Corticosteroids have received increasing focus as an emerging therapy in COVID-19, particularly in patients receiving respiratory support as demonstrated by the RECOVERY trial.1 Previous evidence already supported the use of steroid treatment in patients with acute respiratory distress syndrome (ARDS) undergoing mechanical ventilation.2,3 However, there is no current consensus regarding the ideal dose and treatment duration in this population.
Furthermore, the focus of the discussion on corticosteroid use in COVID-19 has been on the timing of treatment initiation according to disease stage, and concerns about steroid-related delay in viral clearance.4 However, it is likely that benefits are dependent on the cumulative dose and duration of corticosteroid therapy, but these questions remain unaddressed in high quality randomized trials.5
To contextualize this problem, we reviewed a retrospective cohort of 65 patients admitted to a secondary referral intensive care unit (ICU) with SARS-CoV-2 pneumonia and ARDS undergoing mechanical ventilation in a 5-month period. This cohort is composed of predominantly male patients (75.4%), with a mean age of 65.1 years [57–74], with a median duration of ventilation of 10 days [5–16] and an ICU mortality rate of 13.9%.
Patients were treated with different doses of corticosteroids according to the evolution of the scientific evidence during the pandemic and the patients’ clinical course. Patients at the beginning of the pandemic did not receive corticosteroids. Patients admitted later during the year received corticosteroids according to the protocol proposed by the Critical Illness-Related Corticosteroid Insufficiency in Critically Ill (CIRCI) Patients Guideline3 or the RECOVERY trial protocol.1
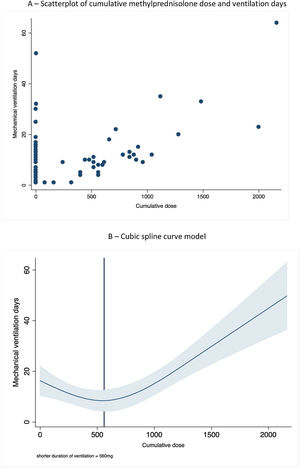
We plotted the duration of mechanical ventilation and cumulative corticosteroid dose (expressed as methylprednisolone equivalent dose) and invasive mechanical ventilation duration (Fig. 1A). We used a cubic spline regression model to plot the relationship between the two variables, using three knots at the 25th, 50th and 75th percentile – namely 160mg, 520mg and 960mg (Fig. 1B). There was a strong correlation between total corticosteroid dose and ventilation days (p<0.001), following a U-shaped pattern. The shortest ventilation time associated with a cumulative dose of 560mg, while higher doses were associated with longer duration of mechanical ventilation. In order to correct for possible longer duration of treatment with corticosteroids due to inertia we looked at the relation between total duration of mechanical ventilation and methylprednisolone-free ventilation days. After controlling for methylprednisolone-free ventilation days the correlation remained significant (p<0.001). There was no correlation with increased infection rates or mortality, but we found a strong correlation between higher cumulative doses of corticosteroids and ICU-acquired myopathy (p=0.03).
This study has some limitations. Given the retrospective nature of the cohort and the change in evidence regarding the efficacy of treatments in COVID-19, treatment was not standardized for all patients. Similarly, the dose and duration of corticosteroid varied among patients and tapering was done based on individual patient condition, as per the CIRCI Guideline. The mean dose of methylprednisolone used in our cohort was 653mg. Despite this heterogeneity, the mortality rate was distributed evenly throughout the 5-month period.
In sum, our analysis suggests that corticosteroid therapy in COVID-19 needs to be carefully titrated and readily tampered when clinical improvement occurs, given the lack of evidence for benefit of higher doses. Considering current evidence, we suggest that prolonged treatment with corticosteroids in COVID-19 should be avoided.
FundingNo funding was received for the completion of this work.
Authors’ contributionsRM, LM, JJM and PTF were responsible for the study conception and manuscript draft. RM and LM collected data. All authors read and approved the final manuscript.
Conflicts of interestNone declared.