Fluid resuscitation is essential for the survival of critically ill patients in shock, regardless of the origin of shock. A number of crystalloids and colloids (synthetic and natural) are currently available, and there is strong controversy regarding which type of fluid should be administered and the potential adverse effects associated with the use of these products, especially the development of renal failure requiring renal replacement therapy. Recently, several clinical trials and metaanalyses have suggested the use of hydroxyethyl starch (130/0.4) to be associated with an increased risk of death and kidney failure, and data have been obtained showing clinical benefit with the use of crystalloids that contain a lesser concentration of sodium and chlorine than normal saline. This new information has increased uncertainty among clinicians regarding which type of fluid should be used. We therefore have conducted a review of the literature with a view to developing practical recommendations on the use of fluids in the resuscitation phase in critically ill adults.
La reanimación con fluidos es esencial para la supervivencia del paciente crítico en shock, independientemente de la causa que lo origine. Hoy en día disponemos de diversos cristaloides y coloides (sintéticos y naturales), existiendo una viva controversia sobre qué tipo de fluidos debemos emplear y los posibles efectos adversos asociados a su uso, especialmente el desarrollo de fracaso renal con necesidad de técnicas de reemplazo renal. Recientemente se han publicado varios ensayos clínicos y metaanálisis que evidencian que el empleo de hidroxietilalmidón (130/0,4) se asocia a un mayor riesgo de muerte e insuficiencia renal, así como datos que muestran un beneficio clínico con el empleo de cristaloides que contienen menor concentración de sodio y cloro que el suero salino. Ello ha contribuido a aumentar la incertidumbre de los clínicos sobre qué tipo de fluido emplear. Por ello, hemos realizado una revisión narrativa de la literatura con el fin de elaborar unas recomendaciones prácticas sobre el empleo de fluidos en la fase de reanimación del paciente crítico adulto y que se presentan en este documento.
The administration of fluids is one of the most common therapeutic practices in the routine care of critically ill patients. Such administration largely takes place in the first hours and days of admission, which is when resuscitation care is provided in patients who are often admitted to the Intensive Care Unit (ICU) due to shock or hypotension of any cause.
It must be clearly kept in mind that fluid administration requires the same caution and knowledge (indications, contraindications, adverse effects) as when any kind of drug is used.1
There are two questions in relation to fluid therapy which clinicians ask themselves daily, and which are reflected in the working hypotheses of the different clinical trials and studies: “What fluid should be provided?” and “How much fluid should be administered and for how long?”
Regarding the first question (on which the present document is centered), it must be mentioned that new solutions are currently found on the market, and recent information is moreover available on the adequacy and suitability of the different solutions in different clinical scenarios. These new data are sometimes contradictory, and firm conclusions are often lacking. This in turn explains the great variety in prescription practices referred to fluid therapy; indeed, while the use of colloids is practically anecdotal in some countries, in others they constitute first line treatment for hypotension.2
The use of synthetic colloids in critical patients is currently subject to strong controversy, due to the adverse effects and even increased mortality associated to the use of some of these products. In fact, a recent consensus report auspiced by the European Society of Intensive Care Medicine (ESICM) considers that synthetic colloids should not be used in the critically ill outside the investigational setting.3 This recommendation consequently limits the treatment options to crystalloids and albumin. The exclusive use of crystalloids is not without risks, particularly the development of interstitial edema.1 On the other hand, it must be taken into account that the term “crystalloid” encompasses a series of solutions with different compositions.
ObjectivesIn view of this situation, a group of specialists in Intensive Care Medicine have conducted a review of the literature with the purpose of establishing a series of practical recommendations on the use of fluids (crystalloids and colloids) in the resuscitation phase of the adult critical patient with hypotension. The review specifically focuses on those studies that evaluate mortality or the impact upon the development of renal failure or the need for extrarenal filtration techniques. This document does not consider pediatric patients, maintenance fluid therapy or the management of hemorrhagic shock.
MethodologyA PubMed literature search was made of all the observational studies and clinical trials (excluding pediatric patients), using the following key words: fluid therapy, colloids, crystalloids, sodium chloride, Ringer, albumin, balanced solution, hetastarch, pentastarch, hydroxyethyl starch, gelatin, AND hyperchloremic acidosis, resuscitation, shock, severe sepsis, septic shock, trauma, major surgery, kidney, renal, mortality, injury, failure, complication, anaphylactoid reactions, adverse, illness, renal replacement therapy, outcome, clinical trials, prospective study, observational study, and metaanalysis. The review was limited to articles published in English or Spanish, with no restrictions regarding the time period covered. The full texts of all the selected articles were retrieved.
On occasion of a meeting held on 30 September 2013, the group reviewed the state of the art of resuscitation with fluids in the critical patient, and debated the identified studies and metaanalyses. All the participants completed a questionnaire addressing different practical aspects of resuscitation therapy. The structure of the present document was approved during the meeting–the different sections being divided up among the participants for the preparation of a first draft (JGM) that was then distributed to the entire group. A second meeting on 18 June 2014 served to discuss this draft document. The final review was prepared with the literature published up until July 2014, and was evaluated and approved by all the signing members.
Available fluids: characteristicsThe fluids used can be classified as crystalloids or colloids. Crystalloids are solutions containing water, electrolytes and/or sugars in different proportions. They can be hypotonic, isotonic or hypertonic with respect to plasma. Their volume expanding capacity is related to the concentration of sodium, which is the factor that conditions the osmotic gradient between the extra- and intravascular compartments.
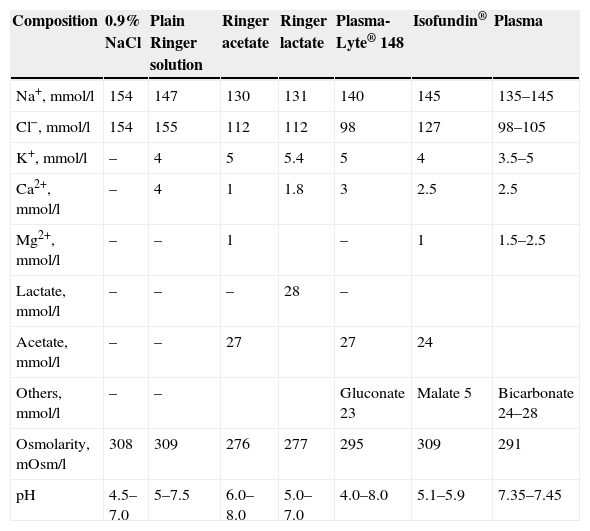
CrystalloidsCrystalloid solutions that are isotonic with plasma distribute within the extracellular fluid compartment, and present a high elimination index. In healthy volunteers, it can be estimated that only 20% of the infused volume remains in the intravascular space 60min after administration.4 Saline solution at a concentration of 0.9%–also known as physiological saline–is slightly hypertonic with respect to the extracellular fluid and has an acid pH value (Table 1).
Composition of the crystalloids and comparison with plasma.
| Composition | 0.9% NaCl | Plain Ringer solution | Ringer acetate | Ringer lactate | Plasma-Lyte® 148 | Isofundin® | Plasma |
|---|---|---|---|---|---|---|---|
| Na+, mmol/l | 154 | 147 | 130 | 131 | 140 | 145 | 135–145 |
| Cl−, mmol/l | 154 | 155 | 112 | 112 | 98 | 127 | 98–105 |
| K+, mmol/l | – | 4 | 5 | 5.4 | 5 | 4 | 3.5–5 |
| Ca2+, mmol/l | – | 4 | 1 | 1.8 | 3 | 2.5 | 2.5 |
| Mg2+, mmol/l | – | – | 1 | – | 1 | 1.5–2.5 | |
| Lactate, mmol/l | – | – | – | 28 | – | ||
| Acetate, mmol/l | – | – | 27 | 27 | 24 | ||
| Others, mmol/l | – | – | Gluconate 23 | Malate 5 | Bicarbonate 24–28 | ||
| Osmolarity, mOsm/l | 308 | 309 | 276 | 277 | 295 | 309 | 291 |
| pH | 4.5–7.0 | 5–7.5 | 6.0–8.0 | 5.0–7.0 | 4.0–8.0 | 5.1–5.9 | 7.35–7.45 |
Hyposaline solution (0.45% NaCl solution) is hypotonic and may be indicated in dehydration with hypernatremia, but not as a plasma expander. Hypertonic saline solutions (3–7.5% NaCl solutions) expand to a greater extent than the infused volume alone, since they result in the transfer of water from the intracellular compartment to the extracellular compartment. Their use in the resuscitation of critical patients is still in the investigational phase, with the possible exception of polytraumatized patients. They are therefore not contemplated in this document.
Crystalloids have been developed with a composition more similar to that of plasma. These are the so-called “balanced solutions” (Table 1). The principal modifications introduced by these solutions are a reduction of the concentrations of sodium and particularly chloride, replacing the latter anion with lactate (Ringer lactate) or acetate, malate or gluconate (new balanced solutions). The pH of these formulations is less acidic than in the case of saline solution, and their sodium and chloride concentrations are more similar to those of plasma. The volume expanding effect achieved with these solutions is very similar to that of saline solution.5
Three Ringer solutions are available on the market (plain Ringer solution, Ringer acetate and Ringer lactate). It must be noted that plain Ringer solution cannot be regarded as a balanced solution due to its sodium and chloride contents, which are very similar to those of saline solution (Table 1). The most widely used formulation is Ringer lactate or Hartmann's solution, which is slightly hypoosmolar with respect to plasma (Table 1) and contains 28mEq of lactate per liter, which is transformed into pyruvate and posteriorly to bicarbonate in the course of its metabolism as part of the Cori cycle. This amount of lactate is present as a mixture of d-lactate and l-lactate. Of these two forms, l-lactate is the most physiological, and is metabolized by lactate dehydrogenase, while d-lactate is metabolized by d-a-dehydrogenase. Ringer acetate is currently not marketed in Spain.6
ColloidsColloids are high molecular weight particles that cross the capillary membrane with difficulty; as a result, they are able to increase the plasma oncotic pressure and retain water within the intravascular space. They produce faster and more persistent hemodynamic effects than crystalloids, and require less administered volume than the latter.7 However, these effects appear to depend on the clinical context: in hypovolemic individuals with low capillary pressure, albumin and the synthetic colloids would offer no hemodynamic advantages over crystalloids. Colloids in turn are either synthetic (gelatins, starches and dextrans) or natural (albumin).
DextransDextrans are mixtures of glucose polymers that are available in two solutions: dextran 40 (mean molecular weight 40kDa) and dextran 70 (mean molecular weight 70kDa). These formulations are associated to a considerable incidence of side effects such as allergic reactions, renal failure or bleeding disorders, and are practically no longer used.2,8
GelatinsThere are two gelatin formulations: polygeline (gelatin linked by urea bonds) and succinylated gelatin. These two formulations differ not only in their chemical characteristics but also in their expansion capacity, electrolyte composition and adverse effects.9 Traditionally, anaphylactic reactions have been the most feared adverse effect associated to gelatin use,8 occurring in 1% of the cases with polygeline and in approximately 0.1% of the cases with succinylated gelatin.10 The molecular weight of succinylated gelatin is about 30kDa, despite which its expansion capacity is similar to that of hydroxyethyl starch (HES) 130 (molecular weight 130kDa).11 Recently, a new formulation has been marketed in Spain with a lesser presence of chloride, which has been replaced by acetate.
Hydroxyethyl starchHydroxyethyl starch (HES) is composed of modified natural polysaccharides obtained from corn or potato starch by replacing the hydroxyl groups with hydroxyethyl ether groups in the glucose molecules of amylopectin. There are two physicochemical features of interest that explain the behavior of this product in the body: its molecular weight and the degree of hydroxylation, which is measured by the molar substitution ratio. The latter is defined as the number of hydroxyethylated glucose units divided by number of glucose units present. The larger the number of hydroxyethylated glucose units, the greater the degree of substitution and the longer the half-life of the molecule in plasma.1
The first generations of HES were characterized by a high molecular weight (450kDa) and substitution ratio (0.7). Posteriorly, other forms with a molecular weight of 200kDa and a substitution ratio of 0.5 were introduced. The long half-life of these products and their accumulation in the tissues as a consequence of their physicochemical properties explain the high incidence of adverse effects, particularly renal failure, associated with their use.12
New generations of HES were subsequently developed, with theoretical advantages over their predecessors, and with a mean molecular weight of 130kDa and a molar substitution ratio of 0.42 (HES 130/0.4). At least in theory this means that they undergo lesser tissue accumulation and produce fewer side effects. However, clinical studies have not confirmed a lesser accumulation of HES with lower molecular weights and substitution ratios compared with higher molecular weight formulations.13
AlbuminAlbumin is a natural colloid available in the form of 4% and 20% solutions. In Spain and generally also in the rest of Europe the tendency is to use the 20% formulation, while in the United States the 4% solution is mainly used. It must be mentioned that the 4% formulation has a high chloride content (120–130mEq/l), while the 20% solution has a low chloride content (20mEq/l).
Albumin solutions have been used in the care of critical patients since the 1940s. However, on the basis of the findings of a metaanalysis published in 1998, with the reporting of an increase in mortality,14 the role of the administration of albumin in the critical patient became the focus of controversy.
It is well known that albumin has a range of physiological effects that are particularly relevant in the critically ill patient,15 including the regulation of colloidosmotic pressure, the plasma transport of drug substances, antioxidant capacity and the modulation of nitric oxide. Ulldemolins et al.16 reported that the protein binding of antibiotics–including ceftriaxone, ertapenem, teicoplanin and aztreonam–is more frequently diminished in patients with hypoalbuminemia. As a result, drug clearance is increased, and this may result in infratherapeutic drug concentrations. However, the relationship between albumin therapy and improvement of some of the known physiological effects of albumin has not been demonstrated. In any case, it has been well established that low albumin levels are associated to a poorer prognosis.17,18
Which of these alternatives should be used in the resuscitation phase of critical care?Having reviewed the general characteristics of the different crystalloids and colloids, our aim was to position each of them in resuscitation of the critical patient, based on the available scientific literature and on our opinion as clinicians. In this regard, a series of questions were defined, and after considering the most relevant data found in the scientific publications, conclusions were drawn on how and when we think that these solutions should be used today.
Is saline solution the crystalloid of choice for starting resuscitation of the critical patient?Among the different options, saline solution traditionally has been the first choice for the resuscitation of patients in shock.1,19 In fact, saline solution has been chosen as comparator in several clinical trials that have evaluated different synthetic or natural colloids in the resuscitation of critical patients.20,21
Saline solution sodium and chloride content is slightly greater than in plasma, and has been associated to hyperchloremic acidosis and probably to the development of renal failure.22 If large amounts of saline solution are infused, the excess chloride of the extracellular fluid displaces bicarbonate, producing hyperchloremic acidosis. This effect has been observed in postsurgical patients23–25 and in polytraumatized individuals,26 though the clinical consequences do not appear to be relevant.27 There is a direct relationship between the amount of chloride administered and the appearance of metabolic acidosis.28
On the other hand, the metabolic acidosis seen in patients with severe sepsis and septic shock is of a multifactorial origin, though hyperchloremic acidosis is one of the predominant contributors, particularly in those patients who die.29 It has recently been shown that the infusion of saline solution in healthy volunteers reduces renal flow and perfusion of the renal cortex–a situation not seen when a balanced saline solution is used.30
Recommendations- 1.
Saline solution at a concentration of 0.9% is a valid option for starting resuscitation of the critical patient, including patients with septic shock.
- 2.
Close monitoring of the appearance of hyperchloremic acidosis is advised.
- 3.
If hyperchloremia with or without associated metabolic acidosis is observed, we recommend the use of fluids with a lesser chloride content (balanced solutions).
- 4.
Saline solution at a concentration of 0.9% is the solution of choice in cases of hypochloremic alkalosis, in hypochloremia of any origin, or in cases of severe hyperpotassemia.
The volume expansion effect achieved with Ringer lactate is very similar to that obtained with saline solution. In an experimental model, the use of Ringer lactate was not found to be associated to the development of hyperchloremic acidosis.31
It must be mentioned that no clinical trials have compared the different crystalloids in the resuscitation of critical patients. In contrast, a number of prospective studies and clinical trials have compared saline solution with Ringer lactate in surgical patients.25,27,28,32–35 These studies concluded that the use of Ringer lactate is associated to a lesser incidence of hyperchloremic metabolic acidosis than saline solution, though without differences in terms of the clinical objectives. Although Ringer lactate contains potassium, its use in patients with renal dysfunction is associated to a lesser increase in serum potassium than when saline solution is used.25 Although the explanation for this is not clear, potassium displacement due to the acute blood hydrogen ion changes may be the underlying cause.
In a recent propensity score-adjusted cohort study in medical patients with septic shock, the use of balanced solutions (over 90% of the fluids received in this group corresponded to Ringer lactate) in the resuscitation phase was associated to a significant decrease in mortality compared with the use of saline solution–though without differences in the incidence of renal failure. A dose-dependent relationship was observed between the administration of balanced solutions and beneficial effects upon mortality, independently of the total amount of fluids administered.36
However, it is theoretically possible that the infusion of large amounts of lactate may exacerbate the lactic acidosis present in septic shock and in other peripheral hypoperfusion conditions. Hepatocellular damage or decreased liver perfusion, in combination with a hypoxic component, could reduce lactate clearance and therefore increase lactic acidosis. On the other hand, hyperlactacidemia upon admission to the ICU (lactacidemia >4mmol/l) is an independent mortality factor in patients with severe sepsis.37
It must be noted that no clinical studies in critical patients or patients with severe sepsis have evaluated this possible undesired effect. A study including 24 healthy volunteers recorded no hyperlactacidemia after infusing a liter of this solution in 1h.38 The use of Ringer lactate produced hyperlactacidemia in polytraumatized patients,26 in gynecological surgery,32 in surgical patients over 60 years of age,27 and in donors subjected to right hepatectomy–though it disappeared on the second postoperative day, and had no impact upon the clinical outcome.39
The relative hypoosmolarity of Ringer lactate constitutes an important limitation for its use in the resuscitation of patients at risk of brain edema.40 It is therefore not advised in patients with traumatic brain injury or in other neurological patients at risk of developing intracranial hypertension.
Recommendations- 1.
Ringer lactate is the crystalloid of choice for starting resuscitation of the critical patient, including patients with septic shock.
- 2.
It is recommended as first option for resuscitation in the case of hyperchloremic metabolic acidosis.
- 3.
Although there are no data contraindicating the use of Ringer lactate in renal dysfunction, its potassium content causes us to recommend avoiding this solution in cases of hyperpotassemia or severe renal failure.
- 4.
It is advisable to consider other crystalloids that do not contain lactate in cases of severe hyperlactacidemia, though on the basis of the existing literature we are unable to recommend a serum lactate threshold beyond which this crystalloid should not be used.
- 5.
It is advisable to avoid Ringer lactate in patients with brain edema or at risk of developing brain edema as a consequence of their background disease condition.
In recent years abundant literature has been produced on the use of these new balanced solutions in the resuscitation of critical patients and the benefits obtained. In a study comparing two periods, the incidence of renal failure and the need for renal replacement techniques were significantly lower in the chloride restriction period (basically, the saline solution was replaced by a balanced crystalloid), though without differences in terms of mortality.22 In contrast, this strategy of using low chloride solutions is associated to an increased incidence of metabolic alkalosis.41
There are also data on the use of balanced solutions in surgical patients. An observational study concluded that surgical complications and mortality were significantly greater when using saline solution for resuscitation versus the use of a balanced solution. After propensity score adjustment, the use of the balanced solution was not seen to be associated to a decrease in mortality, though the incidence of complications was lower.42
A randomized, double-blind clinical study compared resuscitation with saline solution (n=30), Ringer lactate (n=30) or a balanced solution without lactate (n=30) in dehydrated patients seen in the emergency care area. The authors did not find any of these solutions to significantly alter the acid-base equilibrium, though there was a tendency toward lower pH values in the group that received saline solution.43 In severely polytraumatized patients, resuscitation with a balanced solution resulted in a lesser incidence of hyperchloremic acidosis than with saline solution. There was no impact in terms of the incidence of renal dysfunction, resource utilization or mortality–though this study was not designed to detect differences in mortality.44
Recent clinical trials have also evaluated these balanced solutions in neurocritical patients. In a randomized, double-blind study of 40 patients with severe traumatic brain injury (Glasgow coma score [GCS] ≤8) or subarachnoid hemorrhage (World Federation of Neurological Surgeons [WFNS] score ≥3), the incidence of hyperchloremic acidosis (primary outcome) was significantly lower with the use of a balanced solution than with the use of saline solution–though without differences in terms of the incidence of intracranial hypertension or mortality.45 Likewise, in a randomized, double-blind clinical trial in patients with subarachnoid hemorrhage, the use of a balanced solution was associated to a lesser incidence of hyperchloremic acidosis, though without the appearance of hyponatremia or hypoosmolarity, which could have negative consequences in patients of this kind.46
Lastly, a recent metaanalysis has evaluated the use of these balanced solutions, comparing them with saline solution in surgical patients. A total of 13 clinical trials were included, and the authors concluded that saline solution therapy is associated to a greater alteration of the acid–base equilibrium, without modifications of the clinical variables–though there was a greater need for platelet transfusion in the patients who were resuscitated with saline solution.47 Likewise, a sophisticated metaanalysis concluded that resuscitation with balanced solutions (defined as all those solutions in which the concentration of sodium was lower than in saline solution) is associated to lesser mortality in patients with sepsis than when unbalanced crystalloids are used.48 However, this conclusion was largely based on indirect evidence (network analysis) of questionable quality.
Recommendations- 1.
The new balanced solutions that do not contain lactate are an option in resuscitation of the critical patient, particularly in cases of septic shock, though they currently cannot be regarded as first choice treatment.
- 2.
These solutions are particularly indicated in patients with severe hyperlactacidemia, where the administration of high chloride levels is to be avoided.
- 3.
Their use is recommended in cases of hyperchloremic metabolic acidosis or following the administration of large amounts of saline solution if hyperchloremia has developed.
- 4.
Because of their potassium content, these solutions are not advised in cases of hyperpotassemia or renal failure.
The use of hydroxyethyl starch (HES) as synthetic plasma expander in critical patients with shock was a widespread practice in the early years of this century. It is well known that the required volume of HES is significantly smaller than in the case of crystalloids.49,50 A survey of 391 ICUs in 25 countries found HES to be used in Europe in 55% of all resuscitation episodes in which colloids were employed.2
However, in recent years a number of clinical trials have questioned the safety of HES, particularly in patients with severe sepsis and septic shock. Furthermore, studies in animals and in humans have shown HES to be deposited in different organs, but especially in the kidneys, persisting even for years. This could explain the adverse effects described with the use of this product, particularly as regards the increase in renal failure rate.51
A German multicenter study comparing HES 200/0.5 versus Ringer lactate in the resuscitation of patients with severe sepsis and septic shock documented a significantly higher renal failure rate and need for renal replacement therapy in the group administered HES. This effect was more pronounced among the patients who received more than 20ml/kg, though the renal failure rate and the need for renal replacement therapy was also significantly greater in those who received <20ml/kg, when compared with the Ringer lactate group.52 In contraposition to these results, a Canadian study found a lesser incidence of renal failure in patients with sepsis resuscitated with crystalloids plus colloids (HES 200/0.5) than in those who received only crystalloids–though without differences in relation to mortality.53
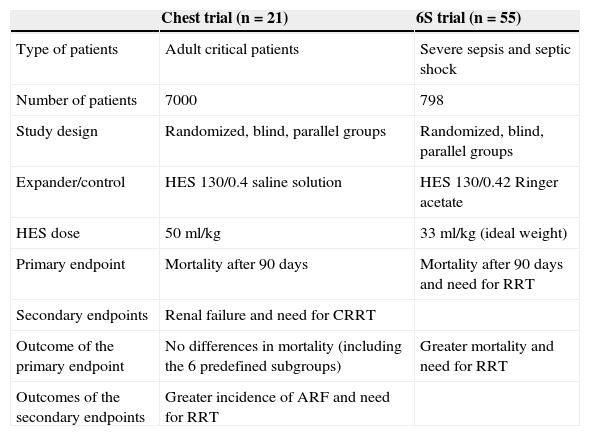
More recently, two clinical trials have evaluated HES of lesser molecular weight and lower molar substitution ratio (HES 130/0.4) in the resuscitation of critical patients or patients with severe sepsis and septic shock (Table 2). The incidence of renal failure and the need for renal replacement therapy were greater in the patients who received HES than in those administered saline solution, though with no differences in mortality after 90 days (primary endpoint).21 In contrast, in the study in patients with severe sepsis, mortality after 90 days and the need for renal replacement therapy were significantly greater among the individuals who received this colloid than in the control group (Ringer acetate). These results were confirmed after adjustment though a multivariate analysis.54
Comparison of two clinical trials that evaluated hydroxyethyl starch (HES) in critical patients.
| Chest trial (n=21) | 6S trial (n=55) | |
|---|---|---|
| Type of patients | Adult critical patients | Severe sepsis and septic shock |
| Number of patients | 7000 | 798 |
| Study design | Randomized, blind, parallel groups | Randomized, blind, parallel groups |
| Expander/control | HES 130/0.4 saline solution | HES 130/0.42 Ringer acetate |
| HES dose | 50ml/kg | 33ml/kg (ideal weight) |
| Primary endpoint | Mortality after 90 days | Mortality after 90 days and need for RRT |
| Secondary endpoints | Renal failure and need for CRRT | |
| Outcome of the primary endpoint | No differences in mortality (including the 6 predefined subgroups) | Greater mortality and need for RRT |
| Outcomes of the secondary endpoints | Greater incidence of ARF and need for RRT |
CRRT: continuous renal replacement therapy (RRT); ARF: acute renal failure.
However, in a randomized, double-blind study of severe trauma patients, the incidence of renal dysfunction was found to be significantly lower with the use of HES 130/0.4 compared with the control group administered saline solution in the patients with penetrating trauma, though without differences in the subgroup of closed trauma patients.50 No differences in mortality were observed. Likewise, a 15-day observational study including 3147 patients (34% of which received HES) concluded that the use of this expander, after adjusting for confounding variables, was not associated to increased deterioration of renal function or a greater need for renal replacement therapy.55
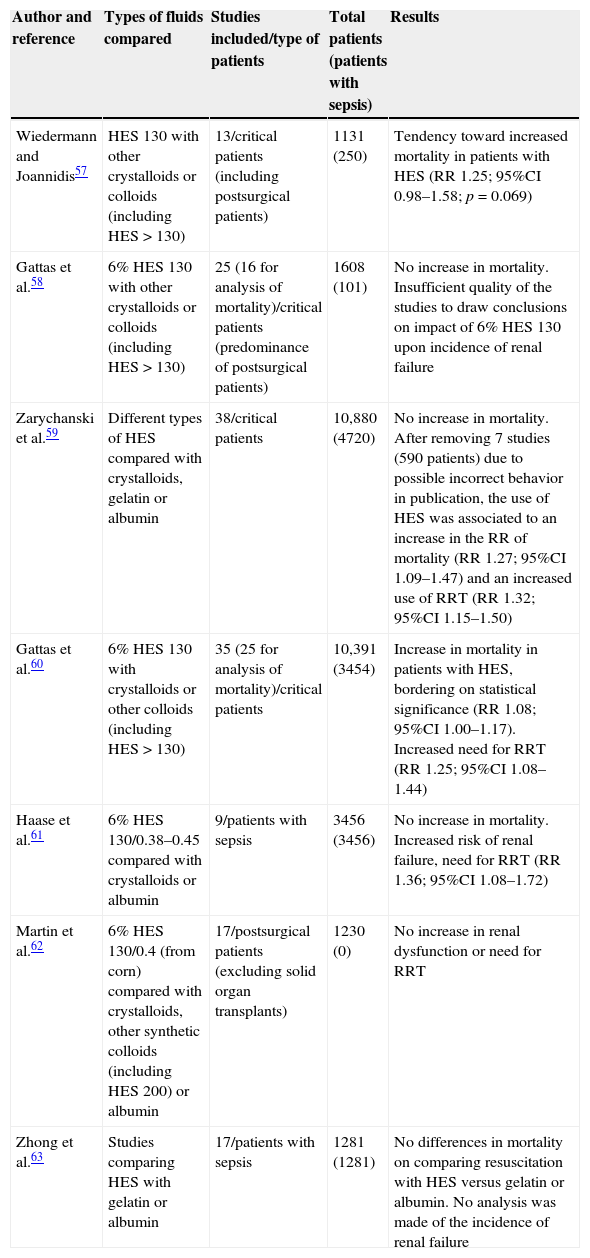
Perhaps there are few treatments as extensively studied as HES and which have generated such a large number of systematic reviews and metaanalyses, with doubts regarding the quality of many of them.56 In this respect, since the year 2012 a total of 6 metaanalyses have evaluated the effects of HES upon renal function and mortality compared with crystalloids and other colloids.57–62 A seventh metaanalysis only included the studies that had contrasted different colloids in the resuscitation of septic patients.63 The characteristics of these metaanalyses and the main conclusions drawn are summarized in Table 3.
Metaanalyses that have analyzed the impact of the use of hydroxyethyl starch (HES) upon mortality and the development of renal failure in critical patients.
| Author and reference | Types of fluids compared | Studies included/type of patients | Total patients (patients with sepsis) | Results |
|---|---|---|---|---|
| Wiedermann and Joannidis57 | HES 130 with other crystalloids or colloids (including HES>130) | 13/critical patients (including postsurgical patients) | 1131 (250) | Tendency toward increased mortality in patients with HES (RR 1.25; 95%CI 0.98–1.58; p=0.069) |
| Gattas et al.58 | 6% HES 130 with other crystalloids or colloids (including HES>130) | 25 (16 for analysis of mortality)/critical patients (predominance of postsurgical patients) | 1608 (101) | No increase in mortality. Insufficient quality of the studies to draw conclusions on impact of 6% HES 130 upon incidence of renal failure |
| Zarychanski et al.59 | Different types of HES compared with crystalloids, gelatin or albumin | 38/critical patients | 10,880 (4720) | No increase in mortality. After removing 7 studies (590 patients) due to possible incorrect behavior in publication, the use of HES was associated to an increase in the RR of mortality (RR 1.27; 95%CI 1.09–1.47) and an increased use of RRT (RR 1.32; 95%CI 1.15–1.50) |
| Gattas et al.60 | 6% HES 130 with crystalloids or other colloids (including HES>130) | 35 (25 for analysis of mortality)/critical patients | 10,391 (3454) | Increase in mortality in patients with HES, bordering on statistical significance (RR 1.08; 95%CI 1.00–1.17). Increased need for RRT (RR 1.25; 95%CI 1.08–1.44) |
| Haase et al.61 | 6% HES 130/0.38–0.45 compared with crystalloids or albumin | 9/patients with sepsis | 3456 (3456) | No increase in mortality. Increased risk of renal failure, need for RRT (RR 1.36; 95%CI 1.08–1.72) |
| Martin et al.62 | 6% HES 130/0.4 (from corn) compared with crystalloids, other synthetic colloids (including HES 200) or albumin | 17/postsurgical patients (excluding solid organ transplants) | 1230 (0) | No increase in renal dysfunction or need for RRT |
| Zhong et al.63 | Studies comparing HES with gelatin or albumin | 17/patients with sepsis | 1281 (1281) | No differences in mortality on comparing resuscitation with HES versus gelatin or albumin. No analysis was made of the incidence of renal failure |
Based on all these data, in late 2013 both the European Medicines Agency (EMA) and the Spanish Drug Agency (AEMPS) recommended the avoidance of solutions containing HES in critical patients. The AEMPS allows the use of HES only in the case of hypovolemia secondary to acute hemorrhage, and with the obligate follow-up of renal function during at least 90 days.64
Posteriorly, a randomized study (the CRISTAL trial) comparing colloids (HES, gelatins, dextrans or albumin) versus crystalloids in the resuscitation of critical patients with hypovolemic shock found no differences in adverse events (including the development of renal failure) or mortality. The mortality rate after 90 days was significantly lower in the group that received colloids, but without differences in mortality after 28 days (primary endpoint). The sub-analysis of those patients who received HES (approximately 60% of the subjects randomized to colloids) did not show its use to be associated to greater mortality or renal failure in either the global cohort or in the patients with sepsis (55% of the sample).65
Recommendations- 1.
It is advisable not to use HES solutions in resuscitation of the critical patient, in view of the evidence relating HES to increased morbidity–mortality (development of renal failure).
- 2.
In particular, its use is not advised in individuals at a high risk of developing renal failure, such as patients with severe sepsis or septic shock.
- 3.
If rapid volume expansion (and therefore the use of a colloid) is considered necessary, we recommend the use of other alternatives such as gelatins or albumin.
The gelatins have been used as plasma expanders for decades. However, in this case the available information is much more limited than in relation to HES, particularly as regards safety issues, as has been evidenced by a recent metaanalysis that has underscored the need for further well designed studies on safety, especially in relation to the development of renal failure.66
A number of studies in postsurgical patients have evaluated the use of gelatins versus crystalloids, and have observed no differences in terms of complications or mortality.7,67 In critical patients, the sub-analysis of those who received gelatin in the CRISTAL trial revealed no differences in mortality, renal failure or the need for renal replacement techniques versus those resuscitated with crystalloids.65
Relatively few clinical trials have compared gelatin versus HES in critical patients. A randomized, open-label trial involving 129 patients with severe sepsis concluded that the use of 4% gelatin is associated to a lesser incidence of acute renal failure than the use of HES 200/0.6, though without differences in terms of mortality.68 A clinical trial comparing these two volume expanders in the resuscitation of brain dead donors found the renal failure rate to be greater among the recipients of kidneys from donors who had been administered HES 200/0.6.69
On the other hand, no clinical trials have compared the use of gelatin versus low molecular weight and substitution ratio HES (130/0.4) in the resuscitation of critical patients. A study contrasting resuscitation with 4% gelatin versus two different HES solutions (130/0.4 and 200/0.5) in patients subjected to surgery of the abdominal aorta concluded that the use of gelatin is associated to a greater incidence of postoperative renal dysfunction, with no differences between the two HES formulations.70
An observational study in patients with severe sepsis found the incidence of renal failure and the need for blood products to be significantly greater with HES 130/0.4 (n=360) or 4% gelatin (n=352) than when administering crystalloids (n=334).71 These same authors conducted an observational study in heart surgery patients, with similar conclusions.72 However, in a study of 1013 patients admitted to the ICU with shock, the administration of hyperoncotic colloids (dextrans or HES) was associated to an increased incidence of renal failure after adjusting for confounding variables (odds ratio [OR]: 2.48 [1.24–4.97])–a situation not seen with the use of hypooncotic colloids (4% gelatin).73
The recommended maximum dose when administering gelatins has not been established. On examining the published studies, the administered doses are seen to vary between 10 and 45ml/kg.7,63,71,72,74 An observational study compared two periods in critical surgical patients. In the first period HES 130/0.4 was used (n=1383), while in the second period 4% gelatin was administered (n=1528). The use of either HES or gelatin was not intrinsically identified as being associated to mortality or renal failure, though an association to both events was identified when either expander was administered at doses >33ml/kg. In the subgroup of patients with sepsis, the mortality rate was higher in the HES group than in the gelatin group (42.5% vs 26.7%; p=0.053). Likewise, in this subgroup of patients with sepsis, the administration of HES or gelatin at doses >33ml/kg was associated to an increased risk of death and renal failure.75
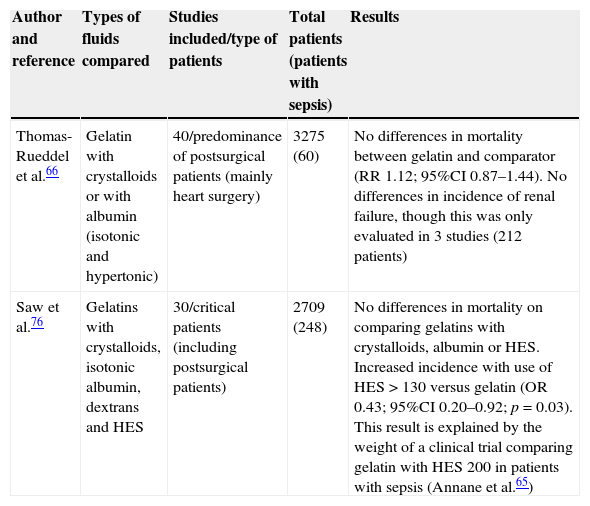
A metaanalysis has examined the prognostic impact of gelatin use in critical surgical patients. When compared with high molecular weight HES, the gelatins were seen to result in lesser renal dysfunction, though no differences in terms of mortality or the development of renal failure were noted on comparing them with crystalloids or albumin. In contrast, a higher incidence of bleeding was recorded.76 The main conclusions of two recent metaanalyses of the efficacy and safety of gelatins are summarized in Table 4. A recent consensus conference does not advocate the use of gelatins in patients with severe sepsis or at a high risk of developing renal failure.3
Summary of the metaanalyses that have evaluated the efficacy and safety of gelatin use.
| Author and reference | Types of fluids compared | Studies included/type of patients | Total patients (patients with sepsis) | Results |
|---|---|---|---|---|
| Thomas-Rueddel et al.66 | Gelatin with crystalloids or with albumin (isotonic and hypertonic) | 40/predominance of postsurgical patients (mainly heart surgery) | 3275 (60) | No differences in mortality between gelatin and comparator (RR 1.12; 95%CI 0.87–1.44). No differences in incidence of renal failure, though this was only evaluated in 3 studies (212 patients) |
| Saw et al.76 | Gelatins with crystalloids, isotonic albumin, dextrans and HES | 30/critical patients (including postsurgical patients) | 2709 (248) | No differences in mortality on comparing gelatins with crystalloids, albumin or HES. Increased incidence with use of HES>130 versus gelatin (OR 0.43; 95%CI 0.20–0.92; p=0.03). This result is explained by the weight of a clinical trial comparing gelatin with HES 200 in patients with sepsis (Annane et al.65) |
- 1.
In patients suffering shock (with the exception of septic shock) and who require rapid volume expansion, the use of 4% gelatin should be considered.
- 2.
If gelatin is chosen, the succinylated form is advised, due to its better safety profile.
- 3.
Because of the uncertainties regarding safety, we do not recommend exceeding a maximum dose of 30ml/kg of 4% succinylated gelatin during the resuscitation phase.
In 2004, the results of the randomized study Saline versus Albumin Fluid Evaluation in 7000 critical patients showed a 4% albumin solution to be as safe as saline solution as resuscitation fluid, though with no impact upon the prognosis.20
However, a sub-analysis of this clinical trial revealed possible benefit from using albumin in the 1218 patients with severe sepsis. In the multivariate analysis, the adjusted mortality risk was significantly lower (OR 0.71; 95% confidence interval [95%CI] 0.52–0.97; p=0.03) in the patients who received albumin than in those resuscitated with saline solution.77 Moreover, a metaanalysis including 17 randomized studies comparing albumin versus other fluids (crystalloids or synthetic colloids) revealed a decrease in mortality (OR 0.82; 95%CI 0.67–1.0; p=0.047).78 In view of the above, different clinical practice guides recommend the use of albumin in the resuscitation of patients with severe sepsis or septic shock, particularly in the event of no response to crystalloid infusion.3,79
More recently, a multicenter, open-label clinical trial (the ALBIOS study) randomized 1818 patients with severe sepsis or septic shock to 300ml of 20% albumin or crystalloids as resuscitation fluid, followed by additional doses of 20% albumin in order to maintain albuminemia >30g/l. There were no differences in mortality on day 28 (32% albumin vs 32% crystalloids) or on day 90 (41% vs 44%). However, the subgroup of patients with septic shock who received albumin suffered less mortality after 90 days (43% vs 48%; p=0.03).80 Lastly, it should be mentioned that in patients with sepsis, a sophisticated metaanalysis concluded that resuscitation with albumin is associated to lesser mortality than the use of crystalloids or staches.48
In a posterior analysis of the Saline versus Albumin Fluid Evaluation study, the patients with traumatic brain injuries treated with albumin showed greater mortality,81 possibly as a result of the fact that albumin can increase intracranial pressure, at least during the first week.82
In another type of patients, Sort et al.83 published the results of a randomized clinical trial involving 126 patients with cirrhosis and spontaneous bacterial peritonitis. The patients who received albumin as plasma expander suffered less renal failure and less in-hospital mortality. A metaanalysis of 16 randomized studies showed albumin to be associated to a decrease in mortality (OR 0.46; 95%CI 0.25–0.86) and renal failure (OR 0.34; 95%CI 0.15–0.75) in patients with cirrhosis and infection.84 Likewise, the use of albumin reduces the development of shock and patient mortality after paracentesis in situations of tense ascites.85 In fact, liver disease is currently the main indication for the use of albumin in Spanish ICUs – its administration in patients with severe sepsis or septic shock being exceptional.86
There are limitations to the use of albumin, such as the low quality of the evidence on its effectiveness, high cost, and the potential risk of microorganism transmission.87 Albumin therefore should not be used as resuscitation fluid on a routine basis, though it can be administered in certain groups of patients.
Recommendations- 1.
The administration of albumin in the critical patient is not associated to demonstrated adverse effects, though it should be reserved for specific patient groups in which it has been shown to offer benefit.
- 2.
It is advisable to consider albumin in the resuscitation of septic shock patients who fail to respond to crystalloids.
- 3.
Albumin should not be used in patients with traumatic brain injuries.
- 4.
The administration of albumin should be considered in patients with cirrhosis and spontaneous bacterial peritonitis.
As has been commented, there are still many unresolved aspects regarding the type of fluids (both crystalloids and colloids) to be used for resuscitation of the critical patient. In relation to colloids, and based on the positive data of the CRISTAL trial,65 we consider that it is not possible to categorically discard the use of such products in patients in shock. We thus propose that further studies should be made to evaluate the efficacy and safety of albumin and gelatins, or that patient subgroups should be identified in which colloids can be used in view of the low risk of renal failure. On the basis of what has been reviewed in the present study, we propose the following future lines of research:
- -
Well designed clinical trials and studies are needed to compare the different crystalloids, evaluating their impact upon clinical parameters, together with cost-effectiveness studies.
- -
Clinical studies are needed to assess the impact upon mortality of the use of gelatins and to confirm their safety, particularly as regards the development of renal dysfunction.
- -
It must be established whether albuminemia is to be considered in the decision to use albumin for resuscitation purposes in general among critical patients, and in particular in cases of severe sepsis or septic shock.
- -
Studies should be made on the usefulness of renal failure biomarkers in defining those patients in which certain solutions associated to increased renal damage are to be avoided, and in establishing whether such solutions can be used in patients with a low risk of developing renal failure.
JGM has participated in counseling activities for B. Braun. EFM is scientific advisor to CSL Behring, B. Braun and a member of the MAB of Pulsion. RFR has participated in counseling activities for Grifols and B. Braun. MHC has participated in counseling activities for B. Braun. JALB has participated in counseling activities for B. Braun. SRS reports no conflicts of interest in relation to this manuscript. AA has participated in counseling activities for B. Braun and Laboratorios Rubio; in conferences organized by Grifols, Astute and Philips, and holds a research grant from Laboratorios Grifols.
The study group wishes to thank Dr. Jaime Latour for critical reading of this manuscript and for his valuable contributions and suggestions.
Please cite this article as: Garnacho-Montero J, Fernández-Mondéjar E, Ferrer-Roca R, Herrera-Gutiérrez ME, Lorente JA, Ruiz-Santana S, et al. Cristaloides y coloides en la reanimación del paciente crítico. Med Intensiva. 2015;39:303–315.