The Spanish Society of Critical Care Medicine (SEMICYUC) has recently published an updated version of Quality Indicators in Critical Care. Daily sedative interruption is included among them. As this practice is controversial, research studies are revised and guidelines for its implementation are proposed.
La SEMICYUC ha publicado recientemente una nueva revisión de los Indicadores de Calidad en el Enfermo Crítico, entre los cuales se cita la Interrupción diaria de la Sedación. Dada la controversia que esta práctica conlleva, se revisan los estudios realizados y se proponen unas recomendaciones para su implementación.
The updated version of the Critical Patient Quality Indicators1 has recently been published, based on the document produced in the year 2005. In this important publication, representatives of the Working Groups (WGs) of the SEMICYUC have collected the evidence and searched for standards with the purpose of offering tools for improving quality–the coordinators of the project recommending each individual Department to adopt those principles which best adapt to the areas where improvement is sought.
Considering the participation of the many experts and the strict methodology used in elaborating the indicators, their suitability and convenience are not questioned. These indicators will allow Intensive Care Units (ICUs) to quantify and improve concrete aspects of critical patient care. The purpose of my comments is to address the implementation of indicator number 65 (daily interruption of sedation (DIS)), which is often a source of controversy.
In the year 2000, Kress published his pioneering work on DIS,2 in the context of a randomized clinical trial (RCT) involving 128 medical patients in which comparison was made of sedoanalgesia management according to the criterion of the attending clinician versus DIS. In the DIS group the perfusions were interrupted daily until the patient woke up, and at this point the decision was made to either extubate or resume sedoanalgesia at half of the previous dose. This approach resulted in a decrease in mechanical ventilation (MV) time from 7.3 to 4.9 days, with a shortening of ICU stay from 9.9 to 6.4 days, no increase in complications, and with a reduction of the altered consciousness evaluation tests.
Immediately after publication of the mentioned study, several authors expressed concern about the safety of the procedure, pointing to the possibility of cardiovascular side effects, confusion and withdrawal syndrome,3 psychological sequelae,4 or oversedation of the control group.5
We will analyze later studies on DIS in terms of whether its effectiveness and safety are generalizable. In addition to mortality, we must document the complications suffered by the patients, including psychological alterations, confusion, patient caused removal of devices (tubes, probes, catheters) and the precipitation of myocardial ischemia. We must assess whether DIS is applicable to all types of patients or whether there are contraindications to its use. Effectiveness would be demonstrated by the capacity to reduce stays and MV time, while efficiency could be assessed from the impact of DIS upon drug consumption and the need for nursing personnel support.
The group led by Kress has published a number of studies addressing these issues. In reference to the psychological repercussions of DIS, a battery of tests was applied by a team of psychologists to a small series of patients6 (18 of the original series and 14 later subjects), assessing awareness of MV, depression, anxiety, post-traumatic stress and residual consequences. Post-traumatic stress was diagnosed in 6 out of 19 cases without DIS versus in none of 13 cases with DIS (p=0.06)–the authors concluded that DIS is favorable in reducing the psychological sequelae of stay in the ICU.
The same group retrospectively analyzed the original series, establishing comparisons between the DIS group and the control group of the incidence of 7 complications: ventilator-associated pneumonia, upper digestive bleeding, bacteremia, barotrauma, venous thromboembolism, cholestasis and sinusitis requiring surgery.7 The authors documented 13 complications in 66 patients in the DIS group versus 26 complications in 60 patients in the control group (p=0.04), and concluded that DIS lessens complications in the critical patient. It can be criticized that this study was made in a pre-selected group of subjects with known differences in the duration of MV and stay. Given the longer exposure time, it is not surprising that there were more complications in the patients with longer stays in the ICU. This protective effect would not appear in Units that do not reduce MV time on applying DIS.
The same research group has designed an observational study assessing the effect of DIS upon myocardial ischemia.8 In 74 patients with at least two cardiovascular risk factors or with antecedents of coronary disease, the authors established comparisons of variables related to myocardial ischemia at baseline and during DIS: blood pressure (BP), respiratory frequency (RF), heart rate (HR), double product (DP) and catecholamine levels (CL). They monitored cardiac enzymes and performed Holter recordings, assessing ST-segment elevations or depressions of >1mm. During DIS, elevations were recorded in BP, RF, HR, DP and CL, but ST-segment alterations appeared indistinctly at baseline or after DIS. Without being able to clarify the chronology of the cardiac enzyme elevations, the authors concluded that DIS is safe in coronary patients, and underscored the high prevalence of silent ischemia at baseline. It is arguable in ventilated patients whether ST-segment alterations can be related to myocardial ischemia when it is not possible to relate such alterations to cardiac enzyme elevations or no contrasts are made with other diagnostic methods such as echocardiography.
In order to evaluate the global applicability of DIS, the experiences of other groups must also be investigated. Mehta9 compared the management of protocolized sedation with or without DIS in 65 medical-surgical patients in three Canadian ICUs. No differences were found in terms of adverse effects, mortality, duration of MV or stay in the ICU. Lesser midazolam doses were used in the DIS group. This was a pilot study showing the applicability of DIS added to routine sedation. The small number of patients involved probably precluded the detection of differences in most of the variables. It is of interest to mention some of the exclusion criteria of the study, since they were referred to patients in which DIS was not considered advisable: neuromuscular block, alcohol or drug abuse, psychiatric disorders, neurological disease, and cardiorespiratory arrest. Posteriorly, acute myocardial infarction was included as an exclusion criterion, on the grounds that one patient suffered infarction upon interrupting sedation. Of note is the high incidence of adverse effects, particularly in relation to device removal, despite the fact that each day 40% of the patients were subjected to physical constraints (6 cases of self-extubation, four cases of nasogastric tube removal, and one case of central venous catheter removal).
On the third day after discharge, a questionnaire was administered to the patients, assessing their experience of stay in the ICU.10 Over one-half of the subjects reported anxiety, pain, fear and lack of sleep. In turn, 29–48% did not remember specific maneuvers (aspirations, daily hygiene, connection to MV). Since only 21 patients were interviewed, it is not possible to establish reliable comparisons between the two protocol arms. Further information probably will become available once the full study has been published, with the estimated inclusion of 410 cases.
A RCT conducted in Greece compared routine sedoanalgesia with DIS in 97 medical, surgical and trauma patients11–no differences being observed in the duration of MV, the stay in ICU, or global hospital stay. It should be noted that routine practice in that Unit comprised analgesia with remifentanil and the adjustment of midazolam or propofol, attempting to minimize diminished consciousness.
Another North American RCT compared DIS with sedation adjustment based on a nursing-implemented sedation algorithm (NSA).12 Before starting the trial, the nursing personnel was trained in the use of the algorithm. Although the trial had been designed for 268 patients, it was concluded due to safety reasons after including 74 cases. In contrast to the findings of previous studies, the DIS group showed a longer duration of MV (6.7 versus 3.9 days, p=0.0003), a longer stay in the ICU (15 versus 8 days, p<0.0001), a longer global hospital stay (23 versus 12 days, p=0.01), and even lesser survival without MV after 28 days (16 days versus 23, p=0.004). The resolution of organ dysfunction as assessed by the SOFA score was faster in the NSA group. In addition to demonstrating that correct perfusion adjustment (for which nursing intervention is essential) can offer advantages over DIS, the study underscored important safety shortcomings associated with the systematic use of DIS. In this context, extreme tachypnea appeared on 22 occasions, and difficult to control patient restlessness on 47 occasions (in reference to 79 DIS episodes), with requests for patient withdrawal from the study made by both the patient relatives and even the supervising physician.
A RCT was carried out in four ICUs in the United States, including the participation of the group led by Kress, in which DIS was associated to the spontaneous ventilation test (SVT).13 An evaluation was made of daily SVT in 336 medical patients randomized to either conventional sedation or DIS−adequate performance being defined by the maintenance of SpO2≥88% with FiO2≤50% and PEEP≤8cm H2O, the presence of inspiratory effort, the absence of myocardial ischemia, no need for high inotropic or vasopressor drug doses, and the absence of intracranial hypertension. SVT consisted of 2h of T-tube with low pressure support–extubation being decided by the supervising clinician, not the investigator. The patients in the DIS group were previously evaluated for suitability of sedoanalgesia suspension–the latter being considered contraindicated in the case of neuromuscular block, alcohol privation, seizures, myocardial ischemia, restlessness or intracranial hypertension. Those patients without these conditions were subjected to DIS, and were followed up on for 4h to decide whether the test had failed (with restoration of perfusion at half the dose) or was successful (followed by evaluation of SVT as previously described). The DIS group showed a reduction of 3.1 days of MV, with ICU discharge 3.8 days earlier, and hospital discharge 4.7 days earlier. The mortality rate after one year was 44% in the DIS group versus 58% in the control group, the calculated number needed to treat (NNT) being 7. Self-extubation was more frequent in the DIS group (10% versus 4%, p=0.03). Another interesting aspect of the study was the systematic assessment of confusion based on the Confusion Assessment Method for the ICU (CAM-ICU), with the observation of a high incidence in both protocol arms (74% DIS and 71% control), with a duration of about two days in both instances.
In this study, DIS showed advantages, shortening MV and stays, though it must be taken into account that sedoanalgesia in the control group was not clearly explained or protocolized. The study did not include surgical patients, who might not tolerate the interruption of analgesia. Moreover, the criteria for SVT are debatable–some authors raising the question of whether the difference in mortality might have been conditioned by multiple failures in insufficiently prepared patients (456 SVTs failed in the group control versus 284 in the DIS group).14
A Turkish RCT compared DIS versus sedation based on the NSA in 50 patients,15 excluding neuromuscular block, severe head injuries and meningoencephalitis. Although no differences were observed in terms of stay or mortality, the patients in the DIS group were sedated for less time and were on MV for shorter periods than in the control group. This study has important design shortcomings (there is no sample size estimation, the patients are not homogeneous, etc.) and implementation problems (non-specified sedation boluses, scantly adequate drug substances, etc.). The authors recognized that the nursing personnel was not well prepared for using the protocol, and that the low ratio between the number of nurses and patients complicated application of the algorithm. Nevertheless, the study does provide clues suggesting that when capacity to strictly adjust sedoanalgesia is inadequate (because of a lack of training, knowledge or resources), DIS can help prevent drug accumulation and thus accelerate weaning from MV.
The first RCT on DIS involving a double-blind design is currently underway in Australia. Only data on the preliminary phase have been published,16 with lesser recruitment than expected, and frequent protocol violations that will delay the definitive results.
A meta-analysis has recently been published17 by authors with experience in applying this methodology to the sedation of critical patients. The analysis included 5 RCTs totaling 699 patients, and has been commented in REMI.18 In this study, DIS yielded no relevant clinical benefits and did not increase the complications. This result is not surprising, given the moderate quality and important heterogeneity of the studies included in the meta-analysis. In view of the lack of conclusive evidence, and considering the findings of the different studies which we have examined, the setting in which DIS is carried out (type of patients, characteristics of the Unit, MV protocol used, drugs selected and the way in which they are adjusted, etc.) probably exerts a decisive influence upon the results obtained with the technique. Based on this same reasoning, Shehabi criticizes the recommendation of DIS as standard practice,19 particularly considering that ICUs outside the United States follow a very different working and organizational model.
The reader may be asking himself whether or not to apply DIS in her/his own Unit, and if so, in what patients. I will try to reason my proposals. We first must take into account that there are patients in whom DIS is not indicated, as confirmed by the authors who developed the methodology.1 Some conditions and disorders are systematically excluded in the published trials, or have been shown to cause complications. Thus, DIS can be regarded as contraindicated in patients with neuromuscular block, intracranial hypertension, seizures, or difficult to control restlessness. Drugs such as remifentanil likewise cannot be suddenly suspended.
DIS can worsen the situation of hemodynamically unstable patients or individuals requiring important ventilatory support. The same applies in severe cases of abstinence syndrome (alcohol or benzodiazepines). In these cases, if the patient is found to be more deeply sedated than would be desirable, we can make use of “modified DIS”, i.e., without waiting for the patient to awaken before resuming perfusion, closely monitoring the depth of sedation, and resuming its administration on reaching the degree established as our objective. Some guides propose the management of sedoanalgesia keeping a Richmond Agitation Sedation Scale (RASS) score of between 0 and −2, and using DIS as rescue for patients that reach a RASS of under −3.20
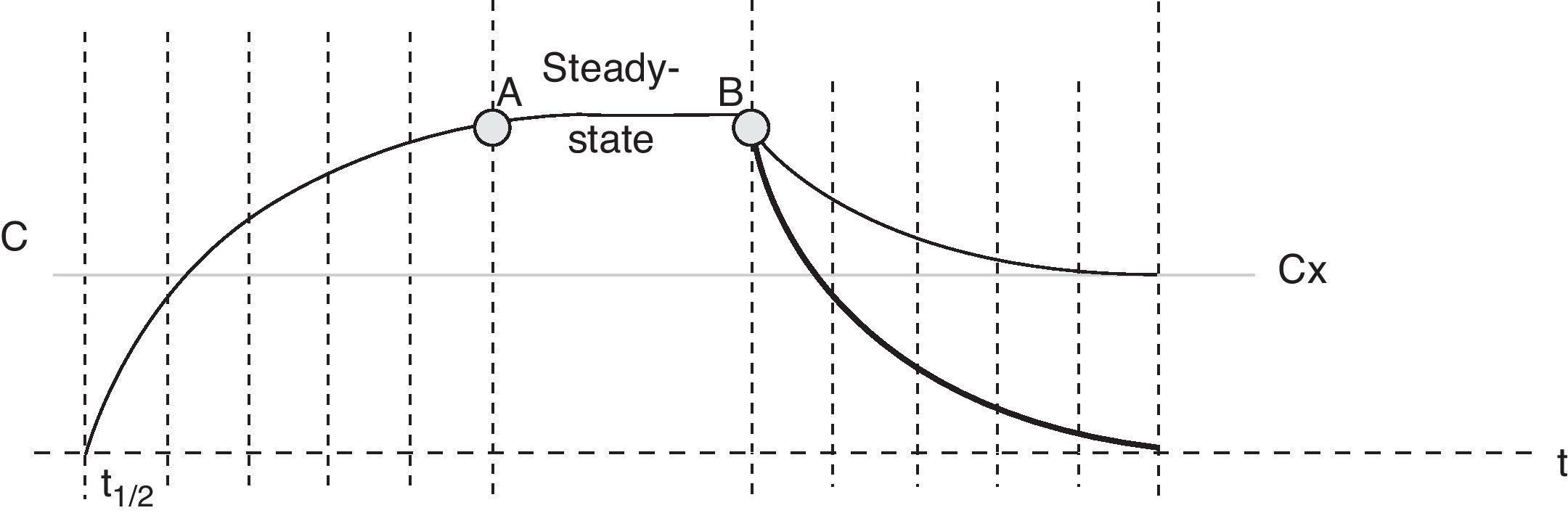
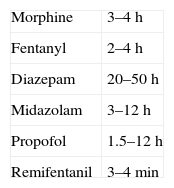
The recommendations of the Sedoanalgesia Working Group of the SEMICYUC stress the need for strategies designed to prevent drug accumulation.21 DIS is one such strategy, and the concept which I have described as “modified DIS” is simply a basic application of pharmacokinetics. When the perfusion of a sedative or opiate is started, the drug is distributed within the different body compartments (including the site of drug action), while at the same time the clearance systems partially eliminated the drug present in the body. The steady-state condition is reached when sufficient drug has been accumulated in the body to cause the perfused dose to equal the amount eliminated. The time taken to reach this equilibrium or balance follows an exponential curve proportional to the elimination half-life of the drug substance (t1/2β)–four or 5 such half-lives being required in order to assume stable conditions (93.75% of plasma concentration in equilibrium with four t1/2β, and 96.875% with 5 half-lives). The same applies when adjusting the perfusion, i.e., we will need 4–5 t1/2β to reach the new steady state. It must be remembered that some commonly used drugs have long t1/2β values (Table 1), and this time is extended even further when prolonged infusions are used. In this case we speak of context-sensitive half-life. As an example, the context-sensitive half-life in the case of fentanyl can triple the t1/2β.22 As can be seen in Fig. 1, the plasma concentration of the drug can be lowered much sooner by stopping than by adjusting perfusion.
Time–plasma concentration curve of a sedating agent administered as a perfusion. After 4–5 half-lives (t1/2), the concentration reaches the steady-state condition (point A), where equal amounts of drug substance are administered and eliminated simultaneously–the plasma concentration thus remaining constant. At point B the decision is made to reduce the amount of drug in the patient with the purpose of reaching the effect corresponding to plasma concentration “x” (Cx). The thin tracing shows the drop in plasma concentration on readjusting the infusion, while the thick tracing corresponds to suspension of the perfusion.
Another important issue is to ensure pain control. In most studies, sedation and analgesia are interrupted simultaneously. This could come into conflict with the recommended practice of ensuring analgesia before assessing the sedation needs,23–27 or with the concept of sedation based on analgesia.28 A way of interpreting incomplete DIS can be to suspend perfusion of the sedative while maintaining perfusion of the opiate. However, certain opiates can accumulate, and the interruption of perfusion can help adjust the correct levels. It must be remembered that monitoring the degree of sedation is not enough: a validated scale for assessing pain must also be used.29 This is a good opportunity to demonstrate that the Quality Indicators can be summative; number 67 refers to Management of analgesia in the patient subjected to MV.1
Mention must be made of the ease with which critical patients experience drug accumulation. Adapting such patients to MV initially may require high sedative and analgesic doses. Posteriorly, despite the lowering of such medication, the drug substances accumulate as a result of dysfunction of the clearance systems–thus producing a delay in regaining levels of consciousness adequate for starting weaning from ventilation, as indicated by Quality Marker number 64 (Adequate sedation), regarded as relevant.1 Excellence in the management of sedoanalgesia consists of keeping the patient free of pain at all times and with the desired degree of sedation (always attempting to secure as little lowering of consciousness as possible); this in turn requires close monitorization, with optimum perfusion adjustment by trained and motivated personnel. However, even in the best of ICUs, some patients may accumulate sedation–hence the possibility of using DIS as a rescue measure. As has been seen in several studies, there is no conflict in the joint adjustment of sedation under strict monitorization with DIS. Even the pioneering authors of the DIS protocol accept this approach.30 In conclusion, Table 2 presents a proposal for integrating both strategies in routine practice, with the hope that it may serve as a working basis for those Units that decide to implement Quality Indicator number 65.
Implementation of DIS added to other recommended sedoanalgesia practices.
| 1. Sedoanalgesia protocol known and accepted by all the healthcare personnel in the ICU. Standardized perfusions. |
| 2. Daily assessment of the sedoanalgesia strategy best suited to each patient, with clear definition of the degree of sedation to be maintained. Assessment of the possibility of interrupting perfusions. Objective of maintaining the patient with as little lowering of consciousness as possible. |
| 3. Adjustment of MV to facilitate adaptation of the patient before deepening the level of sedation. |
| 4. Systematic nursing monitorization of analgesia and sedation (and of neuromuscular block and confusion, where applicable) using validated tools, with graphic registry. |
| 5. Maintenance of the objectives of avoiding pain, with adequate sedation, based on nursing adjustments of the perfusions and/or the administration of boluses following an easily interpretable algorithm. |
| 6. Alertness to the ease with which critical patients accumulate sedatives and opiates. |
| 7. In the case of deeper sedation than desired, despite two or more successive adjustments, interrupt perfusion of the sedative and analgesic, unless there are contraindications. |
| 8. If the patient course suggests that weaning from MV can be tolerated, interrupt perfusion of the sedative and analgesic, unless there are contraindications. |
| 9. Whenever perfusion of the sedative and/or analgesic is interrupted, maintain close vigilance: |
| - Assess the hemodynamic (including signs of myocardial ischemia) and respiratory repercussions |
| - Readjust MV to facilitate adaptation |
| - Detect signs of abstinence |
| - Detect pain |
| - Detect restlessness |
| - Evaluate the possibility of spontaneous ventilation testing |
| 10. If the patient requires the resumption of sedation, consider the possibility of a new strategy: |
| - Define a new target sedation level |
| - Vary the drug doses |
| - Switch to drugs with a shorter half-life (sequential sedation) |
| - Switch to drugs with a different clinical profile (remifentanil, dexmedetomidine, etc.) |
The author is a member of the Advisory Board of Laboratorios Orion-Pharma, S.L.
Please cite this article as: Muñoz-Martínez T. Interrupción diaria de la sedación; ¿ siempre es un indicador de calidad? Med Intensiva. 2012;36:288–93.