Dysregulation of the hypothalamic–pituitary–adrenal axis is a well-documented phenomenon in critically ill patients, and cortisol, its end product, plays a crucial role in the physiological response to stress.1 Experiencing critical illness requiring admission to an intensive care unit (ICU) can be a stressful, even traumatic, experience, especially for patients requiring invasive mechanical ventilation (IMV).2 Brain dysfunction is also common during and after ICU stay,3 but its biological mechanisms remain unclear. A recent study identified that systemic inflammation and endogenous anticoagulant activity are associated with delirium in critically ill patients.4 However, this study did not measure the stress hormone cortisol, which previous research has shown to be associated with brain dysfunction.1 Importantly, cortisol has already been observed to be higher in critically ill patients who develop delirium, regardless of inflammatory status, but no relationship with subjective cognition has been found.5 To date, no study has specifically explored the relationship between cortisol and objective cognition (OC) in critically ill patients. A prospective observational pilot study was conducted to explore whether cortisol, measured in hair (cumulative pre-ICU stress) and blood (acute ICU stress), and OC are related in ICU survivors.
Between November 2018 and February 2020, a convenience sample of 26 adult patients with >24 h of IMV in a medical-surgical ICU was recruited. Patients with a history of neuropsychiatric disease, including previous cognitive impairment, or with severe alopecia were excluded. Written informed consent was obtained from all patients or from an authorized surrogate, such as a family member. If consent was initially obtained from an authorized surrogate, for example in the case of a deeply sedated or delirious patient, we ratified the patients’ consent once they were mentally competent, defined by having a normal mental status and being free of delirium according to the Richmond Agitation-Sedation Scale (RASS = 0)6 and the Confusion Assessment Method for the ICU-7 (CAM-ICU-7 ≤ 2).7 Patients whose authorized surrogates refused to participate, those who did not sign the informed consent, or those who refused to participate despite prior consent from their authorized surrogates were also excluded. Cortisol was determined in a hair sample (pre-ICU cumulative stress) and in a blood sample (basal acute ICU stress), obtained 24–48 h after ICU admission. Cortisol was also measured in a second blood sample obtained at ICU discharge to analyze the rate of change of acute stress during their ICU stay according to the formula: (basal blood cortisol-blood cortisol at discharge)/basal blood cortisol. OC was assessed with the Montreal Cognitive Assessment (MoCA) test at discharge if the patient was mentally competent, defined as RASS = 0 and CAM-ICU-7 ≤ 2.8 The association between the different cortisol measures and OC at discharge was analyzed by age-adjusted partial correlations and linear regressions. Ethical approval was obtained from the local ethics committee (2018/542) and the study was registered at clinicaltrials.gov (NCT03736135).
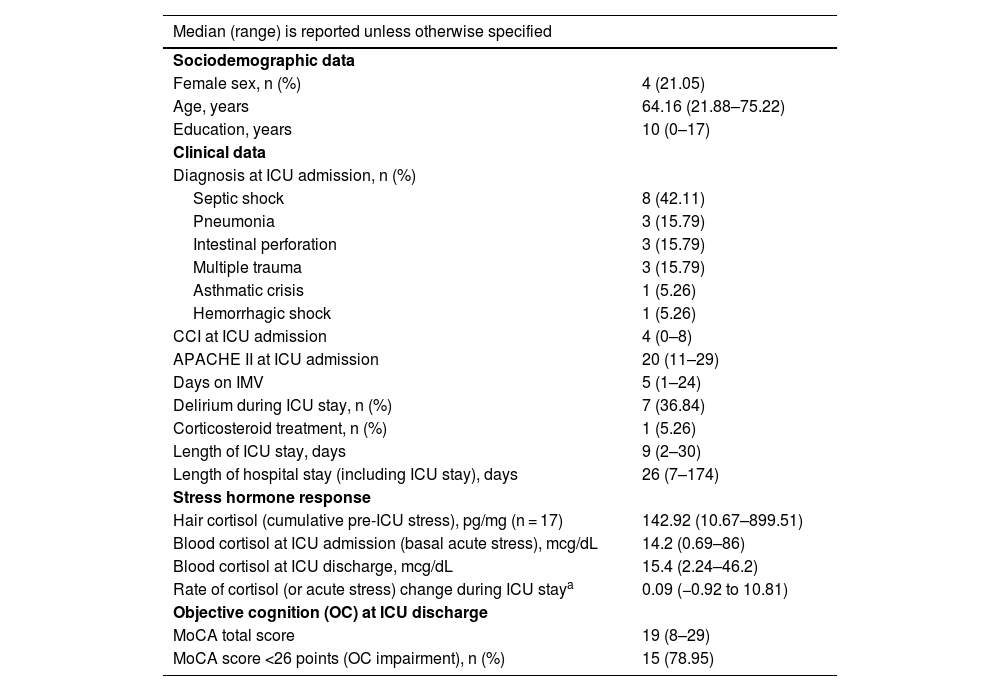
Of the 26 patients included, four (15.38%) died during their ICU stay and three (11.54%) were unable to complete the MoCA test due to altered mental status (RASS ≠ 0) or delirium (CAM-ICU-7 > 2). Data from 19 (73.08%) patients were analyzed. One (5.26%) patient received corticosteroid treatment during their ICU stay and 15 (78.95%) had altered OC at discharge (MoCA score < 26 points) (Table 1). The rate of change of blood cortisol during ICU stay proved to be the only significant predictor of MoCA performance at discharge, as shown by both age-adjusted partial correlations (r = −0.530, p = 0.024) and linear regression analyses (B = −1.638, p = 0.024). No significant associations were observed between hair cortisol or blood cortisol, whether measured at admission or discharge, and MoCA scores at discharge (Tables S1–S5, Supplementary material). Given the potential impact of corticosteroid treatment on cortisol level and adrenal function,1 the analyses were repeated after excluding the patient receiving corticosteroid treatment during their ICU stay. The results remained consistent (Tables S6–S10, Supplementary material), further reinforcing the robustness of the findings.
Patient characteristics (n = 19).
| Median (range) is reported unless otherwise specified | |
|---|---|
| Sociodemographic data | |
| Female sex, n (%) | 4 (21.05) |
| Age, years | 64.16 (21.88–75.22) |
| Education, years | 10 (0–17) |
| Clinical data | |
| Diagnosis at ICU admission, n (%) | |
| Septic shock | 8 (42.11) |
| Pneumonia | 3 (15.79) |
| Intestinal perforation | 3 (15.79) |
| Multiple trauma | 3 (15.79) |
| Asthmatic crisis | 1 (5.26) |
| Hemorrhagic shock | 1 (5.26) |
| CCI at ICU admission | 4 (0–8) |
| APACHE II at ICU admission | 20 (11–29) |
| Days on IMV | 5 (1–24) |
| Delirium during ICU stay, n (%) | 7 (36.84) |
| Corticosteroid treatment, n (%) | 1 (5.26) |
| Length of ICU stay, days | 9 (2–30) |
| Length of hospital stay (including ICU stay), days | 26 (7–174) |
| Stress hormone response | |
| Hair cortisol (cumulative pre-ICU stress), pg/mg (n = 17) | 142.92 (10.67–899.51) |
| Blood cortisol at ICU admission (basal acute stress), mcg/dL | 14.2 (0.69–86) |
| Blood cortisol at ICU discharge, mcg/dL | 15.4 (2.24–46.2) |
| Rate of cortisol (or acute stress) change during ICU staya | 0.09 (−0.92 to 10.81) |
| Objective cognition (OC) at ICU discharge | |
| MoCA total score | 19 (8–29) |
| MoCA score <26 points (OC impairment), n (%) | 15 (78.95) |
APACHE-II, Acute Physiology and Chronic Health Evaluation II; CCI, Charlson Comorbidity Index; ICU, intensive care unit; IMV, invasive mechanical ventilation; MoCA, Montreal Cognitive Assessment; OC, objective cognition.
This study explored the relationship between cortisol and OC in survivors of critical illness requiring IMV. The results show that a reduction in the blood level of cortisol, the stress hormone, during the ICU stay was associated with better OC at discharge, highlighting the role of cortisol dynamics on cognitive outcomes in ICU survivors. This finding complements previous research that identified that systemic inflammation and endogenous anticoagulant activity during ICU stay are associated with brain dysfunction, primarily delirium, in critically ill patients.4 It also complements other research that has shown that elevated cortisol levels during ICU stay is associated with delirium, independent of inflammatory status, but not with subjective cognition.5 In the present study, the rate of change of blood cortisol during ICU stay proved to be the only biological measure significantly associated with OC at discharge. This is a novel contribution, as previous investigations had mainly focused on delirium or subjective cognition, and not on OC.4,5 Importantly, no significant associations were observed between hair cortisol (reflecting cumulative pre-ICU stress) or blood cortisol measured at admission or discharge (reflecting acute ICU stress) and OC. These results suggest that it is the rate of change in blood cortisol (or cortisol dynamics) during the ICU stay that may have a more immediate and influential impact on OC at discharge, rather than prior chronic stress (or hair cortisol) or isolated measures of acute stress (or blood cortisol) taken at ICU admission or discharge. The main limitation of the study is the small sample size, which may affect the generalizability of the results. Although the analyses were adjusted for age –a key predictor of OC– other important factors, such as medical comorbidities, illness severity, duration of IMV, duration of delirium, and analgosedation, among others, could not be taken into account,3 as the limited sample size restricted the number of independent variables that could be included in the models to no more than two. In addition, the use of the MoCA test, while valuable, has inherent limitations, as it is primarily a screening tool and may not fully capture the breadth or depth of OC impairment.8
In conclusion, this pilot study highlights the potential of cortisol dynamics during ICU stay as a biomarker to stratify the risk of OC deterioration in survivors of critical illness requiring IMV. A decrease in blood cortisol level during ICU stay could indicate a lower risk of OC deterioration at discharge, whereas an increase could signal a higher risk. This finding also suggests the potential for targeted interventions, such as pharmacological modulation of the hypothalamic–pituitary–adrenal axis, to mitigate the impact of critical illness on brain function. Larger, well-powered studies controlling for more demographic and clinical variables are needed to validate these results and explore additional therapeutic strategies, such as early cognitive stimulation and stress reduction techniques, to reduce brain dysfunction during and after ICU stay.9,10
CRediT authorship contribution statementConceptualization: L.B., J.L.A., S.F.G.; Data curation: G.N.V., S.F.G.; Formal analysis: G.N.V., J.L.A., S.F.G.; Funding acquisition: S.F.G.; Investigation: G.N.V., M.G.G., S.F.G.; Methodology: G.N.V., J.L.A., S.F.G.; Project administration: S.F.G.; Resources: L.B., J.L.A., S.F.G.; Supervision: S.F.G.; Validation G.N.V., J.L.A., S.F.G.; Visualization: G.N.V., M.G.G.; Writing – original draft: G.N.V.; and Writing – review & editing: G.N.V., M.G.G., L.B., J.L.A., S.F.G.
Ethical standardsThe authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Declaration of Helsinki of 1964 and its subsequent revisions.
Declaration of Generative AI and AI-assisted technologies in the writing processNo form of Generative AI or AI-assisted technologies were used in the conduct of the study or in the production of the text.
Funding and role of the funding sourceThis work was supported by Taulí Research and Innovation Grants (CIR2017/032); CERCA Programme/Generalitat de Catalunya; Fundació Institut d’Investigació i Innovació Parc Taulí-I3PT; CIBER – Consorcio Centro de Investigación Biomédica en Red – CB06/06/1097, ISCIII, Ministerio de Ciencia e Innovación and Unión Europea-European Regional Development Fund. The funders played no role in study design, data collection and analysis, manuscript preparation, or decision to publish.
The authors declare that they have no conflict of interest.
We would like to thank all the patients who participated in the study. We also thank Dr. Antonio Armario and his research group on Neurobiology of stress and vulnerability to psychopathology at the Universitat Autònoma de Barcelona (UAB) who collaborated in the study by performing the techniques for measuring capillary cortisol concentration levels (CCC).
The data sets used and/or analyzed during the present study are available to the corresponding author, M.G.G. (mrgodoy@tauli.cat), upon reasonable request.