The purpose of this study was to analyze the differences in the effectiveness and complications of CPAP versus non-invasive ventilation on bilevel positive airway pressure (BiPAP) in the treatment of COVID-19 associated acute respiratory failure (ARF).
DesignRetrospective observational study.
SettingICU.
PatientsAll COVID-19 patients, admitted to an ICU between March 2020 and February 2023, who required CPAP or BiPAP were analyzed.
InterventionsUse of CPAP or BiPAP in COVID-19 associated ARF.
Main variables of interestInitial clinical variables, CPAP and BiPAP failure rate, complications, in-hospital mortality.
Results429 patients were analyzed, of whom 328 (76.5%) initially received CPAP and 101 (23.5%) BiPAP. Initial respiratory rate was 30&#¿;±&#¿;8 in the CPAP group and 34&#¿;±&#¿;9 in BiPAP (p&#¿;<&#¿;0.001), while PaO2/FiO2 was 120&#¿;±&#¿;26 and 111&#¿;±&#¿;24&#¿;mmHg (p&#¿;=&#¿;0.001), respectively. The most frequent complication related to the device was claustrophobia/discomfort, 23.2% in CPAP and 25.7% in BiPAP (p&#¿;=&#¿;0.596), while the most frequent complications not related to the device were severe ARDS, 58.6% and 70.1% (p&#¿;=&#¿;0.044), and hyperglycemia, 44.5% and 37.6%, respectively (p&#¿;=&#¿;0.221). After adjusting by propensity score matched analysis, neither failure of the device (OR 1.37, CI 95% 0.72–2.62) nor in-hospital mortality (OR 1.57, CI 95% 0.73–3.42) differed between both groups.
ConclusionsEither non-invasive ventilatory device failure or mortality rate differed in patients initially treated with CPAP versus BiPAP.
El objetivo del estudio ha sido analizar las diferencias en la efectividad y complicaciones de CPAP versus ventilación no invasive en modo doble nivel de presión (BiPAP) en el tratamiento de la insuficiencia respiratoria aguda (IRA) relacionada con COVID-19.
DiseñoEstudio observacional retrospectivo.
ÁmbitoUCI.
PacientesFueron analizados todos los pacientes COVID-19, ingresados en UCI entre Marzo de 2020 y Febrero de 2023, que requirieron CPAP o BiPAP.
IntervencionesUso de CPAP o BiPAP en la IRA relacionada con COVID-19.
Variables de interés principalesVariables clínicas iniciales, fracaso de la CPAP o BiPAP, complicaciones, mortalidad hospitalaria.
ResultadosFueron analizados 429 pacientes, de ellos 328 (76,5%) inicialmente recibieron CPAP y 101 (23,5%) BiPAP. La frecuencia respiratoria inicial era de 30&#¿;±&#¿;8 en el grupo CPAP y 34&#¿;±&#¿;9 en BiPAP (p&#¿;<&#¿;0,001), mientras la PaO2/FiO2 era 120&#¿;±&#¿;26 y 111&#¿;±&#¿;24&#¿;mmHg (p&#¿;=&#¿;0,001), respectivamente. La complicación más frecuente relacionada con el dispositivo fue claustrofobia/malestar, 23,2% en CPAP y 25,7% en BiPAP (p&#¿;=&#¿;0,596), mientras que las complicaciones más frecuentes no relacionadas con el dispositivo fueron SDRA severo, 58,6% y 70,1% (p&#¿;=&#¿;0,044), e hiperglucemia, 44,5% y 37,6%, respectivamente (p&#¿;=&#¿;0,221). Tras ajustar mediante análisis de propensión apareado, ni el fracaso del dispositivo (OR 1.37, IC-95% 0,72 a 2,62) ni la mortalidad hospitalaria (OR 1,57, IC-95% 0,73 a 3,42) mostraron diferencias entre ambos grupos.
ConclusionesNi el fracaso del dispositivo no invasive ni la mortalidad difirieron entre los pacientes inicialmente tratados con CPAP o BiPAP.
Respiratory infection due to SARS-CoV-2, COVID-19 disease, can cause severe hypoxemic acute respiratory failure (ARF), requiring admission to intensive care unit (ICU) and respiratory support.1 At the beginning of the pandemic, the use of non-invasive ventilatory devices (NIVD) was discouraged due to the lack of clear evidence of their efficacy in the treatment of severe hypoxemic ARF, the possible increase in the spread of the virus to the environment, the hypothetical risk of developing patient self-induced lung injury, and the worse prognosis derived from a delay in intubation.2–4 Thus, high-flow oxygen therapy through nasal cannula (HFNC), CPAP, and non-invasive ventilation in bilevel positive airway pressure (BiPAP) were rarely used, with initial recommendations favoring early intubation and invasive mechanical ventilation (IMV).5 After the first wave of the pandemic, and when there was evidence of a very high mortality in patients on IMV, together with a low contagion in duly protected health personnel, the use of CPAP and BiPAP became generalized.6,7
Multiple observational series as well as randomized controlled trials analyzing the use of NIVD in COVID-19 have shown very different results. In observational series, the CPAP or BiPAP failure rate is very variable, depending on multiple factors,7 but it is considerably high, around 30–50%,8,9 reaching 88% in a multicenter study.10 In clinical trials, the intubation rate of patients randomized to CPAP or BiPAP ranged from 30% to 47%.11–15 Due to these high failure rates, a recent consensus of different Spanish scientific societies concluded that during viral pandemics, the use of CPAP or BiPAP can be considered in carefully selected patients treated in centers with extensive experience and with optimized measures to prevent contagion.16 In addition, it is common to use different types of NIVD sequentially in the same patient, in relation to the clinical response or the presence of complications associated with the device, fundamentally those related to the interface and pressure levels.6,17
In the treatment of hypoxemic ARF, CPAP or BiPAP can be used. CPAP improves arterial oxygenation by recruiting non-functioning areas of the lung without providing inspiratory support. BiPAP, in addition to recruiting non-functioning alveoli by applying positive pressure at the end of expiration, can reduce inspiratory effort by applying inspiratory positive pressure and thus be the mode of choice in patients with chronic respiratory disease with hypercapnia.18
Different consensus recommended the use of CPAP as the first line treatment in patients with severe ARF.19,20 Despite this, multiple clinical series using BiPAP have been published.6 This variability may be related to the preferences and trust of the physicians and the availability of resources, but also to the degree of impairment of the patient's respiratory function.3,8
We hypothesize that BiPAP as first line of treatment is as effective as CPAP in hypoxemic ARF due to severe COVID-19. The primary objective of this study was to compare the rate of NIVD failure in patients with ARF due to COVID-19. As secondary objectives, we analyzed differences between the following four groups: patients who only received CPAP, those who only received BiPAP, those who initially received CPAP and were crossover to BiPAP, and those initially with BiPAP and who were crossover to CPAP. In addition, the differences between ICU and in-hospital mortality, and complications related to NIVD were also analyzed.
Material and methodsWe conducted a retrospective observational study with a prospective database in an ICU of a University Hospital. The study was approved by the Institution's Ethical Committee.
PatientsIn this study, all consecutive patients admitted for ARF secondary to COVID-19 disease, between March 11, 2020 and February 11, 2023 and treated with CPAP or BiPAP as first line or after failure of HFNC were analyzed. The diagnosis required microbiological confirmation of the disease by polymerase chain reaction (PCR) test (B Analitica™ REALQUALITY RQ-2019-nCov and QIAGEN® QuantiTect Probe RT-PCR Kit), together with the presence of pulmonary infiltrates in an imaging test. In all cases, the diagnosis of ARF required arterial blood gas analysis prior to the start of NIVD. In addition, another arterial blood gas analysis was performed one hour after starting NIVD in order to calculate CPAP or BiPAP failure prediction through the HACOR score.
Patients were included if they required non-invasive CPAP or BiPAP. The criteria for starting CPAP or BiPAP are shown in Table S1 (Supplementary Material). The initial NIVD strategy was chosen by the attending physician, although the use of BiPAP was preferred if the respiratory rate was greater than 30, there were signs of muscle fatigue, respiratory acidosis on arterial blood gases, or history of chronic respiratory disease. The need for immediate intubation due to respiratory exhaustion or cardiorespiratory arrest were considered the only absolute contraindications to the use of CPAP or BiPAP. Patients were excluded if, despite positive PCR, they did not present ARF.
Treatment and CPAP or BiPAP protocolCPAP or BiPAP was performed using specific ventilators (VISION® ventilator by Respironics™, and V60® ventilator by Phillips Respironics™). In the CPAP mode the initial positive pressure was 10 cmH2O, with the possibility of rising to 15 cmH2O. When using BiPAP, the initial EPAP level was 10 cmH2O, up to a maximum of 15 cmH2O. The IPAP level used did not exceed the EPAP level more than 5 cmH2O. In all cases, the initial FiO2 was 1, with a subsequent decrease to maintain SpO2 between 92 and 96%. The interface used was a total facemask.
When a patient was initially treated with CPAP, crossover to BiPAP was performed if the patient presented, for more than 4&#¿;h, a respiratory rate >30&#¿;bpm despite a fentanyl bolus (50 micrograms), developed respiratory acidosis or signs of muscle fatigue with accessory muscle utilization. When a patient was initially treated with BiPAP, crossover to CPAP was performed in the presence of BiPAP-related complications, agitation or intolerance.
Initial CPAP or BiPAP treatment was applied continuously, without interruption, until the patient's respiratory rate was less than 25 breaths per minute and the required FiO2 to maintain SpO2 within the established targets was less than 0.5. In this case, weaning was performed with low-flow oxygen therapy or with HFNC depending on the required FiO2 to maintain oxygenation targets.
The treatment protocol with anti-inflammatory, antibiotic, and analgesic/sedative medications is shown in Table S1.
Criteria for endotracheal intubation (ETI) are shown in Table S1. In patients with do-not-intubate (DNI) order, CPAP or BiPAP was maintained until the patient's improvement or death.
Variables analyzedOn admission and during hospital stay, sociodemographic, clinical and analytical variables were analyzed. The severity of the patients was determined by SAPS II index and the SOFA index of multi-organ failure. The patient's comorbidity was determined by the Charlson index not adjusted for age. The definitions of the main comorbidities and complications analyzed are shown in Table S2.
CPAP or BiPAP failure was defined as patient requiring ETI-IMV or patient’s death when presenting a DNI order.
Statistic analysisWe analyzed all patients admitted in successive waves between the referred period. The patients were grouped according to the initial treatment, CPAP vs BiPAP. In addition, the differences between the four groups resulting from the NIVD crossover were analyzed.
Quantitative variables are expressed as means&#¿;±&#¿;standard deviation or median (interquartile range), and qualitative variables as absolute and relative frequencies. The comparison between categoric variables was performed using the Pearson's Chi2 test or Fisher's exact test. The parametric or nonparametric distribution of a continuous quantitative variable was performed by applying the Kolmogorov Smirnov test. Comparison between a quantitative and a categoric variable of two options was performed using the Student’s t test or Mann–Whitney test, and ANOVA or the Kruskal–Wallis test if the qualitative variable had three or more options. Bonferroni correction was used to multiple comparisons. Propensity score matching was produced using nearest-neighbor model without replace, with a 1:1 ratio. Each patient with BiPAP was matched to one CPAP patient. Variables used to match were: age, gender, ARDS, SAPS II, initial SOFA, Charlson index, basal PaO2/FiO2, basal respiratory rate, location of the patient before admission to the ICU and DNI order. A second propensity matched analysis was performed adjusting for the same variables except DNI order. A caliper width of 0.1 of the standard deviation of the logit of the propensity score was used for the matching. To determine the effectiveness of propensity score matching for controlling the differences between groups, standardized mean differences (SMDs) were calculated for each variable before and after matching. SMDs less than 10% indicated successful propensity scores matching and balancing between the two groups. For comparisons in the matched cohorts Student’s paired t-test, Wilcoxon signed rank test or McNemar test were used. The relationship between the NIVD used and the time-course of the in-hospital mortality patients was performed using the Kaplan Meier method with comparison using the log rank test. A sensitivity analysis was performed excluding patients with previous treatment with CPAP or BiPAP before admission to the ICU. Adjustment of confounding variables in the subgroup analysis was performed by calculating the odds ratio (OR) and its 95% CI, using Inverse Probability Weighting (IPW). In the overall sample, we performed Cox analysis to the variables related to the timing of in-hospital mortality, adjusting for the same variables as the propensity score matching in addition to NIVD failure and development of nosocomial infection, calculating the hazard ratio (HR) and its 95% confidence intervals (95% CI 95%).
The analysis was performed using the SPSS 27.0® program (IBM™, Armonk, NY) and R version 3.4.0® (Copyright 2017 The R Foundation for Statistical Computing Platform™).
All analysis has been performed by two tailed contrast and the statistical significance was determined for a value of p&#¿;<&#¿;0.05.
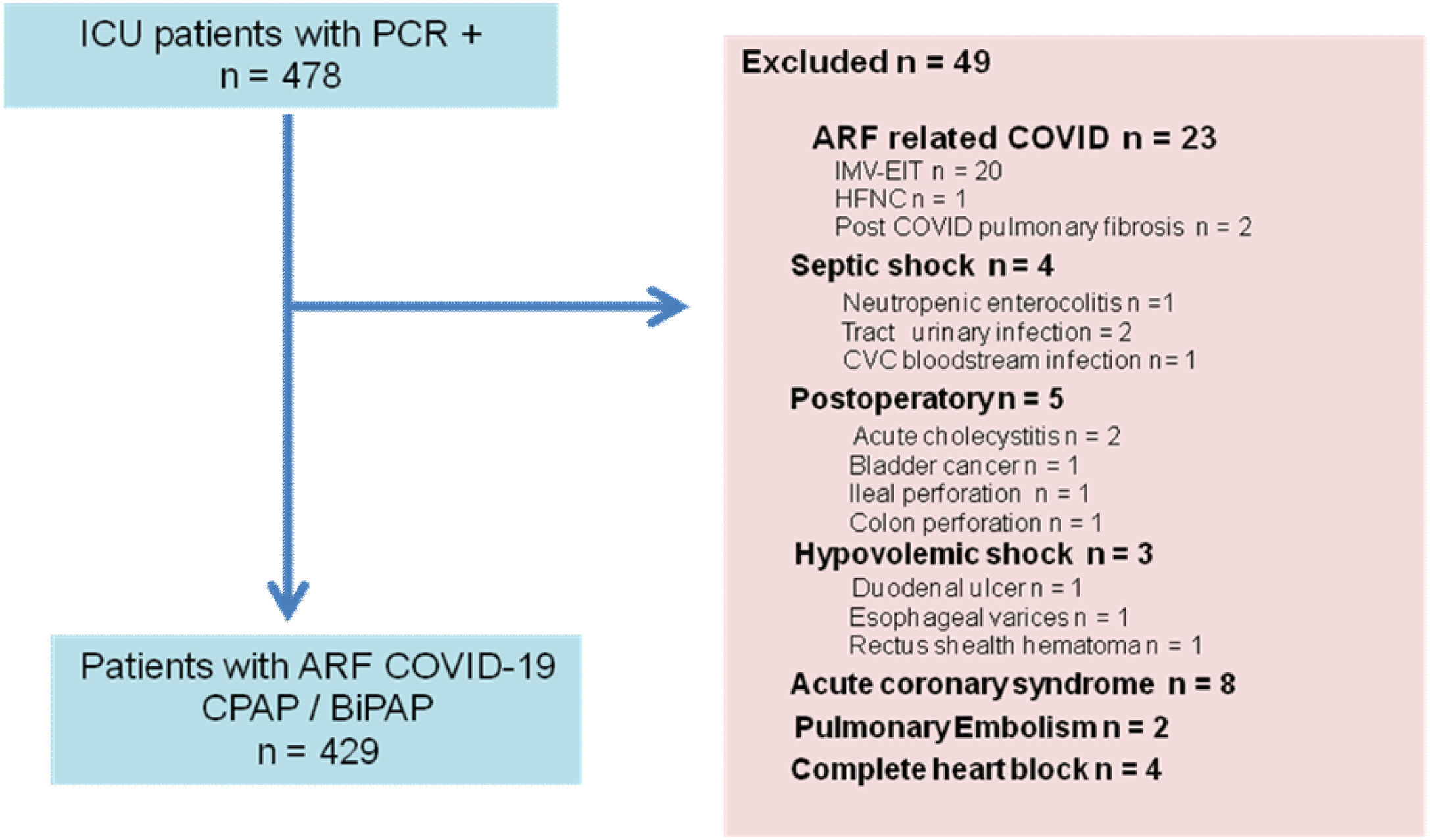
ResultsDuring the study period 478 patients were admitted with positive PCR, of which 49 were excluded (Fig. 1). Of the 452 patients admitted with COVID-19 related ARF, 429 (94.9%) were analyzed. Three hundred and twenty-eight (76.5%) patients initially received CPAP, and 101 (23.5%) BiPAP. During the ICU stay, some patients crossover from one type of respiratory device to the other, in such a way that 120 (28%) patients only received CPAP, 64 (14.9%) only BiPAP, and 245 (57.1%) received both types of NIVD: 208 (48.5%) crossover from CPAP to BiPAP and 37 (8.6%) from BiPAP to CPAP.
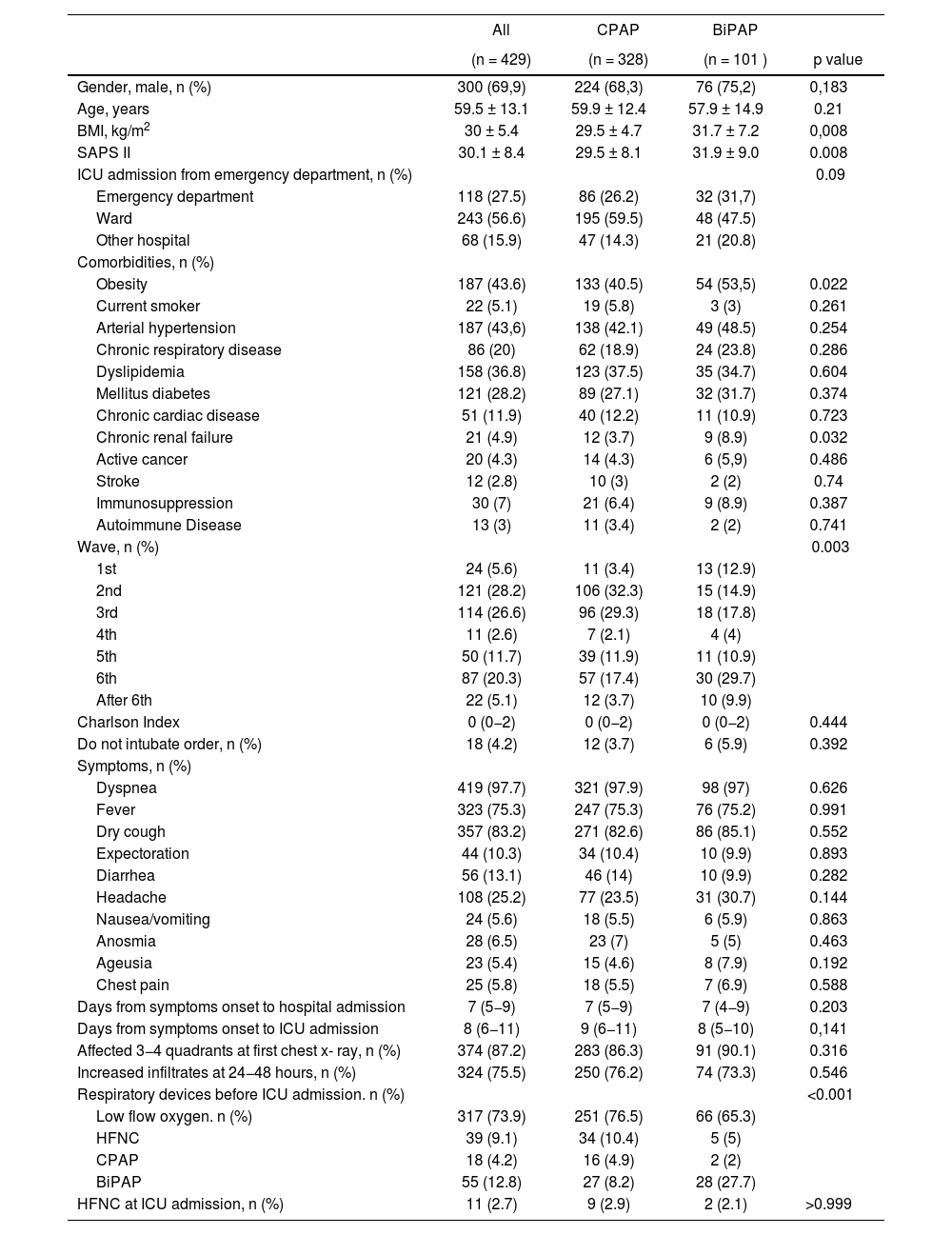
The main sociodemographic and clinical characteristics among patients treated with CPAP vs. BiPAP are shown in Table 1. The type of NIVD was not related to age nor gender. Of the antecedents analyzed only the body mass index and the presence of obesity were more frequent in the BiPAP group. Initial severity, measured by the SAPS II index, was higher in the BiPAP group than in the CPAP group (31.9&#¿;±&#¿;9.0 and 29.5&#¿;±&#¿;8.1, respectively; p&#¿;=&#¿;0.008). Usual medication and SARS-CoV-2 vaccination status prior to admission are shown in Table S3.
Sociodemographic and clinical characteristics.
| All | CPAP | BiPAP | ||
|---|---|---|---|---|
| (n = 429) | (n = 328) | (n = 101 ) | p value | |
| Gender, male, n (%) | 300 (69,9) | 224 (68,3) | 76 (75,2) | 0,183 |
| Age, years | 59.5 ± 13.1 | 59.9 ± 12.4 | 57.9 ± 14.9 | 0.21 |
| BMI, kg/m2 | 30 ± 5.4 | 29.5 ± 4.7 | 31.7 ± 7.2 | 0,008 |
| SAPS II | 30.1 ± 8.4 | 29.5 ± 8.1 | 31.9 ± 9.0 | 0.008 |
| ICU admission from emergency department, n (%) | 0.09 | |||
| Emergency department | 118 (27.5) | 86 (26.2) | 32 (31,7) | |
| Ward | 243 (56.6) | 195 (59.5) | 48 (47.5) | |
| Other hospital | 68 (15.9) | 47 (14.3) | 21 (20.8) | |
| Comorbidities, n (%) | ||||
| Obesity | 187 (43.6) | 133 (40.5) | 54 (53,5) | 0.022 |
| Current smoker | 22 (5.1) | 19 (5.8) | 3 (3) | 0.261 |
| Arterial hypertension | 187 (43,6) | 138 (42.1) | 49 (48.5) | 0.254 |
| Chronic respiratory disease | 86 (20) | 62 (18.9) | 24 (23.8) | 0.286 |
| Dyslipidemia | 158 (36.8) | 123 (37.5) | 35 (34.7) | 0.604 |
| Mellitus diabetes | 121 (28.2) | 89 (27.1) | 32 (31.7) | 0.374 |
| Chronic cardiac disease | 51 (11.9) | 40 (12.2) | 11 (10.9) | 0.723 |
| Chronic renal failure | 21 (4.9) | 12 (3.7) | 9 (8.9) | 0.032 |
| Active cancer | 20 (4.3) | 14 (4.3) | 6 (5,9) | 0.486 |
| Stroke | 12 (2.8) | 10 (3) | 2 (2) | 0.74 |
| Immunosuppression | 30 (7) | 21 (6.4) | 9 (8.9) | 0.387 |
| Autoimmune Disease | 13 (3) | 11 (3.4) | 2 (2) | 0.741 |
| Wave, n (%) | 0.003 | |||
| 1st | 24 (5.6) | 11 (3.4) | 13 (12.9) | |
| 2nd | 121 (28.2) | 106 (32.3) | 15 (14.9) | |
| 3rd | 114 (26.6) | 96 (29.3) | 18 (17.8) | |
| 4th | 11 (2.6) | 7 (2.1) | 4 (4) | |
| 5th | 50 (11.7) | 39 (11.9) | 11 (10.9) | |
| 6th | 87 (20.3) | 57 (17.4) | 30 (29.7) | |
| After 6th | 22 (5.1) | 12 (3.7) | 10 (9.9) | |
| Charlson Index | 0 (0−2) | 0 (0−2) | 0 (0−2) | 0.444 |
| Do not intubate order, n (%) | 18 (4.2) | 12 (3.7) | 6 (5.9) | 0.392 |
| Symptoms, n (%) | ||||
| Dyspnea | 419 (97.7) | 321 (97.9) | 98 (97) | 0.626 |
| Fever | 323 (75.3) | 247 (75.3) | 76 (75.2) | 0.991 |
| Dry cough | 357 (83.2) | 271 (82.6) | 86 (85.1) | 0.552 |
| Expectoration | 44 (10.3) | 34 (10.4) | 10 (9.9) | 0.893 |
| Diarrhea | 56 (13.1) | 46 (14) | 10 (9.9) | 0.282 |
| Headache | 108 (25.2) | 77 (23.5) | 31 (30.7) | 0.144 |
| Nausea/vomiting | 24 (5.6) | 18 (5.5) | 6 (5.9) | 0.863 |
| Anosmia | 28 (6.5) | 23 (7) | 5 (5) | 0.463 |
| Ageusia | 23 (5.4) | 15 (4.6) | 8 (7.9) | 0.192 |
| Chest pain | 25 (5.8) | 18 (5.5) | 7 (6.9) | 0.588 |
| Days from symptoms onset to hospital admission | 7 (5−9) | 7 (5−9) | 7 (4−9) | 0.203 |
| Days from symptoms onset to ICU admission | 8 (6−11) | 9 (6−11) | 8 (5−10) | 0,141 |
| Affected 3−4 quadrants at first chest x- ray, n (%) | 374 (87.2) | 283 (86.3) | 91 (90.1) | 0.316 |
| Increased infiltrates at 24−48 hours, n (%) | 324 (75.5) | 250 (76.2) | 74 (73.3) | 0.546 |
| Respiratory devices before ICU admission. n (%) | <0.001 | |||
| Low flow oxygen. n (%) | 317 (73.9) | 251 (76.5) | 66 (65.3) | |
| HFNC | 39 (9.1) | 34 (10.4) | 5 (5) | |
| CPAP | 18 (4.2) | 16 (4.9) | 2 (2) | |
| BiPAP | 55 (12.8) | 27 (8.2) | 28 (27.7) | |
| HFNC at ICU admission, n (%) | 11 (2.7) | 9 (2.9) | 2 (2.1) | >0.999 |
Data are expressed as means&#¿;±&#¿;standard deviation or median (interquartile range).
Definition of abbreviations: ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blocker; BiPAP: bilevel positive airway pressure; BMI: body mass index; CPAP: continuous positive airway pressure; HFNC: high flow through nasal cannula; ICU: intensive care unit; NSAID: Nonsteroidal anti-inflammatory drugs; SAPS: Simplified Acute Physiology Score.
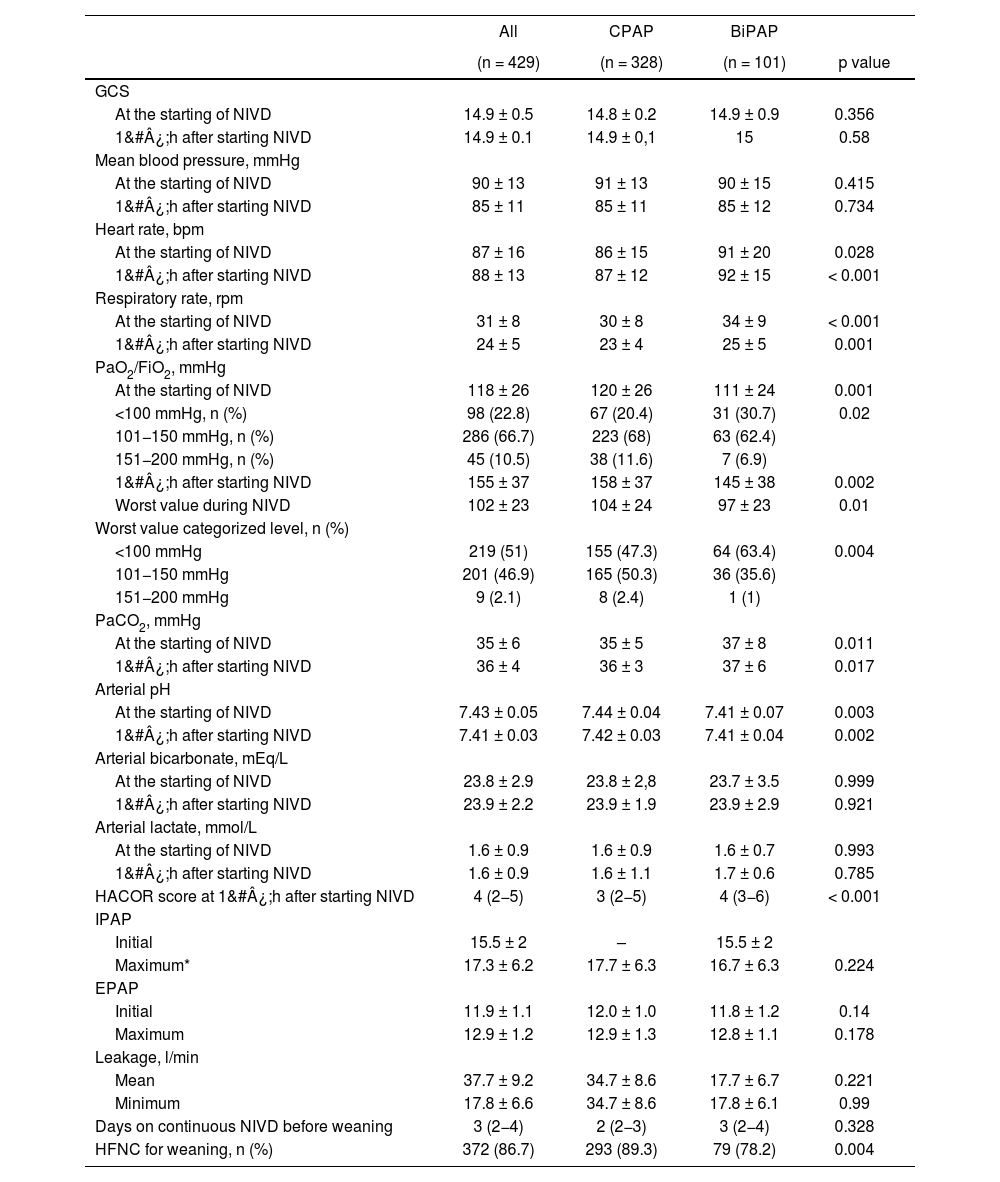
Of the multiple biochemical, hematological and hemostasis determinations, only the ultrasensitive troponine level differed between the two groups (Table S4). However, the physiological variables, especially the respiratory ones, showed greater alterations in the patients from the BiPAP group than in CPAP (Table 2). The patients treated with BiPAP showed a higher respiratory rate before starting NIVD (p&#¿;<&#¿;0.001) as well as a worse oxygenation: PaO2/FiO2 level was 111&#¿;±&#¿;24&#¿;mmHg in BiPAP group and 120&#¿;±&#¿;26 in CPAP group (p&#¿;=&#¿;0.001). The predictive index of NIVD failure, HACOR score, were worse in the BiPAP group (Table 2).
Neurologic, hemodynamic and respiratory variables.
| All | CPAP | BiPAP | ||
|---|---|---|---|---|
| (n = 429) | (n = 328) | (n = 101) | p value | |
| GCS | ||||
| At the starting of NIVD | 14.9 ± 0.5 | 14.8 ± 0.2 | 14.9 ± 0.9 | 0.356 |
| 1&#¿;h after starting NIVD | 14.9 ± 0.1 | 14.9 ± 0,1 | 15 | 0.58 |
| Mean blood pressure, mmHg | ||||
| At the starting of NIVD | 90 ± 13 | 91 ± 13 | 90 ± 15 | 0.415 |
| 1&#¿;h after starting NIVD | 85 ± 11 | 85 ± 11 | 85 ± 12 | 0.734 |
| Heart rate, bpm | ||||
| At the starting of NIVD | 87 ± 16 | 86 ± 15 | 91 ± 20 | 0.028 |
| 1&#¿;h after starting NIVD | 88 ± 13 | 87 ± 12 | 92 ± 15 | < 0.001 |
| Respiratory rate, rpm | ||||
| At the starting of NIVD | 31 ± 8 | 30 ± 8 | 34 ± 9 | < 0.001 |
| 1&#¿;h after starting NIVD | 24 ± 5 | 23 ± 4 | 25 ± 5 | 0.001 |
| PaO2/FiO2, mmHg | ||||
| At the starting of NIVD | 118 ± 26 | 120 ± 26 | 111 ± 24 | 0.001 |
| <100 mmHg, n (%) | 98 (22.8) | 67 (20.4) | 31 (30.7) | 0.02 |
| 101−150 mmHg, n (%) | 286 (66.7) | 223 (68) | 63 (62.4) | |
| 151−200 mmHg, n (%) | 45 (10.5) | 38 (11.6) | 7 (6.9) | |
| 1&#¿;h after starting NIVD | 155 ± 37 | 158 ± 37 | 145 ± 38 | 0.002 |
| Worst value during NIVD | 102 ± 23 | 104 ± 24 | 97 ± 23 | 0.01 |
| Worst value categorized level, n (%) | ||||
| <100 mmHg | 219 (51) | 155 (47.3) | 64 (63.4) | 0.004 |
| 101−150 mmHg | 201 (46.9) | 165 (50.3) | 36 (35.6) | |
| 151−200 mmHg | 9 (2.1) | 8 (2.4) | 1 (1) | |
| PaCO2, mmHg | ||||
| At the starting of NIVD | 35 ± 6 | 35 ± 5 | 37 ± 8 | 0.011 |
| 1&#¿;h after starting NIVD | 36 ± 4 | 36 ± 3 | 37 ± 6 | 0.017 |
| Arterial pH | ||||
| At the starting of NIVD | 7.43 ± 0.05 | 7.44 ± 0.04 | 7.41 ± 0.07 | 0.003 |
| 1&#¿;h after starting NIVD | 7.41 ± 0.03 | 7.42 ± 0.03 | 7.41 ± 0.04 | 0.002 |
| Arterial bicarbonate, mEq/L | ||||
| At the starting of NIVD | 23.8 ± 2.9 | 23.8 ± 2,8 | 23.7 ± 3.5 | 0.999 |
| 1&#¿;h after starting NIVD | 23.9 ± 2.2 | 23.9 ± 1.9 | 23.9 ± 2.9 | 0.921 |
| Arterial lactate, mmol/L | ||||
| At the starting of NIVD | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.7 | 0.993 |
| 1&#¿;h after starting NIVD | 1.6 ± 0.9 | 1.6 ± 1.1 | 1.7 ± 0.6 | 0.785 |
| HACOR score at 1&#¿;h after starting NIVD | 4 (2−5) | 3 (2−5) | 4 (3−6) | < 0.001 |
| IPAP | ||||
| Initial | 15.5 ± 2 | – | 15.5 ± 2 | |
| Maximum* | 17.3 ± 6.2 | 17.7 ± 6.3 | 16.7 ± 6.3 | 0.224 |
| EPAP | ||||
| Initial | 11.9 ± 1.1 | 12.0 ± 1.0 | 11.8 ± 1.2 | 0.14 |
| Maximum | 12.9 ± 1.2 | 12.9 ± 1.3 | 12.8 ± 1.1 | 0.178 |
| Leakage, l/min | ||||
| Mean | 37.7 ± 9.2 | 34.7 ± 8.6 | 17.7 ± 6.7 | 0.221 |
| Minimum | 17.8 ± 6.6 | 34.7 ± 8.6 | 17.8 ± 6.1 | 0.99 |
| Days on continuous NIVD before weaning | 3 (2−4) | 2 (2−3) | 3 (2−4) | 0.328 |
| HFNC for weaning, n (%) | 372 (86.7) | 293 (89.3) | 79 (78.2) | 0.004 |
Data are expressed as means&#¿;±&#¿;standard deviation or median (interquartile range).
Definition of abbreviations: BiPAP: bilevel positive airway pressure; bpm: beat per minute; brpm: breaths per minute; CPAP: continuous positive airway pressure; FiO2: Fraction of inspired oxygen; GCS: Glasgow coma score; HFNC: high flow through nasal cannula; l/min: liter per minute; mEq/L: milliequivalents per liter; mmHg: millimeters of mercury; mmol/L: millimoles per liter; NIVD: non-invasive ventilatory device.
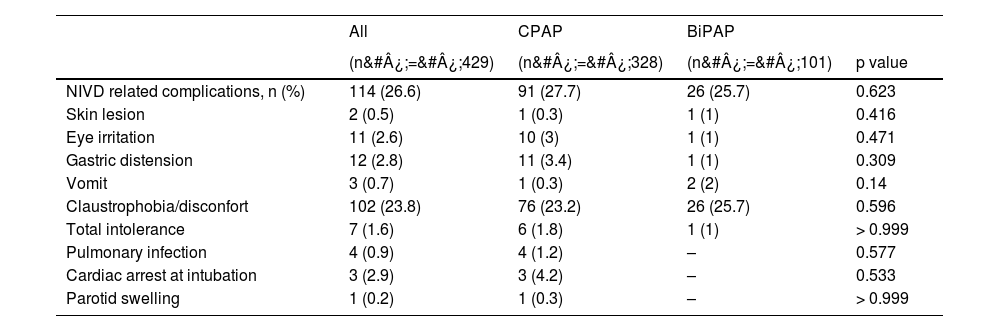
The most frequent NIVD-related complication was the development of claustrophobia/discomfort, occurring in 23.2% of patients receiving CPAP and 25.7% in the BiPAP group (p&#¿;=&#¿;0.596). The remaining complications also showed no significant differences between the two groups (Table 3). Among the complications not related to the use of NIVD (Table S5), the presence of severe ARDS was more frequent in the BiPAP group (70.1%) than in CPAP (58.6%) [p&#¿;=&#¿;0.044]. The second most common complication not related to the use of NIVD was hyperglycemia, 44.5% in CPAP and 37.6% in BiPAP (p&#¿;=&#¿;0.221).
Complications related to non-invasive ventilatory device.
| All | CPAP | BiPAP | ||
|---|---|---|---|---|
| (n&#¿;=&#¿;429) | (n&#¿;=&#¿;328) | (n&#¿;=&#¿;101) | p value | |
| NIVD related complications, n (%) | 114 (26.6) | 91 (27.7) | 26 (25.7) | 0.623 |
| Skin lesion | 2 (0.5) | 1 (0.3) | 1 (1) | 0.416 |
| Eye irritation | 11 (2.6) | 10 (3) | 1 (1) | 0.471 |
| Gastric distension | 12 (2.8) | 11 (3.4) | 1 (1) | 0.309 |
| Vomit | 3 (0.7) | 1 (0.3) | 2 (2) | 0.14 |
| Claustrophobia/disconfort | 102 (23.8) | 76 (23.2) | 26 (25.7) | 0.596 |
| Total intolerance | 7 (1.6) | 6 (1.8) | 1 (1) | > 0.999 |
| Pulmonary infection | 4 (0.9) | 4 (1.2) | – | 0.577 |
| Cardiac arrest at intubation | 3 (2.9) | 3 (4.2) | – | 0.533 |
| Parotid swelling | 1 (0.2) | 1 (0.3) | – | > 0.999 |
Definition of abbreviations: BiPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; NIVD: non-invasive ventilatory device.
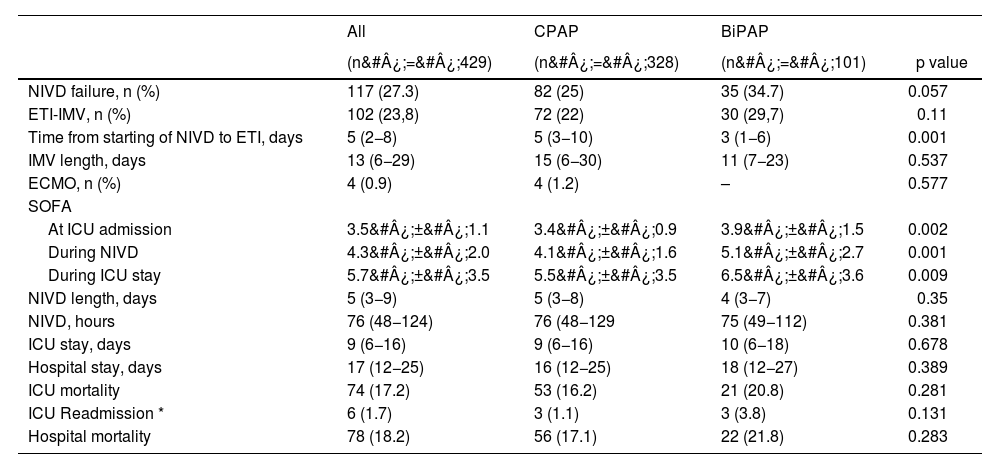
Outcomes of both groups are shown in Table 4. BiPAP showed a higher failure rate than CPAP, but without reaching statistical significance (unadjusted OR 1.59, 95% CI 0.98–2.57; p&#¿;=&#¿;0.058). In-hospital mortality did not differ between the two groups (unadjusted OR 1.35, CI 95% 0.78–2.35; p&#¿;=&#¿;0.283).
Outcomes.
| All | CPAP | BiPAP | ||
|---|---|---|---|---|
| (n&#¿;=&#¿;429) | (n&#¿;=&#¿;328) | (n&#¿;=&#¿;101) | p value | |
| NIVD failure, n (%) | 117 (27.3) | 82 (25) | 35 (34.7) | 0.057 |
| ETI-IMV, n (%) | 102 (23,8) | 72 (22) | 30 (29,7) | 0.11 |
| Time from starting of NIVD to ETI, days | 5 (2−8) | 5 (3−10) | 3 (1−6) | 0.001 |
| IMV length, days | 13 (6−29) | 15 (6−30) | 11 (7−23) | 0.537 |
| ECMO, n (%) | 4 (0.9) | 4 (1.2) | – | 0.577 |
| SOFA | ||||
| At ICU admission | 3.5&#¿;±&#¿;1.1 | 3.4&#¿;±&#¿;0.9 | 3.9&#¿;±&#¿;1.5 | 0.002 |
| During NIVD | 4.3&#¿;±&#¿;2.0 | 4.1&#¿;±&#¿;1.6 | 5.1&#¿;±&#¿;2.7 | 0.001 |
| During ICU stay | 5.7&#¿;±&#¿;3.5 | 5.5&#¿;±&#¿;3.5 | 6.5&#¿;±&#¿;3.6 | 0.009 |
| NIVD length, days | 5 (3−9) | 5 (3−8) | 4 (3−7) | 0.35 |
| NIVD, hours | 76 (48−124) | 76 (48−129 | 75 (49−112) | 0.381 |
| ICU stay, days | 9 (6−16) | 9 (6−16) | 10 (6−18) | 0.678 |
| Hospital stay, days | 17 (12−25) | 16 (12−25) | 18 (12−27) | 0.389 |
| ICU mortality | 74 (17.2) | 53 (16.2) | 21 (20.8) | 0.281 |
| ICU Readmission * | 6 (1.7) | 3 (1.1) | 3 (3.8) | 0.131 |
| Hospital mortality | 78 (18.2) | 56 (17.1) | 22 (21.8) | 0.283 |
Data are expressed as means&#¿;±&#¿;standard deviation or median (interquartile range).
Definition of abbreviations: BiPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; ECMO: extracorporeal membrane oxygenation; ETI: endotracheal intubation; ICU: intensive care unit; IMV: invasive mechanical ventilation; NIVD: non-invasive ventilatory device; SOFA: sequential organ failure assessment.
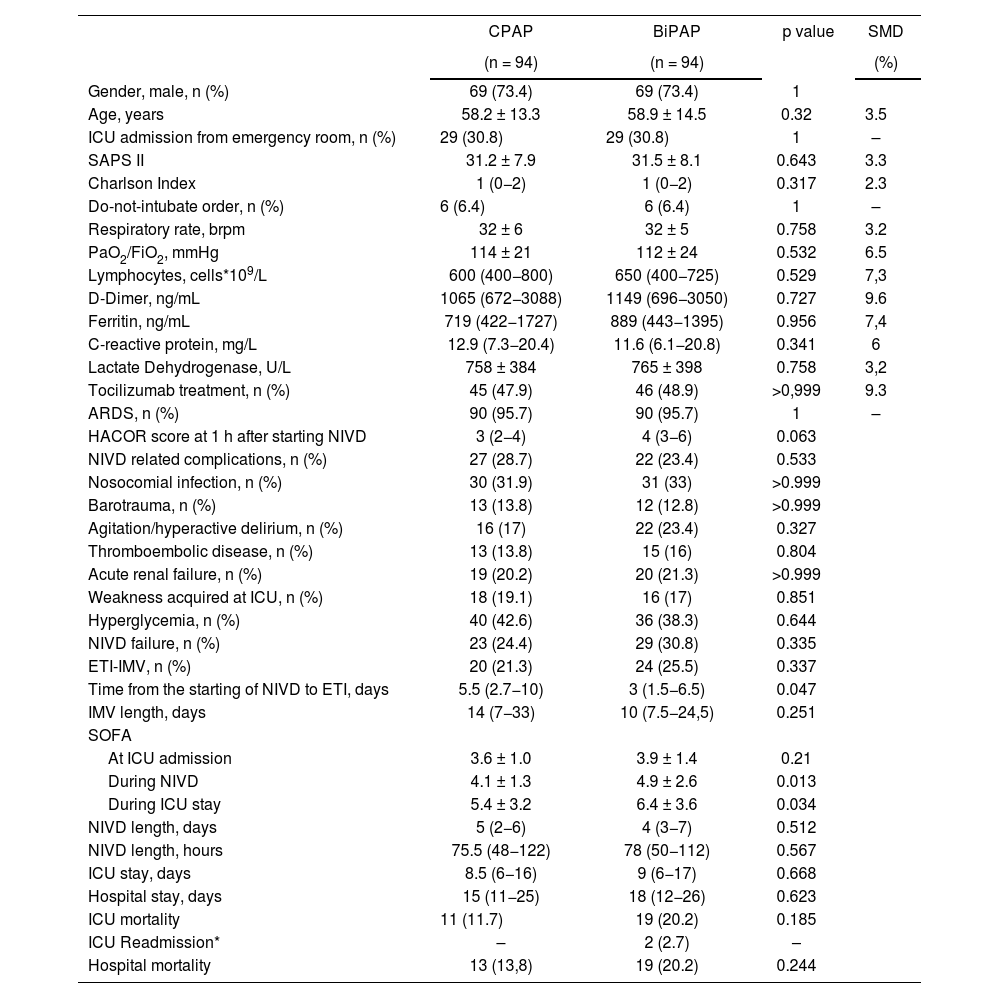
The comparison by means of propensity score matched analysis showed that neither the rate of failure of the device (OR 1.37, CI 95% 0.72–2.62) nor in-hospital mortality (OR 1.57, CI 95% 0.73–3.42) differed between patients treated with CPAP versus BiPAP (Table 5). Sensitivity analysis, after excluding patients on CPAP or BiPAP prior to ICU admission, also showed no differences between the two groups for NIVD failure (OR 1.15, 95% CI 0.55–2.41) or in-hospital mortality (OR 1.64, 95% CI 0.68–3.97) [Table S6]. Survival analysis did not show a relationship between hospital time-mortality with the type of NIVD used (HR&#¿;=&#¿;0.892, 95% CI&#¿;=&#¿;0.699–1.137) [Figure S1]. Propensity-matched analysis, not adjusted for DNI order, showed similar results (OR 1.49, 95% CI 0.80–2.79) and in-hospital mortality (OR 1.69, 95% CI 0.82–3.49) [Table S7].
Comparison between CPAP and BiPAP by Propensity Score–Matched Analysis.
| CPAP | BiPAP | p value | SMD | |
|---|---|---|---|---|
| (n = 94) | (n = 94) | (%) | ||
| Gender, male, n (%) | 69 (73.4) | 69 (73.4) | 1 | |
| Age, years | 58.2 ± 13.3 | 58.9 ± 14.5 | 0.32 | 3.5 |
| ICU admission from emergency room, n (%) | 29 (30.8) | 29 (30.8) | 1 | – |
| SAPS II | 31.2 ± 7.9 | 31.5 ± 8.1 | 0.643 | 3.3 |
| Charlson Index | 1 (0−2) | 1 (0−2) | 0.317 | 2.3 |
| Do-not-intubate order, n (%) | 6 (6.4) | 6 (6.4) | 1 | – |
| Respiratory rate, brpm | 32 ± 6 | 32 ± 5 | 0.758 | 3.2 |
| PaO2/FiO2, mmHg | 114 ± 21 | 112 ± 24 | 0.532 | 6.5 |
| Lymphocytes, cells*109/L | 600 (400−800) | 650 (400−725) | 0.529 | 7,3 |
| D-Dimer, ng/mL | 1065 (672−3088) | 1149 (696−3050) | 0.727 | 9.6 |
| Ferritin, ng/mL | 719 (422−1727) | 889 (443−1395) | 0.956 | 7,4 |
| C-reactive protein, mg/L | 12.9 (7.3−20.4) | 11.6 (6.1−20.8) | 0.341 | 6 |
| Lactate Dehydrogenase, U/L | 758 ± 384 | 765 ± 398 | 0.758 | 3,2 |
| Tocilizumab treatment, n (%) | 45 (47.9) | 46 (48.9) | >0,999 | 9.3 |
| ARDS, n (%) | 90 (95.7) | 90 (95.7) | 1 | – |
| HACOR score at 1 h after starting NIVD | 3 (2−4) | 4 (3−6) | 0.063 | |
| NIVD related complications, n (%) | 27 (28.7) | 22 (23.4) | 0.533 | |
| Nosocomial infection, n (%) | 30 (31.9) | 31 (33) | >0.999 | |
| Barotrauma, n (%) | 13 (13.8) | 12 (12.8) | >0.999 | |
| Agitation/hyperactive delirium, n (%) | 16 (17) | 22 (23.4) | 0.327 | |
| Thromboembolic disease, n (%) | 13 (13.8) | 15 (16) | 0.804 | |
| Acute renal failure, n (%) | 19 (20.2) | 20 (21.3) | >0.999 | |
| Weakness acquired at ICU, n (%) | 18 (19.1) | 16 (17) | 0.851 | |
| Hyperglycemia, n (%) | 40 (42.6) | 36 (38.3) | 0.644 | |
| NIVD failure, n (%) | 23 (24.4) | 29 (30.8) | 0.335 | |
| ETI-IMV, n (%) | 20 (21.3) | 24 (25.5) | 0.337 | |
| Time from the starting of NIVD to ETI, days | 5.5 (2.7−10) | 3 (1.5−6.5) | 0.047 | |
| IMV length, days | 14 (7−33) | 10 (7.5−24,5) | 0.251 | |
| SOFA | ||||
| At ICU admission | 3.6 ± 1.0 | 3.9 ± 1.4 | 0.21 | |
| During NIVD | 4.1 ± 1.3 | 4.9 ± 2.6 | 0.013 | |
| During ICU stay | 5.4 ± 3.2 | 6.4 ± 3.6 | 0.034 | |
| NIVD length, days | 5 (2−6) | 4 (3−7) | 0.512 | |
| NIVD length, hours | 75.5 (48−122) | 78 (50−112) | 0.567 | |
| ICU stay, days | 8.5 (6−16) | 9 (6−17) | 0.668 | |
| Hospital stay, days | 15 (11−25) | 18 (12−26) | 0.623 | |
| ICU mortality | 11 (11.7) | 19 (20.2) | 0.185 | |
| ICU Readmission* | – | 2 (2.7) | – | |
| Hospital mortality | 13 (13,8) | 19 (20.2) | 0.244 | |
Data are expressed as means&#¿;±&#¿;standard deviation or median (interquartile range).
Definition of abbreviations: ARDS: acute respiratory distress syndrome; brpm: breaths per minute; BiPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; ETI: endotracheal intubation; ICU: intensive care unit; IMV: invasive mechanical ventilation; L: liter; ml: milliliter, mmHg: millimeter of mercury; ng: nanogram, NIVD: non-invasive ventilatory device; SAPS: Simplified Acute Physiology Score; SMD: standardized mean difference; SOFA: sequential organ failure assessment.
The comparison between the four previously mentioned groups showed multiple differences in the analyzed variables (Table S8 and S9). Patients initially treated with CPAP and who did not need crossover to BiPAP presented a better prognosis, with lower CPAP failure (2.5%) and mortality rates (2.5%). Patients with the need to crossover from CPAP to BiPAP and those who needed BiPAP on an ongoing basis, had the highest NIVD failure rate (38% and 54.7%, respectively) and in-hospital mortality (22% and 32.8%, respectively).
Subgroup analysis (Table S10) showed that female patients, patients aged ≤ 65 years old and those without chronic respiratory disease had a lower rate of non-invasive respiratory device failure when initially receiving CPAP. However, after adjustment for IPW, only patients without chronic respiratory disease treated with CPAP had a lower failure rate (OR 0.68, 95% CI 0.26 to 0.96).
Cox analysis, in the total sample, showed that the most important independent factor related to in-hospital mortality was NIVD failure (HR 15.69, 95% CI 6.02–40.86) (Table S11).
DiscussionIn this study, it was shown that the management of COVID-19 associated ARF requiring NIVD can be performed using CPAP or BiPAP with similar effectiveness and safety.
NIV has shown efficacy in preventing endotracheal intubation in patients with acute on-chronic respiratory failure.21 However, evidence of benefit has only been demonstrated in mild-to-moderate hypoxemic ARF.21 Several recent systematic reviews have evaluated the role of NIV in the treatment of hypoxemic ARF, in both COVID-19 and non-COVID-19 settings.22–24 The results of a network meta-analysis suggest that NIV, HFNC, and conventional oxygen therapy are comparable in terms of treatment failure and mortality in COVID-19.22 Pitre et al. demonstrated that Helmet NIV strategies are probably effective at reducing mortality, while HFNC, facial-mask NIV, helmet bilevel ventilation, and CPAP reduce the need for IMV compared to standard oxygen-therapy.23 In the study by Sakuraya et al., a decrease in mortality was observed with the use of CPAP, but not with NIV.24
Some studies associate the use of NIV with a worse prognosis.25–27 In a multicenter study performed on patients in and outside ICU during the first wave COVID-19, CPAP failure rate was 36.8%, while NIV failure rate was 60.8%.25 Franco et al., in a multicenter Italian study, conducted during the first wave, found a higher rate of mortality/intubation in patients receiving NIV than CPAP (47.3% versus 53%), but the differences disappeared after adjusting for confounding variables.26 In another multicenter observational study, carried out in high dependency units, with 54% of patients having a DNI order, Crisafulli et al. showed a higher failure rate, defined as intubation or death, when NIV was used initially or during the hospital stay, compared to CPAP (34% versus 60%).27 Like these studies, our work also showed a higher failure rate in the BiPAP group, however, it was lower than in the previous studies: 34,7%, while CPAP failure rate was 25%. However, the adjustment of variables showed that mortality did not differ between the two types of NIVD.
Although the characteristics of the patients in the mentioned studies were quite similar, the management protocols of NIVD clearly differed between them, as well as the type of hospital unit to which the patients were admitted. In a recent observational study in COVID-19 patients with mild-moderate ARF, the failure rate of CPAP was 3.9%.28 The lower failure rate of CPAP in this series compared to the one from our study may be related to the lower severity of the patients (initial PaO2/FiO2 of 193&#¿;mmHg versus 120&#¿;mmHg).
Currently, there is no clear standardization for the use of NIVD in the treatment of ARF, neither in relation to the time of initiation nor with the type of respiratory device to be used, nor with the programmed parameters. A wide variability is shown in this area,6,11–15 both in randomized controlled trials and in observational studies. Most studies that have compared CPAP with BiPAP in adult patients have been in cardiogenic pulmonary edema,29 and those performed in patients with ARF of other etiologies have been scarce and have not shown differences between both modalities.30
We advocate for a sequential treatment of ARF, in which HFNC, CPAP and BiPAP are used depending on the patient's condition. During ARF, variations in the patient's clinical situation are frequent, due to the evolution of the disease itself, and due to the presence of complications associated with the NIVD and interfaces used, fundamentally the presence of claustrophobia, pain or intolerance.17 Because of this, even though most patients initially receive CPAP, crossover to NIV is frequent when the clinical situation does not improve. Likewise, it is frequent crossover from NIV to CPAP in the presence of complications related to NIV. In a recently published pilot study on the timing of starting CPAP or NIV in patients with ARDS due to COVID-19, the initial treatment with CPAP was crossover to NIV due to an inadequate respiratory response.31 Crossover from CPAP to NIV, and from NIV to CPAP was frequent in the study by Crisafulli et al.27 The failure rate of BiPAP in our study was slightly higher than CPAP. However, this higher failure rate with BiPAP may be explained, at least partially, by a greater severity of the patients who initially received BiPAP. The pressure support used during BiPAP can also cause an increase in tidal volume (promoting volutrauma), which has been related to BiPAP failure.32 Finally, a greater effect of patient self-induced lung injury cannot be ruled out due to an increase in inspiratory effort that could occur during the use of NIVD. In the case of BiPAP it may be related to patient-ventilator desynchrony, whose effect may be potentiated by higher tidal volumes associated with pressure support.33 However, the difficulty of measuring inspiratory effort during NIVD, as well as the lack of a definitive treatment to reduce patient self-induced lung injury make it difficult to know the exact role that this process plays in NIVD failure.34 Despite these considerations, when adjusting for different factors that may condition a worse prognosis, through paired propensity analysis, the differences in the failure rate between BiPAP and CPAP diminished (OR 1.37, CI 95% 0.72–2.62).
However, there were two groups of patients with a worse prognosis: those with continuous need for BiPAP, which had a failure rate of 54.7% and in-hospital mortality of 32.8%, and those with a need for crossover from CPAP to BiPAP, with a failure of 38% and in-hospital mortality of 22%. These hallmarks were also shown in a study by Nevola et al., where the need for crossover from CPAP to NIV was a risk factor for NIV failure in the univariate analysis, although not in the multivariate analysis, while the need for continuous NIV was an independent predictor of poor prognosis.31 Crossover to more complex therapies usually indicate greater severity and worse prognosis.35 In our series, few patients were treated with ECMO. Only four patients received this therapy, all of them under 60 years old and without relevant comorbidities. All four were intubated and died in the hospital.
Few studies have analyzed the relationship between the type of NIVD and complications. In a small study, Pontes et al. found a greater number of complications in patients treated with BiPAP than with CPAP.36 The use of NIV leads in many cases to the appearance of asynchrony between the patient and the ventilator, which is one of the factors that can cause intolerance. Despite this consideration, in a series of patients with NIVD, intolerance was slightly more frequent in patients treated with CPAP than with NIV.37 In our study, total intolerance was very low (1.6%), despite the duration of NIVD, probably related to the systematic use of opiates.
One of the reasons for not using NIVD at the beginning of the COVID-19 pandemic was the possible association between a delay in endotracheal intubation and a worse prognosis. This relationship, shown in de novo ARF patients of different etiologies treated with and without NIV3,38 has been showing contradictory results in ARF related to COVID-19.30,38–40 In our work, the average time to intubation was high, being greater in patients initially treated with CPAP (median of 5 days in overall population and 5.5 days in population adjusted through propensity) even though mortality did not differ between both groups.
This study has several limitations. First, it is an uncontrolled observational study. The effectiveness of a treatment must be evaluated through a well-designed controlled and randomized trial, where the confusion variables, known and not known, are controlled through an appropriate randomization. In observational studies, control of some of the confusion variables can be attempted to imply causality through different techniques, including propensity score-matched analysis, but even in these circumstances, since there is the possibility that some of the analyzed variables were not considered adequately (and that there might be other confusion variables which were not measured), the conclusions must be evaluated carefully, and the results of these analyzes must not be considered definitive. On the other hand, it must be noted that the comparison between the adjusted groups may lead to problems arising from over-adjustment. Also, the number of patients in the sample may not be sufficient to ensure adequate statistical power. Secondly, our patient management protocol includes several novel points, such as the use of full-face mask, the systematic use of perfusion and bolus of fentanyl, the use of HFNC in weaning or in the presence of complications, which may be controversial since there is no clear evidence for its use. This makes it difficult to extrapolate the results to other protocols on the use of non-invasive respiratory devices. Finally, the study was carried out in a single center, and in an ICU with extensive experience in the use of NIV in hypoxemic ARF patients, with highly trained nursing staff, an adequate nurse-patient ratio, and high-performance ventilators and devices, which can make it difficult to extrapolate the results to other units with fewer resources or experience.
Our results point to the possibility of using CPAP or BiPAP in patients with moderate-severe COVID-19 associated ARF. Bearing in mind that the use of CPAP is more common than that of BiPAP, the selection between them will tend to involve the experience of healthcare teams, the severity and antecedents of the patient, as well as the availability of resources, ventilators, monitoring and health staff, as well as the place/unit to which the patients was admitted to. Also, respiratory management must be individualized, depending on the pathological and clinical characteristics of the patient, and on the type of NIVD (and the parameters to be programmed), to ensure that the patient is not intubated and can be discharged alive from the hospital.
ConclusionsCOVID-19 associated hypoxemic ARF can be treated initially with non-invasive positive pressure respiratory devices with a high success rate and avoidance of endotracheal intubation and death. These findings suggest that while both CPAP and BiPAP can be effective in managing acute respiratory failure, the choice of treatment should be tailored to the patient's clinical profile, with close monitoring for those who may require escalation of care.
CRediT authorship contribution statementAndrés Carrillo Alcaraz: Data mining Statistical analysis. Elaboration and drafting of the article.
Miguel Guia: Revision and drafting of the article.
Laura Lopez-Gomez: Data mining. Drafting.
Pablo Bayoumy: Data mining. Drafting.
Aurea Higon-Cañigral: Data mining. Drafting.
Elena Carrasco González: Data mining. Drafting.
Pilar Tornero Yepez: Data mining. Drafting.
Juan Miguel Sánchez-Nieto: Data mining. Drafting.
FundingNone.