Coronavirus disease 2019 (COVID-19) is a respiratory tract infection caused by a newly emergent coronavirus, that was first recognized in Wuhan, China, in December 2019. Currently, the World Health Organization (WHO) has defined the infection as a global pandemic and there is a health and social emergency for the management of this new infection. While most people with COVID-19 develop only mild or uncomplicated illness, approximately 14% develop severe disease that requires hospitalization and oxygen support, and 5% require admission to an intensive care unit. In severe cases, COVID-19 can be complicated by the acute respiratory distress syndrome (ARDS), sepsis and septic shock, and multiorgan failure. This consensus document has been prepared on evidence-informed guidelines developed by a multidisciplinary panel of health care providers from four Spanish scientific societies (Spanish Society of Intensive Care Medicine [SEMICYUC], Spanish Society of Pulmonologists [SEPAR], Spanish Society of Emergency [SEMES], Spanish Society of Anesthesiology, Reanimation, and Pain [SEDAR]) with experience in the clinical management of patients with COVID-19 and other viral infections, including SARS, as well as sepsis and ARDS. The document provides clinical recommendations for the noninvasive respiratory support (noninvasive ventilation, high flow oxygen therapy with nasal cannula) in any patient with suspected or confirmed presentation of COVID-19 with acute respiratory failure.
This consensus guidance should serve as a foundation for optimized supportive care to ensure the best possible chance for survival and to allow for reliable comparison of investigational therapeutic interventions as part of randomized controlled trials.
La enfermedad por coronavirus 2019 (COVID-19) es una infección del tracto respiratorio causada por un nuevo coronavirus emergente que se reconoció por primera vez en Wuhan, China, en diciembre de 2019. Actualmente la Organización Mundial de la Salud (OMS) ha definido la infección como pandemia y existe una situación de emergencia sanitaria y social para el manejo de esta nueva infección. Mientras que la mayoría de las personas con COVID-19 desarrollan solo una enfermedad leve o no complicada, aproximadamente el 14% desarrollan una enfermedad grave que requiere hospitalización y oxígeno, y el 5% pueden requerir ingreso en una unidad de cuidados intensivos. En casos severos, COVID-19 puede complicarse por el síndrome de dificultad respiratoria aguda (SDRA), sepsis y shock séptico y fracaso multiorgánico. Este documento de consenso se ha preparado sobre directrices basadas en evidencia desarrolladas por un panel multidisciplinario de profesionales médicos de cuatro sociedades científicas españolas (Sociedad Española de Medicina Intensiva y Unidades Coronarias [SEMICYUC], Sociedad Española de Neumología y Cirugía Torácica [SEPAR], Sociedad Española de Urgencias y Emergencias [SEMES], Sociedad Española de Anestesiología, Reanimación y Terapéutica del Dolor [SEDAR]) con experiencia en el manejo clínico de pacientes con COVID-19 y otras infecciones virales, incluido el SARS, así como en sepsis y SDRA. El documento proporciona recomendaciones clínicas para el soporte respiratorio no invasivo (ventilación no invasiva, oxigenoterapia de alto flujo con cánula nasal) en cualquier paciente con presentación sospechada o confirmada de COVID-19 con insuficiencia respiratoria aguda.
Esta guía de consenso debe servir como base para una atención optimizada y garantizar la mejor posibilidad de supervivencia, así como permitir una comparación fiable de las futuras intervenciones terapéuticas de investigación que formen parte de futuros estudios observacionales o de ensayos clínicos.
The present document has been developed by consensus among the scientific societies involved in acute respiratory failure in adult patients, and seeks to provide a more detailed description of the recommendations on the use of non-invasive respiratory support (NIRS) in the management of acute respiratory failure (ARF) secondary to infection by the newly emergent SARS-CoV-2 coronavirus, which causes so-called COVID-19 disease, as a complement to the information emitted by the Spanish Ministry of Health, Consumer Affairs and Social Wellbeing (Ministerio de Sanidad, Consumo y Bienestar Social [MSC]),1,2 which is frequently updated and establishes a series of general recommendations.
The World Health Organization (WHO) has recently declared SARS-CoV-2 (COVID-19) disease3 as an international alert and public health emergency. This ongoing COVID-19 pandemic is devastating, despite the widespread adoption of control measures. In fact, there are important regional differences in the availability and accessibility of medical resources among the more than 70 currently affected countries. These differences could in part explain the low mortality rates despite the high incidence of cases. In this regard, the different health authorities and governments have developed contingency plans to deal with the local outbreaks of the disease.4 Such measures are essential to control the epidemic, protect the healthcare professionals at the frontline, and mitigate the seriousness of the patient outcomes.
The results of a recent analysis of the clinical characteristics of a selected cohort of 1099 patients with COVID-19 throughout China5 have evidenced that up to 15% (173/1099) developed serious disease according to the clinical criteria of severe community-acquired pneumonia of the American Thoracic Society.6 Of these patients with serious disease, 24.8% (43/173) had the composite outcome of admission to the Intensive Care Unit (ICU) or the use of mechanical ventilation (both invasive and non-invasive), or death. On the other hand, 2.9% (5/173) required extracorporeal oxygenation measures.
This scenario, when extrapolated to the situation of the current disease outbreak in Spain, points to the need to anticipate and demand a contingency plan from the national and regional health authorities for the management of healthcare resources and the safety of the professionals – including the use of specific areas in hospital centers, such as expert units capable of assisting ventilated patients (ICU, Intermediate Respiratory Care Units [IRCUs]), Emergency Care Departments, hospitalization wards equipped with means and healthcare professionals capable of safely and efficiently dealing with the epidemiological challenge of controlling and treating the COVID-10 disease outbreak in Spain. Furthermore, during the previous viral epidemics in the form of SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome), the healthcare workers suffered high infection rates of 18.6% during the MERS outbreak and 21% during the SARS outbreak.7,8
Therefore, from the working groups and supervising scientific societies, contingency plans must be established, contemplating eventual critical situations in order to optimize the human and material resources and be prepared in advance for potential and unexpected scenarios of overwhelming demand. Specifically, from the Consensus Group, and within this contingency plan, we feel that the following must be included:
- 1.
Guarantees that the hospital management bodies have an established protocol referred to personal protective equipment (PPE) for healthcare workers. Verification that the healthcare staff is trained in fitting and removing PPE.
- 2.
Utilization of education, capacitation and the simulation of scenarios to assess response capacity in different outbreak scenarios in the healthcare centers. Optimization and anticipation of human healthcare resources to improve the safety of the healthcare staff, and contemplation of possible sick leaves in the event of confirmed disease cases.
- 3.
Identification of reference hospital centers that can safely manage a possible increase in the number of cases.
- 4.
Boosting of the total capacity of the ICUs and IRCUs, with preparation in advance of the material resources, healthcare staff and equipment requirements, and hospitalization and emergency care areas where to group COVID-19-positive patients in each hospital, if needed.
- 5.
Definition of a classification protocol for the identification of suspected, probable and confirmed cases, in order to guide correct management circuits within the hospital.
- 6.
Generation of a pre-established protocol for the management of cases of suspected COVID-19 with serious disease criteria.
- 7.
Definition of clear criteria for the care of patients and their families, and of admitted patient visiting policies.
In conclusion, we cannot predict how many seriously ill patients with COVID-19 we are going to receive, but we need to anticipate the possible scenarios, adjust the resources as rationally as possible in concordance with the experiences of other affected countries, do the best we can to be prepared, and work jointly to overcome the epidemic.
The recommendations of the present consensus document, developed among the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]), the Spanish Society of Pneumology and Thoracic Surgery (Sociedad Española de Neumología y Cirugía Torácica [SEPAR]), the Spanish Society of Urgencies and Emergency Care Medicine (Sociedad Española de Medicina de Urgencias y Emergencias [SEMES]) and the Spanish Society of Anesthesiology, Resuscitation and Pain Therapy (Sociedad Española de Anestesiología, Reanimación y Terapia del Dolor [SEDAR]), are based on the studies of other viral pandemics (influenza, SARS, MERS) and on the latest publications referred to COVID-19. Therefore, the current level of evidence is low, given the lack of methodological robustness and the inherent nature of the disease. This document will be brought up to date as knowledge evolves and with the changes in the recommendations of the national and international organisms and societies.
General recommendations for the management of acute respiratory failure secondary to COVID-19Objectives- •
To adequately identify patients with ARF amenable to the start of noninvasive respiratory support (NIRS).
- •
To know the processes underlying high risk of failure of NIRS.
Acute respiratory failure is the most common cause of death among patients with influenza and viral infections in general.9 With regard to COVID-19 (the infectious disease caused by SARS-CoV-2), 3.4% of the patients infected in China presented acute respiratory distress syndrome (ARDS), representing 40.3% of the patients with a serious disease.5 It is therefore very important to adopt a therapeutic strategy for ARF secondary to COVID-19 infection.
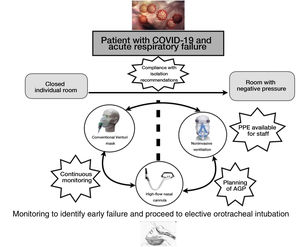
A fundamental element in the management of ARF in COVID-19 patients in monitoring – preferably of a non-invasive nature. These patients need to be located in individual rooms, ideally with negative pressure. This implies the need for centralized monitoring control (pulsioxymetry, respiratory frequency), preferably with video monitoring.
Therapeutic strategy of NIRS in COVID-19In the same way as in other processes that cause ARF, in patients with SARS-CoV-2 we can use the respiratory management strategy proposed by Scala and Heunks.10 Conventional oxygen therapy involves the administration of oxygen at different concentrations, and constitutes the basis of the therapeutic pyramid. The next step is high-flow nasal cannula (TAFCN) therapy. This technique involves the use of a gas mixture at high flow rates (up to 60lpm), with variable proportions (FiO2) of air and oxygen administered through a nasal cannula. The administered gas must be warm and with 100% humidification.11 The advantages with respect to conventional oxygen are a constant FiO2, dead space reduction, and the generation of a positive pressure that implies redistribution of intraalveolar fluid and alveolar recruitment.12 The next step is non-invasive mechanical ventilation (NIMV). The main characteristic of NIMV is its use under spontaneous breathing conditions; sedation is therefore null or low.13 The penultimate step is invasive mechanical ventilation (IMV). In this case ventilation in controlled mode is usually used, and tracheal intubation is required.10 The last step is extracorporeal membrane oxygenation (ECMO).14 At present, the respiratory therapies that define NIRS and HFNC and NIMV.
As an orientation and taking the above into account, the criteria for starting respiratory support in ARF secondary to COVID-19 would be the following15:
Clinical criteria:
- •
Moderate to severe dyspnea with signs of labored breathing and the use of accessory muscles, or paradoxical abdominal motion.
- •
Tachypnea in excess of 30rpm.
Blood gas criteria:
- •
PaO2/FiO2 <200 (or the need to administer FiO2 >0.4 to secure SpO2 of at least 92%).
- •
Acute ventilatory failure (pH <7.35 with PaCO2 >45mmHg).
In patients without any of the above criteria, the initially indicated treatment would be conventional oxygen therapy. In the presence of any of the above criteria, respiratory support would be indicated – whether invasive or non-invasive. The use of NIRS for SARS and other viral pandemics is subject to controversy, with NIMV failure rates of about 30%.16
More recently, NIMV has also been used in patients with ARF due to influenza A H1N1, with failure rates of between 13 and 77%.17–19 Despite the uncertainty of the evidence and the lack of randomized clinical trials, the positive data obtained by most of the observational studies suggest that its use can be considered in carefully selected patients in experienced centers and in a protected environment (ideally rooms with negative pressure). In the present Chinese epidemic, 5.1% of the patients required NIMV, 2.3% IMV and 0.5% ECMO.5 Accordingly, the treatment choice will also depend on the patient background disease (mainly of a respiratory nature), the location of the patient (room with negative pressure, closed individual room with air renewal), and the possibility of an eventual need for aerosol generating procedures. Thus, globally speaking, we may encounter three different clinical scenarios:
- 1.
Patients without prior disease (de novo ARF) with hypoxemic respiratory failure, and therefore amenable to escalate treatment up to ECMO. The NIRS failure rate in this clinical scenario – mainly in relation to NIMV – is extremely high. Furthermore, there is evidence of increased mortality if the start of IMV is delayed. We therefore do not recommend the use of NIRS in these patients.1,20,21 It could only be contemplated in carefully selected patients, and provided all the following criteria are met19,21–27:
- •
PaO2/FiO2 >100 despite conventional oxygen therapy.
- •
Absence of multiorgan failure (APACHE score <20).
- •
A team with expertise in continuous monitoring is required. It is therefore advisable to perform the technique in special units with a pre-specified nursing staff ratio as in ICUs and IRCUs.
- •
Early orotracheal intubation (OTI) in the following hour, in the absence of criteria of improvement. In this regard, and in addition to the traditional intubation criteria, we could consider intubation in patients treated with HFNC presenting a respiratory rate-oxygenation (ROX) index ([SpO2/FiO2]/respiratory frequency) of <3, <3.5 and <4 at 2, 6 and 12 hours after the start of HFNC treatment. Likewise, intubation could be considered in patients with a heart rate, acidosis, consciousness, oxygenation, and respiratory rate (HACOR) index of >5 one hour after the start of NIMV therapy.
Extrapolating the evidence referred to de novo ARF, the use of HFNC would be the first choice.21,28 Non-invasive mechanical ventilation is defined as the second option in the event of insufficient patient response and without immediate criteria for IMV.
- 2.
Patients with hypoxemic failure, instructions against OTI and therapeutic ceiling in NIMV. The start of NIRS would be indicated in these patients, provided the preventive measures are ensured. In this context it is essential to establish the treatment objectives with the patient and family, delimiting the therapeutic ceiling. In general, it is advisable to start treatment with HFNC before resorting to NIMV.21,28 We recommend the following considerations for the use of NIRS29,30:
- •
Adjust FiO2 to secure a target SpO2 of about 95%.31
- •
If HFNC is used, it is advisable to administer flows of over 50lpm; if possible, start with 60lpm.
- •
If NIMV is decided, it is advisable to use positive end-expiratory pressure (PEEP) values and low assist pressures (in order to obtain a high end-tidal volume (VTe) of <9ml/kg ideal body weight).31,32
- 3.
Patients with severe exacerbation of chronic obstructive pulmonary disease (COPD) with acute or exacerbated hypercapnic respiratory failure: perform a NIRS therapeutic test, especially with NIMV. High-flow nasal cannulas may be useful in these patients in the event of intolerance to NIMV, or for the lowering of NIMV.33–36
To limit transmission of the infection to both the healthcare staff and to other patients.
BackgroundThe use of NIRS is a practice of special risk of SARS-CoV-2 transmission.37–40 The capacity of SARS-CoV-2 to infect healthcare workers has been confirmed, though it is still too soon to establish comparisons with MERS and SARS.40 Thus, the Spanish Ministry of Health, Consumer Affairs and Social Wellbeing has defined a series of recommendations for management of the disease, and this document seeks to disclose the measures that limit transmission of the infection to the healthcare staff.2,39 In order to be able to apply MIRS to a patient with suspected or confirmed SARS-CoV-2 infection, it is crucial to follow the general preventive recommendations. In this regard, the public administrations must be in possession of the materials required for safe NIRS use.
General recommendations- 1.
The healthcare staff assisting cases under investigation for COVID-19 infection or with already confirmed infection subjected to NIRS must wear personal protective equipment (PPE) for the prevention of infection2,22,37,39,41 during aerosol-generating procedures that have been associated to an increased risk of air transmission of pathogens. The preventive measures should be targeted to microorganisms that are transmitted via droplets and through contact, and are to include:
- •
A high-efficacy FFP2 mask or preferably an FFP3 mask (if available).
- •
Integral-frame protective goggles.
- •
Retraction of long hair into a knot or tail; a surgical cap can be used in this regard.
- •
Gloves.
- •
Long-sleeved, impermeable microbiological protective gowns.
- 2.
Patient location within the hospital setting is related to the possibility of performing aerosol-generating procedures. In this respect, the available evidence on the use of NIRS devices as invasive procedures and the risk of pathogen transmission to the healthcare professionals is stronger in cases of orotracheal intubation and patients with an artificial airway, as well as in noninvasive ventilation – though these data come from limited studies of very low quality. Interpretation of the data is therefore difficult (Table 1). Therefore, in mild cases it is advisable to keep the patient in a room with negative pressure. If no such rooms are available, the patient can be located in an individual room with bathroom. The door to the room should be kept closed at all times.39 Patients with severe hypoxemia are to be admitted to special units.
Table 1.Summary of aerosol generation procedures and risk of transmission of acute respiratory diseases.
Aerosol generation procedures Type of studies Odds ratio (95%CI) Orotracheal intubation 4 cohort studies 3.0 (1.4–6.7) 22.8 (3.9–131.1) 13.8 (1.2–167.7) 5.5 (0.6–49.5) Pooled effect (I2=39.6%) 6.6 (2.3–18.9) Orotracheal intubation 4 case–control studies 0.7 (0.1–3.9) 9.2 (4.2–20.2) 8.0 (3.9–16.6) 9.3 (2.9–30.2) Pooled effect (I2=61.4%) 6.6 (4.1–10.6) Aspiration before intubation 2 cohort studies 13.8 (1.2–161.7) 1.7 (0.7–4.2) Pooled effect (I2=59.2%) 3.5 (0.5–24.6) Aspiration after intubation 2 cohort studies 0.6 (0.1–3.0) 1.8 (0.8–4.0) Pooled effect (I2=28.8%) 1.3 (0.5–3.4) Nebulizations 3 cohort studies 6.6 (0.9–50.5) 0.1 (0–1.0) 1.2 (0.1–20.7) Pooled effect (I2=73.1%) 0.9 (0.1–13.6) Bronchoscopy 2 cohort studies 3.3 (0.2–59.6) 1.1 (0.1–18.5) Pooled effect (I2=0%) 1.9 (0.2–14.2) Endotracheal aspirations 1 cohort study 1.0 (0.2–5.2) High-flow nasal cannula therapy 1 cohort study 0.4 (0.1–1.7) Noninvasive mechanical ventilation 2 cohort studies 2.6 (0.2–34.5) 3.2 (1.4–7.2) Pooled effect (I2=0%) 3.1 (1.4–6.8) Oxygen administration 1 case–control study 1.0 (0.3–2.8) Chest compressions 1 case–control study 4.5 (1.5–13.8) Chest compressions 2 cohort studies 3.0 (0.4–24.5) 0.4 (0.0–7.8) Pooled effect (I2=27.3%) 1.4 (0.2–11.2) Defibrillation 2 cohort studies 0.5 (0.0–12.2) 7.9 (0.8–79.0) Pooled effect (I2=55.3%) 2.5 (0.1–43.9) Tracheotomy 1 case–control study 4.2 (1.5–11.5) NIMV mask manipulation 1 cohort studies 4.3 (0.6–27.4) Mechanical ventilation 1 cohort studies 0.9 (0.4–2.0) Manual ventilation before intubation 1 cohort studies 2.8 (1.3–6.4) Sputum sample collection 1 cohort studies 2.7 (0.9–8.2) Insertion of nasogastric tube 2 cohort studies 1.7 (0.2–11.5) 1.0 (0.2–4.5) Pooled effect (I2=0%) 1.2 (0.4–4.0) I2: statistical heterogeneity index; CI: confidence interval.
Results of studies selected in the systematic review that measured the risk of SARS transmission to healthcare professionals exposed to the cited procedures versus those not exposed to the same procedures.48,49
- 3.
When in-hospital transfer is required, both the patient and the professional in charge of moving the patient are to wear surgical masks. During transfer, the patient bed is to be covered with a disposable clean sheet that should be eliminated afterwards as group III waste.39
Although the Spanish Ministry of Health, Consumer Affairs and Social Wellbeing recommends the administered of oxygen using masks equipped with an exhaled air filter, such masks are not universally available in our setting. If these masks are not available, a surgical mask may be safely used over the nasal cannula or oxygen mask in order to limit viral dispersion. No studies have compared the safety in reducing SARS-CoV-2 dispersion between the former type of mask and the surgical mask,39 but oxygen administration is considered to be a low-risk aerosol-generating procedure.39,40
Therapy with high-flow nasal cannulasIt is advisable to follow the mentioned general recommendations and keep a distance of at least two meters with respect to other patients and healthcare staff lacking adequate protection.42 Although there is still uncertainty regarding particle dispersion with this therapeutic modality, placing a surgical mask over the nasal cannula – although not investigated to date – could be an option in extreme situations.
Non-invasive mechanical ventilationIt is advisable to keep a distance of at least two meters with respect to other patients and healthcare staff lacking adequate protection.
In general, and according to the available evidence, there are no contraindications to the use of NIRS in patients with COVID-19. However, the respiratory therapy used depends not only on the severity of respiratory failure but also on the availability of an area or location that meets the isolation and safety recommendations of the WHO. Probably the most serious cases, in which probable and rapid intubation is anticipated, should be admitted to the ICU in order to avoid possible delays in intubation that are negative for the patient outcome (Fig. 1).
Choice of respirator configuration- •
Although there is still uncertainty regarding particle dispersion in COVID-19, during the SARS epidemic a number of articles demonstrated particle dispersion with single-circuit and expiratory port NIMV of no more than four feet (1.25m).8,43,44
- •
Dual-arm configurations are to be preferred, since they afford sealing of the circuit in both the inspiratory and the expiratory phase. High-efficiency antimicrobial filters are to be positioned in the expiratory arm in order to avoid inverse infection from the patient to the respirator.39
- •
If dual-arm systems are not available and single-arm ventilators must be used, the expiratory orifice should be placed in the single tubing, with the fitting of a high-efficiency and low-resistance antimicrobial filter to minimize dispersion of the exhaled gas that could contaminate the environment. It likewise seems feasible to interposition a T-piece in the circuit to place the filter and intentional escape valve distal to the latter – though the resulting increase in dead space must be taken into account.
- •
If a high-efficiency antimicrobial filter cannot be fitted to the expiratory orifice, a high-efficiency antimicrobial filter should be placed between the patient/ventilator interface (without expiratory orifices) and the circuit. In this regard, the increase in resistance may need a change in the ventilator parameters, incrementing the level of pressure support.
- •
A feasible alternative to dual- or single-arm systems with escape valve is to use single-arm systems with an active valve and the placement of an antimicrobial filter at the outlet of the active valve.
- •
We do not recommend the use of heat and moisture exchangers (HMEs).45
The interface is the connecting device that facilitates the physical but also the functional relationship between two independent elements: the ventilator and the patient. It is a key element in NIMV, serving to vehiculize the negative pressure toward the patient with no artificial component positioned in the airway. The recommendations for use of the interface in SARS-CoV-2 infection are40,41,46,47:
- •
The recommended interface comes without an expiratory orifice, with no use of accessory ports, if any.
- •
Use of the helmet should be a priority if the interface is available, with correct knowledge of the pertinent fitting and maintenance technique.
- •
In general terms, it is advisable to use a full face mask as first alternative, or an oronasal mask if not available.
- •
Strict monitoring of leakage points around the mask is required, particularly at the oronasal interfaces, in order to avoid skin damage to patients with adequate protection, and also to maintain the circuit airtight in order to avoid exhalation of the infected air. Protective patches are to be avoided, due to the risk of increased leakage; repeated application of hyperoxygenated oils is advised.
- •
Use of the nasal interface is not advised, since it generates more aerosols, and furthermore in general in SARS-CoV-2 infection the problem is acute hypoxemic respiratory failure.
- •
We recommend the use of an elbow without anti-asphyxia valve. These are generally of a blue color. The use of elbows of this kind requires close patient monitoring due to possible ventilation system malfunction. Considering the risk/benefit ratio (asphyxia versus dispersion), and the fact that these patients are located in very complex rooms under the continuous care of specialized medical staff, it is unlikely for accidental and undetected or uncorrected disconnection to occur, but a minimum safe nursing ratio is needed.
- •
We misadvise the use of an anti-rebreathing elbow (which also houses an anti-asphyxia valve), due to the risk of increased expired air dispersion.
The current COVID-19 pandemic requires greater infection control precautions. Nebulizers generate aerosol particles measuring 1–5(m in size that can carry bacteria and viruses into the lungs. The risk of infection transmission through droplets and aerosols may increase during nebulizer treatment, due to the potential for generating a large volume of respiratory aerosols that can be ejected over a greater distance than in the natural dispersion pattern. Furthermore, the larger particles may stimulate cough in patients and by-passers, and thus increase the risk of disease spread. Nebulizer therapy in patients with pandemic COVID-19 infection may transmit potentially viable viruses to susceptible individuals.
In recent years some centers have experienced a change from the use of nebulizers to metered-dose inhalers (MDIs) with valved-holding chambers (VHCs). The administration of inhalatory therapy preferably should be made using an MDI device and VHC. However, it is important to underscore that it is unlikely for patients with acute respiratory failure to be able to receive the medication in an effective manner with these devices.
Nebulization therapy should only be used under the following conditions:
- -
Serious and potentially fatal respiratory disease (e.g., patients with hypoventilation or impaired ventilation, severe COPD or cystic fibrosis).
- -
Uncooperative patients or individuals unable to follow the required instructions for an MDI with VHC.
- -
Patients with a poor response to MDI with VHC.
However, despite the important body of evidence suggesting an absence of either superiority or inferiority in comparison to MDI with VHC, the open nebulizer is still widely used in hospital centers.
During the current COVID-19 outbreak, and in order to reduce the risk of transmission of all infectious respiratory processes, we encourage all healthcare professionals to seriously consider avoiding the use of open nebulizers in patients under spontaneous breathing if the aforementioned devices are not available. Preserving the safety of the patients and healthcare staff must be our priority concern.
Therefore, if aerosol therapy is needed, we recommend the use of vibrating mesh nebulizer devices with a mask or buccal pipette, limiting dispersion and fitting a surgical mask over the device. It must be taken into account that if a buccal pipette with anti-dispersion system is used, the deposited drug dose (particularly important in the case of beta2-adrenergic bronchodilators) may be greater, and require dose adjustment therefore may prove necessary.
Jet systems are disadvised due to the greater capacity to disperse particles into the environment. If such systems are necessary, it is essential to fit a surgical mask on the patient during nebulization.
Observation of the following points is recommended for the use of inhalatory therapy together with NIRS:
- •
The general recommendation for administering inhalatory therapy is to use pressurized cartridges with VHC or adaptor. If NIMV is used, it should be placed in the inspiratory arm of the circuit, coordinating pulsation with patient inspiration.
- •
If aerosol therapy is used, vibrating mesh nebulizers with adaptation to the elbow of the interface is the option of choice. As a second option we can use a vibrating mesh nebulizer with a T-piece fitted to the NIMV circuit. Since this is a “closed system”, there is no dispersion into the environment provided leakage at the mask periphery is well controlled.
- •
Jet-type nebulizers with a T-tube generate greater turbulence and particles of larger size, as well as increased ease of particle dispersion.
- •
If HFNC is used, we ideally should employ pressurized cartridges with VHC, a pipette with a vibrating mesh nebulizer or mesh device fitted to the dry arm of the water reservoir chamber.
- •
In general, we should reduce the pressure support used in NIMV and the temperature if HFNC is used.
This consensus document arises from a situation of social and healthcare alarm, and from the demands of the professionals for management guidelines; it therefore focuses on specific situations that are outside the context of routine clinical practice in hospital centers. It therefore should be adapted to the particular circumstances found in each concrete scenario.
Please cite this article as: Cinesi Gómez C, Peñuelas Rodríguez Ó, Luján Torné M, Egea Santaolalla C, Masa Jiménez JF, García Fernández J, et al. Recomendaciones de consenso respecto al soporte respiratorio no invasivo en el paciente adulto con insuficiencia respiratoria aguda secundaria a infección por SARS-CoV-2. Med Intensiva. 2020;44:429–438.
The recommendations in the present document are under constant revision and may be modified if the epidemiological situation and therapeutic options so require.