Delirium reveals itself in an acute and fluctuating clinical presentation whose most important characteristic is inattention accompanied by disorganized thinking or an altered level of consciousness. It can be hyperactive, hypoactive (the most common one) or else have a mixed clinical presentation. The prevalence of delirium in the intensive care setting (ICS) is extremely variable: it affects 30%–80% of critically ill patients and its presence is associated independently—according to some authors—with a higher mortality rate, higher healthcare costs, and an extended length of stay. Also, these patients have a long-term higher risk of cognitive impairment, and a higher incidence rate of mental state disorders like anxiety and depression.1
Advanced age, the existence of high severity scores, and prolonged mechanical ventilation are among the risk factors associated with the development of delirium especially in patients with acute respiratory distress syndrome (ARDS), deep sedation, and use of benzodiacepines.2
The COVID-19 pandemic caused by SARS-CoV-2 has been associated with an extremely high prevalence of delirium especially in patients with ARDS on mechanical ventilation who have been affected in up to 80% of the cases.1
The type of predominant delirium in these patients was the hyperactive type characterized by a state of unusual agitation after the withdrawal of sedatives that is difficult to control and sometimes has serious repercussions like self-extubations.
The biggest prevalence of delirium in these patients with COVID-19 is due to a confluence of factors that have traditionally been associated with the development of delirium and other aspects that are very present in this group of patients like pain, fear, anxiety, familial isolation, sleeplessness, and prolonged immobilization.3 This adds to the damage caused to the central nervous system by SARS-CoV-2 or direct neural damage4,5 or immonulogical mechanisms. Therefore, all these patients should be considered high-risk patients for the development of delirium, which leads to having to implement early on measures for identification, prevention, and treatment purposes.6
Identifying delirium in the critically ill patient can be done fast and easy using validated scales like the CAM-ICU (Confusion Assessment Method for ICU) or the ICDSC (Intensive Care Delirium Screening Checklist). Despite of these scales, in general, the search for delirium is a rare practice in the ICS,7 something that has probably exacerbated during the COVID-19 pandemic due to, among other factors, issues with prioritizing and isolating patients, the collapse of the healthcare system, the hiring of untrained personnel, and the use of personal protection equipment.8 Therefore, it is likely that the prevalence of delirium in critically ill patients infected with SARS-COV-2 may have been higher than previously thought, meaning that its long-term consequences can also be significant.1
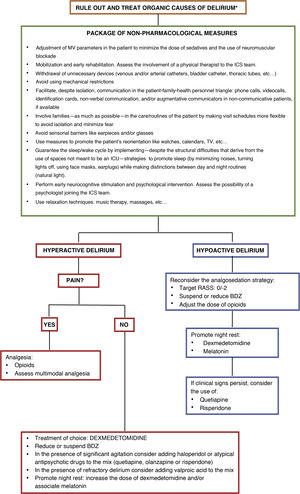
Although, to this date, no pharmacological treatment has proven effective to prevent delirium in patients with SARS-CoV-2, the implementation of general non-pharmacological measures—recommended both for prevention and treatment purposes—can be effective (Fig. 1)3 paying special attention to a few distinctive aspects of this pandemic.
Treatment of delirium. Non-pharmacological recommendations and pharmacological approach.
* Rule out organic causes of delirium such as hypoxemia, hypercapnia, kidney or liver failure, shock, sepsis, metabolic acidosis or hiydroelectrolytic alterations. Dose of dexmedetomidine for the management of hyperactive delirium: between 0.2 mcg/kg and 1.4 mcg/kg per hour. Dose of haloperidol: 2.5 mg–5 mg IV. It can be repeated every 10 min–30 min up to a cumulative dose of 30 mg. Early dose of quetiapine: 25 mg/8−12 h PO increasing by 25 mg per dose on a daily basis. Early dose of olanzapine: 5 mg/24 h PO. Early dose of risperidone: 1 mg/24 h PO. Dose of valproic acid: 1200–1600 mg/day IV throughout 3–4 takes that can be preceded by a 28 mg/kg load. Dose of melatonin: starting from 2 to 4 mg PO administered 1 to 2 h before night rest.
BDZ, benzodiazepines; RASS, Richmond Agitation Sedation Scale.
Regarding the traditional package of ABCDEF measures that has been associated with less delirium in critically ill patients on mechanical ventilation,9 its implementation poses multiple barriers in patients with COVID-19.3 Among them the need for deep sedation and neuromuscular blockade, patients being spread out across different units, the hiring of new and untrained personnel, the heavier workloads sustained, and the use of personal protection equipment. It all adds to the fact that family visits were absolutely restricted.3,4 Therefore, it is necessary to adequate the ABCDEF measures to the special characteristics of patients with SARS-CoV-2 by incorporating a new letter (R) that refers to how important adaptation to mechanical ventilation is in these patients to avoid asynchronies (ABCDEF-R, Table 1).10
Package of ABCDEF-R measures adapted to patients with COVID-19.
| A: Analgesia as the priority in the patient with COVID-19 | 1. Try monitoring pain on a routine basis (at least every 8 h) despite the existence of barriers |
| Pain as a significant cause for delirium | • Communicative patients: numerical scales |
| In patients with COVID-19, the continuous monitorization of pain becomes complicated by factors such as isolation, work overload or the lack of experience of the new personnel involved, among others | • Non-communicative patients: behavioral scales (BPS, CPOT, BPS) |
| Analgesia should be a priority and pain should be presumed in all the patients. If possible, pain should be anticipated, and pain assessment should be made especially in patients with severe ARDS, deep sedation, neuromuscular blockade or prone position | • RASS − 4/−5: objective methods (ANI®/NOL® or video pupillometry) |
| 2. Drugs: | |
| • Opioids like drugs of choice | |
| • In patients with an extended administration of opioids in whom progressive increases of the doses are needed the appearance of tolerance phenomena should be anticipated | |
| • Use multimodal analgesic strategies to help reduce the dose of opioids, side effects, and any tolerance phenomena that can occur | |
| • Identify and treat neuropathic pain (gabapentin, carbamazepine or pregabalin), which is common in patients with COVID-19 due to the possible viral invasion of peripheral nerves, prolonged immobilization, and use of the prone position | |
| • Avoid using NSAID as much as possible since they are associated with phenomena of coagulopathy, platelet dysfunction, kidney failure, and deleterious effects on prostaglandins | |
| 3. Consider non-pharmacological interventions to reduce pain and spare opioids (massages, music therapy, and relaxation techniques) | |
| B: Daily search for cooperative sedation and weaning from MV | 1. Monitor sedation, at least, 3 times a day: |
| Prevent the systematic use of deep sedation in all patients with COVID-19 on MV | • Scales: RASS or SAS |
| In cases where deep sedation is required—whether associated or not with NMB—the daily reassessment of the actual need for these measures is advised, as well as the use of objective monitorization to prevent phenomena of under and oversedation | • In deep sedation (RASS −4/−5) and NMB monitorization with BIS® is advised (target values: 40−60) to prevent under and oversedation phenomena |
| 2. Establish daily targets regarding deep sedation adapted to the patient’s clinical situation by prioritizing cooperative/dynamic sedation (RASS −2/−3) and sparing deep sedation for patients with severe ARDS or need for NMB only. | |
| 3. Use dynamic and sequential sedation strategies | |
| C: Proper selection of analgesic drugs and sedatives | 1. Prioritize the use of propofol in deep sedation and dexmedetomidine/remifentanil or propofol in moderate/mild sedation depending on the individual characteristics of each patient |
| When possible, avoid using benzodiazepines and use short half-life drugs (dexmedetomidine, remifentanil, propofol) to achieve sequential and dynamic sedation and early weaning from MV. | 2. Patients with COVID-19 can develop cytokine storm, which is somehow similar to hemophagocytic lympohistiocytosis and elevates the levels of triglycerides. Some authors recommend tolerating triglyceride levels of up to 800 μg/dL before propofol is withdrawn |
| 3. Consider the use of inhalation sedation (preferably isoflurane because it can be used for longer periods of time with fewer side effects) in deep sedation or in cases of difficult sedation due to its short wake-up time, lack of accumulation, non-use of sedatives and opioids, and their possible fewer effects on the long-term cognitive function. Although data is still scarce on this regard, inhalation sedation seems to be associated with anti-inflammatory properties and promote less airway resistance and pulmonary vasodilation, and an improved ventilation/perfusion ratio in patients with ARDS | |
| 4. Consider the use of ketamine as adjuvant drug in the presence of difficult sedation or in patients with refractory bronchospasm | |
| D: Diagnose, prevent, and treat delirium | 1. Monitor delirium using scales at admission, on a daily basis, and whenever there is a change in the patient’s mental status: |
| Prioritize the identification of delirium | • CAM-ICU |
| Encourage the use of prevention strategies to initiate early treatment measures, reduce the duration of delirium, and improve the short and long-term prognosis of these patients | • ICDSC |
| 2. Establish preventive measures of delirium in all the patients: | |
| Non-pharmacological measures (Fig. 1) | |
| Pharmacological measures: prevent or reduce the dose of sedatives, hypnotic, and anticholinergic drugs as much as possible | |
| 3. Apply early treatment with special emphasis in non-pharmacological measures (Fig. 1). | |
| 4. Monitor the QT interval regularly since the association of certain drugs used for the management of COVID-19 with opioids, haloperidol, and methadone can extend the QTc-duration. | |
| E: Promote rehabilitation and early moving | 1. Passive physical therapy in patients on deep sedation |
| Immobilization as an independent risk factor of delirium | 2. Respiratory physical therapy and extremity therapy when the clinical situation is favorable |
| Initiate early rehabilitation to prevent long-term cognitive impairment | |
| Get the physical therapist involved in the ICS | |
| F: Family involvement in the care and rehabilitation process of the patients | 1. Elaborate specific protocols regarding family visits that should be dynamically adapted to the evolution of the pandemic and abide by the current legislation |
| Family plays a key role to prevent delirium, and long-term cognitive impairment | 2. Whenever possible adopt flexible policies that, at the same time, should minimize the risk of infection to patients, family members, and healthcare workers by facilitating personal protection equipment (PPE) to family members and/or caregivers while teaching them how to use these |
| 3. In cases when on-site accompaniment is not possible (eg, quarantines) other communication alternatives should become available to facilitate contact between patients and their families like videocalls, letters, e-mails, phone calls, etc. | |
| 4. Always prioritize family accompaniment with proper PPE in patients at the end of their lives | |
| R: Adapt the ventilator to the patient, not vice versa | 1. Use MV strategies to prevent or minimize asynchronies |
| We should not assume that all patients on MV will require deep sedation and muscle relaxants | 2. Use a spontaneous ventilation modality with support when the clinical situation is favorable |
| 3. Prevent deep sedation and continuous NMB as much as possible | |
ANI®, Analgesia Nociception Index; ARDS, acute respiratory distress syndrome; BIS®, bispectral index; BPS, Behavioral Pain Scale; CAM-ICU, Confusion Assessment Method for ICU; CPOT, critical care pain observational tool; ICDSC, Intensive Care Delirium Screening Checklist; MV, mechanical ventilation; NMB, neuromuscular blockade; NOL®, NOciception Level index; NSAID, non-steroidal anti-inflammatory drugs; RASS, Richmond Agitation Sedation Scale; SAS, Sedation-Agitation Scale.
Also, there are few specific recommendations on the management of delirium (Fig. 1) for patients with COVID-19 admitted to the ICS.3,5 Same as it happens with its prevention, non-pharmacological strategies with multicomponent interventions can be useful to control delirium.
Before initiating pharmacological treatment, other organic causes or trigger factors of delirium should be ruled out such as pain, fear, anxiety (more common in patients with hyperactive delirium) or oversedation (more prevalent in cases of hypoactive delirium). If delirium persists after solving and ruling all these aspects of delirium out pharmacological treatment should be initiated based on the existing recommendations for the management of delirium in critically ill patients.5
In case of hyperactive delirium, the use of IV dexmedetomidine especially in patients whose delirium becomes complicated or delays the process of weaning from mechanical ventilation. Typical (haloperidol) or atypical (quetiapine, olanzapine) antipsychotic drugs should be spared for patients with hyperactive delirium as bailout drugs in situations of agitation, anxiety or hallucinations, and only as long as these symptoms persist.5 Valproic acid can be an option for patients with refractory symptoms to the usual antipsychotic drugs while melatonin can be useful in patients with COVID-19 and cytokine storm thanks to its immunomodulatory, neuroprotective, and sleep-regulatory properties, especially in elderly patients with low levels of melatonin, something that contributes to the appearance of delirium.3
Regarding the pharmacological treatment of hypoactive delirium, the best therapeutic options available are dexmedetomidine, quetiapine or risperidone to treat persistent symptoms plus adjusted analgosedation.
In conclusion, delirium is a common problem in the ICS, and it is even more common in patients with COVID-19. This added to the difficulties associated with this disease require a great effort regarding the prevention, diagnosis, and early management of delirium. Since, for the time being, we don’t know of any specific measures for the management of delirium in patients with SARS-CoV-2 the existing general recommendations should be implemented in critically ill patients while making a few adaptations based on the individual characteristics of each individual patient.
FundingNone whatsoever.
Conflicts of interestCarola Giménez-Esparza Vich received honoraria from Orion Pharma and Sedana Medical for her participation in lectures and congresses.
Sara Alcántara Carmona declared having received speaker fees as a lecturer and course manager from Orion Pharma.
Manuela García Sánchez declared having received speaker fees from Orion Pharma and Medtronic for his participation in lectures and congresses.
Please cite this article as: Giménez-Esparza Vich C, Alcántara Carmona S, García Sánchez M. Delirium y COVID-19. Aspectos prácticos de una frecuente asociación. Med Intensiva. 2022;46:336–340.