD in 9 Spanish centers, including neurocritical patients with GCS<8 admitted both to the ICU and to other areas of the hospital. One out of every 5 patients (24.2%) admitted to these centers evolved toward BD, and a little over one-half of them were finally organ donors (56.4%).
In contrast to other studies, calculation of the potential and effectiveness of donation in these centers was made on a prospective basis. Most previous studies designed to evaluate donation potential have involved retrospective analyses (based on case history reviews) corresponding to cases of patients who died in ICUs or in other areas of the hospital.7 Senouci et al.6 conducted a similar prospective predictive study but only included patients admitted to the ICU. The authors found that subjects with clinical criteria of BD represented 15.1% of the patients admitted to the ICU with GCS<8. The fact that the proportion of patients with GCS<8 admitted to hospital and who died in BD in our series was higher than in the study of Senouci et al. suggests that there is a proportion of patients who might not be admitted to intensive care due to different reasons and who presumably could evolve toward BD. In the present study, the proportion of neurocritical patients admitted to the ICU was less than 70%, which leaves a significant proportion of patients who could possibly serve as donors in other areas of the hospital outside the ICU.
Le Conte et al.9 found that 78.8% of the patients who died in the emergency service had been subjected to some type of LSTL, particularly individuals over 80 years of age, with a history of metastatic cancer, or with previous functional limitations. A posterior analysis conducted by the same group18 found that 7% of the elderly patients who die in the emergency service with neurological disease could have been selected and evaluated as potential organ donors–though the authors did not include information referred to the full diagnosis of BD.
It is well known that among patients admitted due to acute disease, acute neurological cases are those most frequently targeted for some type of LSTL in the ICU.8 Although we have tools for predicting a fatal outcome in diseases that most often produce neurological damage (brain hemorrhage based on the ICH score,19 clinical and electrophysiological data in anoxic encephalopathy,20,21 for example), the early decision to apply LSTL in different disease conditions has been widely debated,22,23 since LSTL has been associated to increased mortality. It is clear that end of life decisions cannot be based only on the degree of severity of the neurocritical patient but must also consider patient evolution during the first few days, neuromonitoring data,24 treatment response25 and, particularly, the wish of the patient (as expressed either personally or through the family) regarding the care received.26
The present study shows that LSTL was applied to one out of every three patients admitted to hospital due to critical neurological disease (GCS<8), and almost one-half of these subjects were not admitted to the ICU. The median time from admission to the application of LSTL was three days. Of the 176 patients in which LSTL was decided, 71 (40.3%) had no contraindications for donation. In the present study, LSTL was associated to a lesser evolution toward BD and, specifically, in 9 patients under 80 years of age without contraindications for organ donation and with GCS≤4, LSTL was applied within the first four days of admission to hospital–this representing a potential loss rate of 12%. The question here is whether these individuals could have been evaluated as possible donors if LSTL had not been applied.
Considering the published articles on the impact upon which early LSTL may have fatal outcome in neurocritical patients and potential donation, we see that more extensive studies are needed to assess the effect of a change in the initial approach toward patients of this kind.
Another important issue is the participation of the transplant coordinator in the decision making process in the end of life care setting in neurocritical patients. In our study group, evaluation by the transplant coordinator in the course of hospital admission was associated to a lesser incidence of LSTL only in the univariate analysis. However, it is important to point out that in 53 cases in which LSTL was decided, the coordinator had not evaluated the patient at any time. Therefore, the patient wish regarding organ or tissue donation had not been examined by talking to the family.
An ethical dilemma remains27–29 regarding the instauration of life supporting treatments not in the benefit of the patient as such but in wait of BD among neurocritical patients with inevitable imminent death, and in which measures for treating the background disease would not be indicated. Although such measures are not adopted with the purpose of healing or improving the patient condition, there is an agreement in our setting that they are justified provided they are made in accordance with the patient's wish referred to organ donation. Talking with the family is needed in this regard, offering clear and transparent information, and including all the possible options referred to end of life care on the basis of patient evolution in the first few days. In this way we can make a decision based on consensus between the medical team and the family of the patient.
The present study did not aim to analyze donation potential in controlled asystolia (Maastricht type III). No analysis was made of the type of LSTL or the timing of death after LSTL. Although donors of this kind have been shown to be less profitable in terms of organ harvesting compared with BD donors, they are a valid option in concrete cases. Specifically, we refer to patients admitted to the ICU with GCS<8, exhibiting a poor prognosis, in which a fatal outcome is expected following trial treatment, management proves futile, and LSTL is decided when it is seen that evolution toward BD does not occur. Evidently, in accordance with current legislation30 and the good donation in asystolia practice recommendations,31 the donation process is independent of the decision to apply LSTL. Donation always must abide with the wish of the deceased patient (as previously expressed by the patient in person or by the family on occasion of the donation interview), with the absence of contraindications.
Our study has a few limitations. The group of hospitals and the causes of coma were heterogeneous, and so the findings cannot be generalized to all neurological disorders and to all settings. However, the large number of cases included allows us to extract important information regarding the donation potential and the incidence of LSTL. Although the cases were included on a prospective basis, no specification was made of the ultimate factors conditioning the decision and the timing of LSTL, or of the specific limited life support measure involved (no start or suspension) in each case. Nevertheless, it has been seen that while LSTL is common in this group of patients, it is less frequent in the first few days of admission.
In conclusion, this study evidences the real potential for organ donation in BD and the high incidence of LSTL in neurocritical patients in our hospitals. It shows that although the application of LSTL is more frequent in patients with contraindications for organ donation, it is associated to evolution toward non-BD, with cases that are limited to the first few days even in the absence of contraindications for organ donation. It is therefore important for health professionals involved in the care of patients with serious brain damage in both the ICU and in other areas of the hospital (particularly the emergency service) to receive adequate training, with the capacity to identify patients in which BD is imminent and the possibility of postulating them as organ donors. The key principal is to provide quality end of life care in line with the current recommendations. The participation of the transplant coordinator is important for including the option of organ donation according to the wish of the patient, and always offering the family sensible, clear and transparent information.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to Dr. Roser Deulofeu and to the Catalan Transplant Organization (Organización Catalana de Trasplantes, OCATT) for support and collaboration in the initial design of the study and in the elaboration of the digital platform.
Please cite this article as: Bodí MA, Pont T, Sandiumenge A, Oliver E, Gener J, Badía M, et al. Potencialidad de donación de órganos en muerte encefálica y limitación del tratamiento de soporte vital en los pacientes neurocríticos. Med Intensiva. 2015;39:337–344.