Catheter-related bloodstream infections (CRBSI) constitute an important cause of hospital-acquired infection associated with morbidity, mortality, and cost. The aim of these guidelines is to provide updated recommendations for the diagnosis and management of CRBSI in adults. Prevention of CRBSI is excluded. Experts in the field were designated by the two participating Societies (the Spanish Society of Infectious Diseases and Clinical Microbiology and [SEIMC] and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units [SEMICYUC]). Short-term peripheral venous catheters, non-tunneled and long-term central venous catheters, tunneled catheters and hemodialysis catheters are covered by these guidelines. The panel identified 39 key topics that were formulated in accordance with the PICO format. The strength of the recommendations and quality of the evidence were graded in accordance with ESCMID guidelines. Recommendations are made for the diagnosis of CRBSI with and without catheter removal and of tunnel infection. The document establishes the clinical situations in which a conservative diagnosis of CRBSI (diagnosis without catheter removal) is feasible. Recommendations are also made regarding empirical therapy, pathogen-specific treatment (coagulase-negative staphylococci, Staphylococcus aureus, Enterococcus spp., Gram-negative bacilli, and Candida spp.), antibiotic lock therapy, diagnosis and management of suppurative thrombophlebitis and local complications.
La bacteriemia relacionada con catéteres (BRC) es una causa importante de infección hospitalaria y se asocia con elevados morbilidad, mortalidad y costes. El objetivo de esta guía de práctica clínica es proporcionar recomendaciones actualizadas para el diagnóstico y tratamiento de la BRC en pacientes adultos. De este documento se excluye la prevención de la BRC. Expertos en la materia fueron designados por las dos Sociedades participantes (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica y Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias). Los catéteres venosos periféricos a corto plazo, los catéteres venosos centrales no tunelizados y de largo plazo, los catéteres tunelizados y los catéteres de hemodiálisis están incluidos en estas guías. El panel identificó 39 temas clave que fueron formulados de acuerdo con el formato PICO. La fuerza de las recomendaciones y la calidad de la evidencia se clasificaron de acuerdo con las directrices de la ESCMID. Se dan recomendaciones para el diagnóstico de BRC con extracción de catéter y sin él, y de la infección en túnel. El documento establece las situaciones clínicas en que es factible un diagnóstico conservador de CRBSI (diagnóstico sin retirada de catéter). También se dan recomendaciones respecto a la terapia empírica, el tratamiento específico según el patógeno identificado (estafilococos coagulasa-negativos, Staphylococcus aureus, Enterococcus spp., bacilos gramnegativos y Candida spp.), la terapia con sellado del catéter y el diagnóstico, así como tratamiento de la tromboflebitis supurativa y las complicaciones locales.
Intravascular devices have become an essential component of modern medicine for the administration of intravenous fluids, medication, blood products and parenteral nutrition and for monitoring hemodynamic status and providing hemodialysis. According to national data supplied by the study of the prevalence of nosocomial infections in Spain (EPINE), it is estimated that about 70% of patients admitted to Spanish hospitals will wear one of these devices at some point during their stay.1 Local or systemic infections represent one of the main associated complications.2 The incidence of catheter-related infections varies considerably depending on the type and intended use, the insertion site, the experience and training of the individual who places the catheter, the frequency with which the catheter is accessed, duration of catheter placement, the characteristics of the patient, and the use of proven prevention strategies. Catheter-related bloodstream infections (CRBSIs) are among the most frequent infections acquired in hospital. Current estimates are that between 15% and 30% of all nosocomial bacteremias are catheter-related.3 CRBSIs have significant associated morbidity, incur increased hospital costs,4 estimated at approximately 18,000 euros per episode, and length of stay.5 Attributable mortality ranges between 12% and 25%.6 In recent years, there has been a remarkable increase in our knowledge of the epidemiology of CRBSI and of the most appropriate methodologies for diagnosis, management and prevention. The vast amount of information accumulated and the inherent complexity of this type of infection make it necessary to sort and analyze the available information. At the same time, there are few current guidelines available on this topic. The last Spanish catheter-related infections guidelines were published in 2004.7 The aim of this new guide is to update recommendations for the diagnosis and management of catheter-related bloodstream infections. This document targets only microbiological diagnosis and antimicrobial therapy; other aspects of infection management and prevention are therefore excluded. Only adult patients with these infections are covered.
MethodsThe two participating Societies (the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica and the Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias) nominated three coordinators for this project (FC, JGM and JLdP: a microbiologist, an intensivist, and an infectious disease physician). This coordinating group selected the rest of the members of the panel, including microbiologists, intensivists, and infectious disease physicians. The Scientific Committees of both Societies approved their proposal. The present Statement was written following the SEIMC guidelines for consensus statements (www.seimc.org) as well as the recommendations of the Agree Collaboration (www.agreecollaboration.org) for evaluating the methodological quality of clinical practice guidelines. The strength of the recommendations and quality of the evidence were graded in accordance with ESCMID guidelines (Table 1).
Strength of recommendation and quality of evidence.
| Category/grading | Definition |
|---|---|
| Strength of recommendations | |
| A | Strongly supports a recommendation for use |
| B | Moderately supports a recommendation for use |
| C | Marginally supports a recommendation for use |
| D | Supports a recommendation against use |
| Quality of evidence | |
| I | Evidence from at least one properly designed randomized, controlled trial |
| II | Evidence from at least one well-designed clinical trial, without randomization; from cohort or case-controlled analytic studies (preferably from 1 center); from multiple time series; or from dramatic results of uncontrolled experiments |
| III | Evidence from opinions of respected authorities, based on clinical experience, descriptive case studies |
The coordinating group identified 39 key topics that were formulated in accordance with the PICO format defining the population, intervention, comparator, and outcome of interest. These key questions were approved by the Scientific Committees of both Societies and then distributed to the different members of the panel (2 or 3 questions each) for further development. The coordinating group wrote the first draft based on the sections submitted by each participant, which was then sent to the panel for critical review. Before its final approval, the document was published on the intranet of both Societies and left open to suggestions and comments from members. All authors and coordinators of the Statement have agreed the contents of the document and the final recommendations. A summary of these recommendations is available in the Supplementary Electronic Material.
Catheter-related bloodstream infection diagnosis (Table 2)General aspectsWhen should catheter-related bloodstream infection be suspected?
CRBSI should be clinically suspected if the patient has fever, chills or hypotension with signs of infection proximal to insertion sites of peripheral venous cannulae or on the skin overlying the subcutaneous tunnel of a tunneled catheter.8 Several circumstances should increase suspicion that a given episode of bacteremia is catheter-related. The most obvious one is a patient with local signs of infection at the catheter. In addition, bloodstream infections are often caused by microorganisms that colonize the skin, such as Staphylococcus aureus, coagulase-negative staphylococci, Corynebacterium spp., Bacillus spp., Candida spp., among others. CRBSI should also be considered in settings of persistent or recurrent blood cultures for given microorganisms.8 Clinical suspicion of CRBSI should also arise in patients with intravenous catheters who have focal infections known to be caused by the hematogenous spread of bacteria (i.e., septic emboli); this is the case in endocarditis or suppurative thrombophlebitis, particularly if caused by Staphylococcus spp. or Candida spp. in patients with venous catheters. Septic emboli secondary to a CRBSI are more frequently found in the lungs,9 although virtually any organ can be affected by septic metastasis arising from an infected catheter.10,11
Summary of main diagnostic methods for catheter-related bloodstream infections.
| Criteria for positivity | Interpretation | Comments | Recommendation | |
|---|---|---|---|---|
| Diagnosis without catheter withdrawal | ||||
| Paired quantitative blood cultures | Ratio ≥3:1 | Both sets are positive for the same microorganism and the set obtained through the catheter has ≥3:1 fold-higher colony count than the peripheral culture | Sensitivity≈79% Specificity≈99% Labor intensive and expensive | A-II |
| Paired blood cultures for differential time to positivity (DTP) | ≥120min | Both sets are positive for the same microorganism and the set obtained through the catheter becomes positive ≥120min earlier | Sensitivity: 72% to 96% Specificity: 90% to 95% Less specificity for long-term catheters The interpretation of DTP should take into account adherence to the technical procedure and the type of microorganism | A-II |
| Endoluminal brushing | >100CFU | Indicative of CRBSI | Sensitivity: 95% to 100% Specificity: 84% to 89% It may underestimate CRBSI in short-term catheters Risk of pathogen dissemination and thrombotic complications | C-III |
| Superficial cultures (semiquantitative cultures of skin surrounding the portal entry and catheter hubs) | ≥15CFU per plate | Indicative of CRBSI | Sensitivity: 78% Specificity: 92% Must be combined with peripheral blood culture | B-II |
| Gram stain-acridine orange leukocyte cytospin of catheter blood | Presence of any microorganisms in a minimum of 100 high-powered fields | Indicative of CRBSI | Sensitivity≈79% Specificity≈87% The technique is simple and rapid, but requires cytospin technology | B-II |
| Diagnosis with catheter withdrawal | ||||
| Semiquantitative catheter culture | ≥15CFU | The same microorganism in at least one percutaneous blood culture and catheter tip culture | Sensitivity≈84% Specificity≈86% This method mainly detects colonization on the external surface | A-II |
| Quantitative catheter segment culture (vortexing or flushing internal surface) | ≥103CFU | The same microorganism in at least one percutaneous blood culture and catheter tip culture | Sensitivity≈83% Specificity≈91% All quantitative methods are time consuming | A-II |
| Quantitative catheter segment culture (sonication) | ≥102CFU | The same microorganism in at least one percutaneous blood culture and catheter tip culture | Sensitivity≈83% Specificity≈91% All quantitative methods are time consuming | A-II |
RECOMMENDATIONS
- 1.
CRBSI should be suspected in patients with intravenous catheters and fever, chills or other signs of sepsis, even in the absence of local signs of infection, and especially if no alternative source is identified (A-III).
- 2.
Clinical suspicion of CRBSI should also arise in patients with intravenous catheters with metastatic infections caused by hematogenous spread of microorganisms (i.e., septic emboli) (A-III).
- 3.
Persistent or recurrent bacteremia caused by microorganisms that colonize the skin in patients with intravenous catheters should lead to CRBSI suspicion (A-III).
How is complicated catheter-related bloodstream infection defined?
There are several factors associated with worse outcomes in patients with CRBSI and identifying these risk factors can help in the management of those patients. There is no universally accepted definition of complicated CRBSI. Endocarditis is one of the main CRBSI-associated complications with a prolonged therapy that requires catheter removal. Suppurative thrombophlebitis also makes CRBSI complicated, as do metastatic foci of infection, which usually require prolonged therapy and catheter removal. Local complications, such as tunnel infection or a port abscess, even in the absence of septic thrombophlebitis, require catheter removal and so complicate a CRBSI.10,11 Systemic severity (septic shock) in patients with suspected CRBSI is another circumstance that should lead to prompt catheter removal. Non-resolving fever or bacteremia (≥72h) should lead to a detailed reassessment of the patient in order to rule out local or distant infectious complications and so should be considered complicated CRBSI. It is very important to closely monitor immunocompromised hosts with CRBSI for possible treatment failure.
RECOMMENDATIONS
- 1.
Patients diagnosed with CRBSI and with endocarditis, suppurative thrombophlebitis, septic metastasis, extraluminal infections, septic shock, non-resolving CRBSI, or immunocompromised patients should be categorized as complicated CRBSI (A-III).
- 2.
Non-resolving fever or bacteremia (≥72h) should lead to a detailed reassessment of the patient in order to rule out local or distant infectious complications and so should be considered complicated CRBSI (A-III).
How should blood cultures be taken?
Because the aim of a blood culture is to detect true bacteremia and avoid contamination leading to unnecessary treatment, a proper diagnostic methodology is needed. This is particularly important when catheter-related bacteremia is suspected, because the common etiologic agents are also the most frequent contaminants.
Conventional blood cultures are currently performed using commercial systems with automated detection of growth. These systems consist of an aerobic and an anaerobic bottle, considered as one blood culture set. Some studies show a sensitivity of <80% for one blood culture set and >99% for 3 or more culture sets.12–14 To ensure optimal detection of bacteremia, the volume of blood is the essential factor. The Clinical and Laboratory Standards Institute (CLSI) recommends therefore that a blood volume of at least 20ml be inoculated into each of 2 blood culture sets (two bottles per set) taken from different venipuncture sites.15
Blood must be obtained using an aseptic methodology to reduce the risk of contamination16–18 to less than 3% of all blood culture sets,19 which is considered to be the acceptable range. The venipuncture should be performed after disinfecting the skin. The three key factors when choosing the antiseptic are: antimicrobial spectrum, method of application, and duration of antimicrobial effect. The most commonly used disinfectants are alcohol-, chlorhexidine- and iodine-based products.20–24 A recent meta-analysis of 6 randomized control trials concluded that: (1) overall, alcohol-based products seemed to be superior to non-alcohol-based solutions, and (2) solutions containing a combination of alcohol and chlorhexidine showed significant reductions in contaminated blood cultures compared with aqueous povidone-iodine.23 The most widely studied concentration is 2% chlorhexidine gluconate in isopropyl alcohol. On the other hand, a recent study showed that choice of antiseptic agent did not impact contamination rates when the blood cultures were collected by a phlebotomy team. Perhaps the single most important aspect is the use of proper technique, which includes time required to perform the procedure and allowing enough time for the disinfectant to exert its antimicrobial effect. Alcohol and chlorhexidine products require 30s to dry, whereas povidone iodine preparations require 1.5–2min. No studies have evaluated the effect of disinfecting catheter access hubs before drawing the blood samples,16 although it seems to be a rational intervention aimed at minimizing risk of contamination.
The timing of blood culture collection may vary. Although most blood culture systems have different methods of minimizing the effect of antibiotics,25,26 the samples should be obtained, if at all possible, before antibiotic therapy is started.16,25–27 Blood cultures obtained from intravascular catheters are associated with higher sensitivity and negative predictive values.17 In patients with suspected CRBSI, two sets of blood cultures should be taken, one from a peripheral vein and the other from the catheter hub. For multiple-lumen venous catheters, several studies suggest that blood cultures be drawn from all lumens (i.e., the same volume from each lumen) to establish a diagnosis of CRBSI. Omitting a culture of samples from one or more lumens is associated with failing to detect a considerable number of CRBSI episodes.28–30
Once drawn, the blood should be immediately inoculated into the blood culture bottles, which should then be appropriately marked (peripheral vein, catheter, etc.) and promptly and simultaneously incubated in the automated machine, in order to interpret the results on the basis of time to positivity of each blood culture set. Because the rubber caps are not sterile, they are usually disinfected with an alcohol solution, which must be dried before inoculation. Since the incidence of true anaerobic bacteremia is low,31 it may be preferable to inoculate the optimal volume of blood into the aerobic bottle first, and then the remaining volume into the anaerobic bottle.
RECOMMENDATIONS
- 1.
Blood cultures should be obtained using an aseptic technique and before the initiation of antimicrobial therapy (A-I).
- 2.
Skin preparation for obtaining blood samples drawn percutaneously should be performed with proper techniques, including the time to perform the procedure and leaving adequate time for the disinfectant to take effect (A-I). Alcohol-containing products are associated with low rates of contamination. Alcohol-chlorhexidine solutions reduce blood culture contamination more efficiently than aqueous povidone-iodine (A-I).
- 3.
Two pairs of blood cultures should be drawn in patients with suspected CRBSI, one from a peripheral vein and the other from the catheter (A-I).
- 4.
For multiple-lumen venous catheters, samples should be obtained from all lumens (A-II).
How should conventional blood cultures be interpreted?
Identification of the microorganism is considered crucial for interpreting the significance of the result. Propionibacterium spp., Bacillus spp., and most Corynebacterium spp. almost always mean contamination.16,26,32 Contamination is defined as the isolation of an organism in a blood culture that is not present in the patient's bloodstream.19 Unfortunately, some of the microorganisms that frequently contaminate blood cultures are also common causes of CRBSI, such as coagulase-negative staphylococci, which is the leading cause of CRBSI. Other organisms that cause bacteremia, such as S. aureus and Enterococcus spp., can also be detected as contaminants, albeit in a low percentage of cases.33 In the case of skin commensals, at least 2 positive blood cultures with an identical strain are required for them to be considered a cause of bacteremia.25
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is one of the most widely evaluated new technologies for the rapid microbial identification of blood culture isolates.34–40 Although the performance of MALDI-TOF-based identification varies depending on the enrichment and purification methods used, this technology has shown high sensitivity and specificity for rapid identification of microbes in positive blood cultures.34–40 MALDI-TOF has some limitations associated with the identification of some Gram-positive microorganisms (Streptococcus spp.), non-fermenting Gram-negatives, and non-albicans Candida species,39 although its use in the clinical setting could improve time to identification of microorganisms, time to effective therapy and time to optimal antimicrobial therapy.41
Detecting the actual time to positivity of each blood culture is considered critical to the diagnosis of CRBSI. Several studies have confirmed that measuring the differential time to positivity (DTP) of blood cultures obtained from a central venous catheter and a peripheral vein is highly diagnostic for suspected CRBSI.42,43 Blot et al.44,45 reported that a DTP cut-off limit of 120min had 94% sensitivity and 94% specificity for catheter-related infection, and 96.4% sensitivity and 100% specificity for catheter-related sepsis. Other studies showed similar results for the same cut-off value, with sensitivities ranging from 72% to 96.4% and specificities between 90.3% and 95%.42,43 Raad et al.46 showed that a DTP of ≥120min was associated with a 81% sensitivity and 92% specificity for short-term catheters (<30 days) and 93% sensitivity and 75% specificity for long-term catheters (>30 days). Although this diagnostic test has been implemented in routine clinical practice, some authors have reported that DTP is not useful for diagnosis of CRBSI in medical surgical intensive care units.47 These differences can be attributed to the definition of CRBSI used48 or to the type of microorganism causing the CRBSI.49–51 A recent report suggested that a DTP of ≥120min was the optimal cut-off point for diagnosis of Candida spp. CRBSI (85% sensitivity and 82% specificity), except for Candida glabrata.51 However, in a study of catheter-related candidaemia (CRC) that included mainly Candida albicans and Candida parapsilosis, Bouza et al.49 found that a DTP of ≥120min had high sensitivity (94.7%) but low specificity (40%). In general, the accuracy of the DTP method requires accurately tracking how long it takes the blood cultures from the source (central venous catheter vs. peripheral vein) to become positive. The method also relies on the cultures being placed in the automated machine at the same time.46
For suspected CRBSI, detection of the identical microorganism in blood cultures obtained via peripheral venipuncture and the suspected catheter was recently evaluated as a means of diagnosing CRBSI without catheter removal. Although most laboratories use antimicrobial susceptibility testing and biochemical identification to establish identity without using molecular techniques, which seems to be the most practical way to compare isolates, the possibility of polyclonal infection should always be considered, as several studies have demonstrated that polyclonal infections are probably more common than previously suspected.52–54
RECOMMENDATIONS
- 1.
Positivity of blood cultures obtained through the catheter ≥120min before those obtained from a peripheral vein with the same microorganism is highly suggestive of CRBSI. An optimal DTP cut-off for the diagnosis of catheter-related candidemia has not been established (A-II).
- 2.
The interpretation of DTP should consider adherence to the procedural technique used and the type of microorganism (A-II).
- 3.
Rapid microbial identification by MALDI-TOF MS from a positive blood culture significantly reduces time to identification of microorganisms and has clinical impact on the management of patients with suspected bloodstream infection (A-II).
How should quantitative blood cultures be taken and interpreted?
The quantitative methodology is based on lysing red blood cells with different detergents, centrifugation (i.e., lysis-centrifugation) and inoculating the sediment into different culture media and in different atmospheres.55,56 This system has shown better results than conventional methods in terms of detection times and specificity, but is relatively complex and the sample must be processed within 20–30min of inoculation of the blood into the tube.26,27 There are no specific guidelines for the procedure of obtaining blood cultures, so that the recommendations for conventional blood cultures above also apply to quantitative blood cultures,15,16,25–27,32 except for inoculation into the bottle. In the lysis-centrifugation system, 10ml of blood is inoculated into the lysis tube, which contains the specific amount of detergent for this volume. After inoculation, the blood and detergent should be gently mixed before centrifugation is performed. Another currently used method for diagnosing CRBSI is the pour plate method.57 Briefly, for each quantitative blood culture, 1–3ml of blood is mixed with 20ml of previously melted brain heart infusion agar at ∼56°C in Petri plates, then the plates are incubated aerobically for 4 days at 35–37°C.
The number of blood cultures required is similar to conventional blood cultures. For diagnosis of CRBSI, several authors have demonstrated that a differential colony count that is (5–10 times) greater for the intravascular catheter blood culture than the peripheral vein culture is indicative of CRBSI.42,58–61 In a meta-analysis performed by Safdar et al.,62 the differential quantitative blood culture (DQBC) was the best approach for diagnosing CRBSI without catheter removal, with a pooled sensitivity of 0.79 (95% CI: 0.74, 0.84), and pooled specificity of 0.99 (95% CI: 0.98, 1.0). There is some controversy about the cut-off point of DQBC. A study that evaluated different cut-off points for paired quantitative blood cultures for the diagnosis of CRBSI showed that the DQBC was not useful with short-term central venous catheters (CVCs), although in long-term CVCs, DQBCs of 2:1 or greater, or 5:1 or greater were sensitive, but associated with low specificity and positive predictive values.61 Quantitative blood cultures are labor intensive and expensive, which makes them less practicable for routine use.
RECOMMENDATION
- 1.
A quantitative blood culture with a colony count 3 times greater in a sample drawn through a catheter than from the peripheral vein supports a diagnosis of CRBSI (A-II).
What particular aspects should be considered for the diagnosis of CRBSI in patients on hemodialysis?
For patients without a functioning vascular access, central venous catheters (CVC) have become an acceptable means of vascular access for hemodialysis (HD), although their clinical usefulness is severely limited by potential infectious complications.63–65 The relative risk of a CVC causing CRBSI in HD patients is estimated to be approximately 10 times higher than the risk of bacteremia in patients with an arteriovenous fistula or graft.63,65,66
In HD patients, particularly in the outpatient setting, it is difficult to meet the standard microbiological criteria of paired quantitative blood cultures and differential time to positivity to confirm diagnosis of CRBSI. The limitations of the standard diagnostic criteria for CRBSI include the following:
- 1.
Obtaining peripheral blood cultures may be impossible in up to 40% of HD patients, either because their peripheral veins have been exhausted or because of the need to avoid venipuncture in veins intended for the future creation of a dialysis fistula or graft.25,66–69
- 2.
If blood cultures are drawn during the dialysis session when systemic blood is circulating through the catheter, there is no significant difference between peripheral and catheter blood culture results, so that peripheral sampling can be omitted.67–69
- 3.
In the absence of concurrent blood cultures from the catheter and a peripheral vein, there is a risk that a positive blood culture corresponds to a source of infection other than the catheter.67,68
- 4.
In the outpatient setting, longer preincubation due to excessive time for transportation may lead to a false-negative DTP.25,69
RECOMMENDATIONS
- 1.
Whenever possible, paired blood samples from the CVC and a peripheral vein should be obtained for CRBSI diagnosis in hemodialysis patients (A-II).
- 2.
Peripheral blood samples should be obtained from veins that are not intended for future creation of dialysis fistulae or grafts. The veins of the hand for outpatients and hand or femoral veins for hospital inpatients should be used to obtain peripheral blood cultures (A-III).
- 3.
If a blood sample cannot be drawn from a peripheral vein, two separate samples should be drawn, 10–15min apart, through the CVC or the dialysis circuit connected to the catheter (B-II).
What other conservative techniques may be used for diagnosis of CRBSI?
Conservative methods for the diagnosis of CRBSI include endoluminal brushing, superficial cultures of the skin around the insertion site and catheter hubs, and the Gram stain with acridine orange leukocyte cytospin (AOLC) test.42,43,70–72 Endoluminal brushing, a method of sampling the internal surface of the catheter, showed high sensitivity (95–100%) and specificity (84–89%) in two studies7,2,73 although the procedure is impractical and unreliable and major side-effects have been reported, such as cardiac arrhythmias and embolization with subsequent bacteremia.56 Superficial cultures (semiquantitative cultures of skin around the catheter insertion site and catheter hubs) have also been proposed for the diagnosis of CRBSI,43 based on a sensitivity and specificity of 78% and 92%, respectively. It has been suggested that superficial and peripheral blood cultures be combined to screen for CRBSI, reserving DQBC as a more specific technique for confirmation. Other authors have also reported on the Gram stain-AOLC test as a rapid method for diagnosis of CRBSI.70 The method requires two 50μL samples of catheter blood. After several steps, including the use of cytospin technology, a monolayer of leukocytes and microorganisms is placed on two slides, then stained with either acridine orange or Gram stain, and viewed by ultraviolet and light microscopy, respectively. The authors reported a 96% sensitivity and 92% specificity.70 In the meta-analysis by Safdar et al.,62 the overall sensitivity and specificity of the AOLC test were 72% and 91%, respectively. Generally speaking, these methods have not been validated by other authors and are not widely used in clinical laboratories. Table 2 gives a brief summary of these conservative methods and those requiring catheter removal.
RECOMMENDATIONS
- 1.
Endoluminal brushing of the internal surface of the catheter may be useful for diagnosis of CRBSI. However, the procedure is impractical and major side-effects have been reported (C-III).
- 2.
Semiquantitative cultures of skin around the catheter insertion site and catheter hubs with ≥15CFU may be indicative for CRBSI. These procedures must be combined with peripheral blood culture (B-II).
- 3.
Gram stain-acridine orange leukocyte cytospin (AOLC) of catheter blood may be used as a rapid method for diagnosis of CRBSI. The presence of any microorganisms in a minimum of 100 high-powered fields may be indicative of CRBSI (B-II).
What is the value of molecular techniques for the diagnosis of CRBSI?
Most molecular techniques for diagnosis of CRBSI without catheter withdrawal are performed directly on blood samples drawn through catheters. Various molecular methods have been applied to different patient populations. A 16S rDNA analysis of blood drawn through vascular access devices in patients with hematologic disorders had a 100% positive predictive value for CRBSI.74,75 Other authors used pulsed-field gel electrophoresis (PFGE) to confirm CRBSI caused by coagulase-negative staphylococci (CoNS) in patients with neutropenia.76 Most studies are based on real-time PCR, such as LightCycler® SeptiFast or Gene Xpert®, which are demonstrated to be a useful complementary diagnostic tool for blood cultures, especially in patients receiving antibiotics.77–80 There is very little data about the use of molecular techniques with samples other than blood to confirm a CRBSI episode.81
Although direct molecular detection techniques for detecting microorganisms in the blood and other samples are a promising approach for improving patient management and outcome by streamlining the diagnosis of CRBSI, they are still currently unable to replace the traditional culture and remain expensive and time-consuming.82,83
RECOMMENDATION
- 1.
There is not enough information to recommend implementing molecular techniques in clinical practice for CRBSI diagnosis (C-II).
When should a catheter tip be sent for culture?
Diagnosis of CRBSI requires establishing the presence of a bloodstream infection (see section How should blood cultures be taken?) and demonstrating that the infection is related to the catheter. As a general recommendation, a catheter culture should only be obtained when a CRBSI is suspected,84 thus avoiding unnecessary cultures. Several factors should be taken into consideration when determining whether the catheter should be removed: the type of catheter, ease of new catheter insertion, immune status, the severity of the underlying illness of the patient, and the presence and severity of sepsis.85–88
RECOMMENDATION
- 1.
Catheter cultures should only be obtained when CRBSI is suspected (A II).
How should a catheter be sent to and processed in the Microbiology Laboratory?
After pulling the catheter, its tip should be cut to a length of 5cm approximately, under sterile conditions and avoiding contact with the patient's skin, and then placed in a dry, sterile container for transport. The catheter tip should be stored at 4–8°C27 while transport to the laboratory is arranged.
The most widely used laboratory technique is the semi-quantitative method described by Maki, in which the catheter segment is rolled across a blood agar plate using sterile forceps. After overnight incubation, the number of colony-forming units (CFU) is counted.89 One limitation of this method is that it mainly detects colonization on the external surface of the catheter. This is more of a concern with long-term catheters, where luminal colonization more frequently leads to bloodstream infections.56,90 In 1980, Cleri described a quantitative culture method to improve the detection of microorganisms progressing inside the catheter lumen.91 Quantitative cultures of the endolumen were obtained by immersing the catheter segment in 2–10ml of tryptic soy broth (TSB), then flushing it three times with a syringe. The broth was serially diluted 100-fold. 0.1ml of each dilution was streaked onto sheep blood agar and the number of CFUs counted after incubation.91
Brun-Bruisson et al.92 simplified Cleri's technique by placing the catheter segments into a test tube with 1ml of sterile distilled water. After vortexing for 1min, 0.1ml of the suspension is plated onto blood agar. Other modifications of quantitative endoluminal cultures include a quantitative sonication technique,93 in which the catheter tip is placed in 10ml of TSB and sonicated for 1min. 0.1ml of both the sonicated broth and a 1:100 dilution of the broth are plated onto blood agar and the number of colony-forming units counted.
In order to distinguish between colonization on the internal and external surfaces of the catheter, Liñares et al.90 used the semiquantitative method for culturing the catheters,89 then a modified quantitative technique, flushing each catheter lumen with 2ml of TSB, which was then serially diluted and plated.
All quantitative methods are time-consuming, whereas the simplicity of semiquantitative techniques has contributed to their widespread use in clinical microbiology laboratories.43,94 Several prospective studies have compared Maki's semiquantitative technique with quantitative methods (sonication and vortexing) for detection of CRBSI and concluded that the three methods exhibited similar reliability, although Maki's semiquantitative technique was simpler to use.95,96
The predictive values of quantitative or semiquantitative methods may vary depending on the type and location of the catheter, the culture methodology used, and the source of catheter colonization.97 For example, skin-colonizing microorganisms are more likely to colonize the external surface of a recently inserted catheter, so that Maki's semiquantitative method would be very sensitive for identifying this colonization. By contrast, a catheter that has been in place for more than a week could become colonized intraluminally via the hub, rendering the roll plate method less sensitive. In this case, methods that obtain samples for culture from both internal and external surfaces are more sensitive.95
RECOMMENDATIONS
- 1.
The most reliable diagnostic methodologies for catheters sent to culture are the semiquantitative (roll plate) or quantitative (vortex or sonication methods) (A-II).
- 2.
Qualitative cultures (culture of the catheter tip by broth immersion) are unreliable for distinguishing between contamination and infection, and are not therefore suitable for the diagnosis of CRBSI (A-II).
How should the results of catheter cultures be interpreted?
A semiquantitative catheter cultures discriminate between catheters as the cause of infection and non-significant colonization. The catheter is considered to be the source of infection if growth from a culture of the catheter tip is ≥15CFU, whereas <15CFU with no associated clinical signs is considered to be catheter colonization.89 The cut-off point of ≥15CFU is significantly associated with clinical signs and bacteremia, with a 76% specificity.89 Subsequent studies have validated the semiquantitative culture technique for evaluating catheter-related infections.98,99 There is no established cut-off point for mycobacteria and fungi.
For quantitative catheter cultures (flushing the internal surface and vortexing), the cut-off point has been established at 103CFU/segment, based again on its association with bacteremia in CRBSI. Colony counts of less than 103CFU are considered intermediate, possible contamination, or the early stages of colonization.91,92 For quantitative cultures based on sonication, a cut-off point of >102CFU was established to discriminate between catheter infection and catheter colonization.93 In general, semiquantitative and quantitative cultures give comparable results, although the semiquantitative procedure is easier and faster in practice.27,100
RECOMMENDATIONS
- 1.
The presence of more than 14CFU per plate by semiquantitative culture (roll-plate) is indicative of significant catheter colonization (A-II).
- 2.
A count of 103CFU/segment or more using quantitative culture methods based on vortexing or flushing the internal surface reflects significant catheter colonization (A-II).
- 3.
Counts above 102CFU/segment for quantitative culture methods based on sonication indicate significant catheter colonization (A-II).
How should a subcutaneous reservoir be processed?
Venous access devices (VADs) are widely used for long-term access to the vascular system, mainly in cancer patients. The diagnosis and management of CRBSI also includes a recommendation to perform a qualitative culture of the port reservoir contents as well as a semiquantitative culture of the catheter tip if VAD-related bloodstream infection (VAD-RBSI) is suspected. This has been thoroughly studied in patients with suspected VAD-RBSI by comparing VAD cultures with blood cultures obtained before removal. In all studies, the catheter tip cultures failed to detect several VAD-RBSI episodes, whereas cultures of the endoluminal content (thrombotic material) had better predictive value.101–104
Bouza et al. assessed the validity values of cultures obtained from multiple sites of 223 VADs that had been withdrawn for some reason and confirmed that the rate of VAD colonization improved when they not only obtained cultures from the catheter tip and the inside of the port, but also from the sonication fluid used to obtain microorganisms from the external surface of the port.105 In addition, del Pozo et al. assessed the yield from the septum of 240 VAPs after sonication. The latter procedure showed the highest sensitivity and specificity (78% and 93%, respectively) for diagnosing VAD colonization with a cut-off of 110CFU/ml.106
These recent findings will probably have an impact on the routine laboratory processing of pulled VADs, since confirmation of VAD-RBSI requires performing cultures of the catheter tip, and the inner and outer surfaces of the port. There is no consensus statement for thresholds for VAD cultures.
RECOMMENDATION
- 1.
Venous access devices removed for suspected CRBSI should be sent to the microbiology laboratory. Routine processing should include a combination of cultures from different parts of the VAD, including a culture after septum sonication and semiquantitative catheter tip cultures (B-II).
What is the present value of molecular techniques for the diagnosis of CRBSI after catheter removal?
Diagnosis of CRBSI requires confirmation that the microorganisms isolated from blood and catheter tip cultures are phenotypically identical. A recent study using quantitative PCR for the detection of CoNS suggested that the role of the catheter as a source of bacteremia may be overestimated.107 Indeed, the conventional microbiological procedures used to diagnose CoNS CRBSI performed badly when compared with an evaluation by PFGE of different morphotypes of CoNS isolated from catheter tip and blood cultures.108 By contrast, using microsatellite markers, the genotypes of Candida isolates recovered from blood cultures and catheter tips were a match in 91% of patients studied.109
Due to its low sensitivity, 16S rRNA polymerase chain reaction (PCR) has not managed to replace the conventional culture and there are at present no data about the application of molecular methods to non-tunneled catheters. On the other hand, the application of 16S rRNA PCR using endoluminal samples increased detection of venous access device-related bloodstream infection (VAD-RBSI) in patients undergoing antibiotic therapy by 21.1%.110
In summary, molecular methods have the potential to improve diagnosis of CRBSI in patients undergoing antibiotic therapy, although these techniques have not been standardized.
RECOMMENDATION
- 1.
16S rRNA PCR could be performed with septum sonication fluid to rule out or confirm VAD-RBSI in patients undergoing antibiotic therapy (C-III).
What samples should be taken and how should they be interpreted when an insertion site infection is suspected?
Insertion site infections are characterized by signs of inflammation, including induration, erythema, warmth, and pain or tenderness within 2cm of the catheter insertion site. They may also be associated with other signs and symptoms of infection, such as fever or purulent discharge from the insertion site, with or without a concomitant bloodstream infection.6,111 A microbiologically documented insertion site infection is defined as exudate with a positive culture at the catheter insertion site.6,111 The sensitivity and positive predictive value of local inflammation for the diagnosis of CRBSI is shown to be very low.112 When catheter infection is suspected and there is exudate at the catheter insertion site, the exudate should be sent for Gram staining, routine culture, and additional culture for fungi as indicated when assessing immunocompromised patients.25 Blood cultures should also be drawn.6,111,112
In the absence of local signs of infection, the results of several studies suggest that semi-quantitative cultures of swabs of skin taken from around the insertion site and surface cultures from the internal surface of the catheter hubs may be useful for ruling out catheter colonization and infection, and so avoiding unnecessary catheter withdrawals.43,81,113–115 For skin samples, a dry cotton swab should be rubbed over a 2cm2 area around the insertion site. For hub samples a small alginate swab should be introduced into each hub and rubbed repeatedly against its inner surface.43,113 Semi-quantitative growth of <15CFU from both the insertion site and the catheter hub enables CRBSI to be ruled out,43,113 although surface cultures show very low specificity and positive predictive value. Combining a semiquantitative culture of the subcutaneous tract with a hub swab culture improves specificity and positive predictive values.116
VAD-related infection should be suspected if a patient exhibits signs of a local infection, such as pain or erythema at the implant site.104 A local complicated infection is defined as infection of the tunnel or pocket, with extended erythema or induration (more than 2cm), purulent collection, skin necrosis and spontaneous rupture and drainage. Clinical signs of local infection, such as redness or purulent exudate, have high specificity but low sensitivity.101,104 A recent study showed that 23% of patients with VAD-related infection had local signs of infection.117 In such cases, a culture of purulent fluid and/or necrotic tissue surrounding the port is required. Blood culture from peripheral veins should also be performed in order to rule out CRBSI.
RECOMMENDATIONS
- 1.
When there is exudate at the catheter insertion site, it should be sent for Gram staining and culture. Blood cultures should also be drawn (A-III).
- 2.
In patients with suspected catheter-related infection but negative superficial cultures (growth of <15CFU from both the insertion site and catheter hub cultures), the possibility of infection can reasonably be ruled out (B-II).
The main antimicrobial drug and dosage regimens that should be used for CRBSI are shown in Table 3.
The main antimicrobial drug and dosage regimens that should be used for catheter-related infections.
| Antimicrobial | Dosage |
|---|---|
| Antibacterials | |
| Amikacin | Loading dose: 25–30mg/kg IV, followed by 15–20mg/kg/d IV |
| Amoxicillin-clavulanate | 2g/200–500mg every 6–8h IV |
| Ampicillin | 2g every 6–8h IV |
| Aztreonam | 1–2g/6–8h IV |
| Cefazolin | 2g every 8h IV |
| Cefepime | 2g/8–12h IV |
| Ceftaroline | 600mg/12h IV |
| Ceftazidime | 2g/8-12h IV |
| Ceftriaxone | 1g every 12h |
| Cefotaxime | 1–2g/6–8h IV |
| Ciprofloxacin | 500mg/12h IV VO |
| Cloxacillin | 2g every 4h IV |
| Colistin | 7–9 MU load, then 4.5 MU every 12h IV |
| Dalbavancin | 1000mg IV, 500mg IV one week apart |
| Daptomycin | 8–10mg/kg/d IV |
| Ertapenem | 1g every 24h IV |
| Fosfomycin | 4g/6–8h IV |
| Gentamicin | 5–7mg/kg/d IV |
| Imipenem-cilastatin | 500mg every 6h IV |
| Levofloxacin | 750mg daily |
| Linezolid | 600mg every 12h |
| Meropenem | 1g every 8h IV |
| Piperacillin-tazobactam | 4/0.5g every 6–8h |
| SMX-TMP | 160–800mg bid 5–10mg/kg/day of TMP |
| Tedizolid | 200mg/d |
| Teicoplanin | 6mg/kg/12h (3 doses), 6mg/kg/d IV |
| Tobramycin | 5–7mg/kg/d IV |
| Vancomycin | Loading dose: 25–30mg/kg IV, then 15–20mg/kg/8 – 12h IV |
| Antifungals | |
| Anidulafungin | 200mg loading dose, 100mg/d IV |
| Caspofungin | 70mg loading dose, 50mg/k/d |
| Fluconazole | 800mg loading dose, then 400mg daily |
| Liposomal amphotericin B | 3–5mg/kg/d |
| Micafungin | 100mg/d IV |
| Voriconazole | 400mg bid×2 doses, then 200mg every 12h 6mg/kg IV every 12h for 2 doses, followed by 4mg/kg IV every 12h |
Note that doses of the drugs are not adjusted for renal or hepatic function.
When can a catheter be retained until blood cultures are available?
Two studies found no differences in outcome when early CVC removal was compared with a watchful waiting strategy for suspected CRBSI in patients with non-tunneled catheters.118–120 These studies excluded patients with neutropenia, solid organ or hematologic malignancy, immunosuppressive drugs or radiation therapy, organ transplants, intravascular foreign bodies, hemodynamic instability, suppuration or frank erythema/induration at the insertion site, as well as bacteremia or fungemia. One of these ICU studies was a randomized single-center clinical trial118 and the other was prospective, observational, and multicenter.119 In the multicenter study, CRBSI was confirmed in only 12% of patients and there was no difference in mortality between immediate and late removal of the CVC. Another randomized trial demonstrated that, with critically ill patients, the DTP method makes it possible to use a watchful waiting strategy up to definitive diagnosis of CRBSI.121 It should be noted that catheter exchange is not without its risks, and severe complications, although fortunately uncommon, can occur.122
RECOMMENDATION
- 1.
Immediate removal of the CVC is not routinely recommended when CRBSI is suspected in patients who are hemodynamically stable, without immunosuppressive therapy, intravascular foreign bodies or organ transplantation, no suppuration at the insertion site or bacteremia/fungemia, (A-I).
When is it safe to perform a catheter exchange over a guidewire?
A CVC replacement can be inserted by percutaneous venipuncture at a new site or by using the Seldinger over-the-guidewire technique. A meta-analysis of 12 randomized controlled trials (RCT)123 that evaluated guidewire exchange versus new-site insertion found non-significant differences between the two for the prevention of CRBSI. Guidewire exchange was associated with fewer mechanical complications (8 RCTs, relative risk=0.48, 95% confidence interval=0.12–1.91) but also a higher rate of catheter colonization (9 RCTs, relative risk=1.26, 95% confidence interval=0.87–1.84), catheter exit-site infections (5 RCTs, relative risk=1.52, 95% confidence interval=0.34–6.73) and catheter-related bacteremia (9 RCTs, relative risk=1.72, 95% confidence interval=0.89–3.33).123 A study of 1598 CVCs in critically ill patients showed that over-the-guidewire exchange was associated with the development of CRBSI.124 On the other hand, inserting tunneled hemodialysis catheters using elective guidewire exchange from non-tunneled catheters was not associated with a higher incidence of catheter infections, and venous access was preserved in these high-risk patients.125
Guidewire exchange is not indicated for patients with documented catheter infections or CRBSI.126 Using guidewire-assisted exchange to replace a malfunctioning catheter is an option if there is no evidence of infection at the catheter site and new percutaneous venipuncture is not recommended because of a high risk of complications (difficult venous access, bleeding diathesis).
RECOMMENDATIONS
- 1.
Routine replacement of a CVC by guidewire exchange is not recommended because this strategy is associated with a higher risk of associated infectious complications. (B-II)
- 2.
Guidewire exchange of a CVC is contraindicated in patients with documented catheter related infections. (A-II)
- 3.
Guidewire exchange should be restricted to patients with very difficult venous access (i.e., extensive burns, morbid obesity, or severe coagulopathy) and without documented catheter infection (B-II). In this case, a meticulous aseptic technique and a culture of the catheter tip are mandatory. (A-III)
- 4.
If the catheter tip culture is positive, the new line, inserted over a guidewire, should be re-placed via a new direct venipuncture. (C-III)
What should be done if the catheter tip culture is positive, but the blood cultures are negative?
There is very limited data about the clinical implications of a positive CVC tip culture with negative blood cultures taken at the time of catheter removal.
Two retrospective studies127,128 concluded that an intravascular catheter colonized with S. aureus is a risk factor for subsequent S. aureus CRBSI. Antibiotic therapy initiated within 24h of catheter removal significantly reduced the risk for subsequent S. aureus bacteremia (SAB).
Another retrospective multicenter study showed a lower incidence of septic complications after the removal of a colonized catheter in patients with early antibiotic treatment (13% vs. 4%) (OR=4.2; 95% CI=1.1–15.6). In that study, exit-site infection was also a risk factor for the development of S. aureus CRBSI (OR=3.39; 95% CI=1.19–9.34).127 A meta-analysis of four retrospective studies yielded a pooled OR of 5.8 (95% CI=2.6–13.2) for SAB when antibiotic therapy was not initiated. The number needed to treat to prevent 1 episode of SAB was 7.4.129 Conversely, a more recent retrospective study concluded that administration of early antistaphylococcal therapy had no impact on outcome, which was defined as S. aureus infection within 3 months of catheter withdrawal or death with no obvious cause. The only factor independently associated with a poor outcome were clinical signs of sepsis at the time the catheter was removed (OR=20.8; 95% CI=2.0–206.1).130,131
A retrospective study of patients with CVC tips colonized with Candida spp. observed that the incidence of subsequent candidemia (SC) was only 1.7% and a multivariate analysis of risk factors for poor prognosis showed that antifungal therapy was not protective in this setting (OR=0.82; 95% CI=0.27–2.47).132 A more recent study showed that the incidence of SC was 2.5% and that administration of antifungals was not protective in 55% of patients.133 Another study however showed that the risk of infectious complications following catheter removal was higher when Candida spp. were involved (7.7%) than in the case of bacterial infection (1.8%) and initiating antifungal therapy was suggested for all patients with positive catheter tip cultures and negative blood cultures.134
No clear recommendations can be given if the catheter is colonized with other microorganisms. The decision should be individualized, although antimicrobial therapy would be justified only in patients with septic shock and no other obvious explanation for the clinical picture.
RECOMMENDATIONS
- 1.
Antibiotic treatment (i.e., 5–7 days) should be given to patients with catheter tip cultures positive for S. aureus and negative blood cultures if the patient shows systemic or local infection (B- II).
- 2.
In non-neutropenic patients or those without valvular heart disease, the presence of a catheter tip culture positive for Candida spp. and negative or unavailable blood cultures should be assessed on an individual basis before starting systematic antifungal treatment. Antifungal treatment should not be prescribed for patients without systemic signs of infection (B-II).
- 3.
No clear recommendations can be given for catheters colonized with other microorganisms (C-III).
What is the empirical antimicrobial therapy for CRBSI?
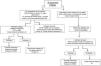
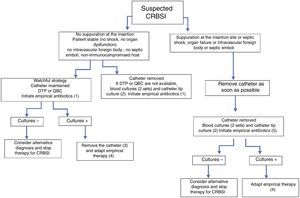
The initial choice of antimicrobial should be based on an assessment of the risk factors for infection, the severity of the clinical picture and the likely pathogens associated with the specific intravascular device. Fig. 1 summarizes the recommended empirical approach for a patient with a high index of suspicion for CRBSI.
Approach to the management of a patient with suspicion of CRBSI. (1) Vancomycin (alternative daptomycin; see text for specific recommendations for this agent) plus antibiotic therapy to cover Gram-negative bacilli if: the femoral catheter is in place, the focus of Gram-negative infection is known, with a high index of colonization by Gram-negative bacilli or prolonged admission in ICU. As the patient is clinically stable, consider antifungal therapy (fluconazole) in patients with total parenteral nutrition, prolonged use of broad-spectrum antibiotics, malignancy, femoral catheterization, colonization due to Candida species at multiple sites or previous anti-anaerobic therapy. (2) Semi-quantitative or quantitative tip culture. (3) Catheter can be maintained only in patients without septic shock secondary to CRBSI, without intravascular devices, and if the culprit pathogen is a CoNS (except Staphylococcus lugdunensis) or a Gram-negative bacilli if the isolate is susceptible to antibiotics that are available for ALT. See Fig. 2 for management. (4) See text and Fig. 2 for choosing targeted treatment, duration of therapy, and need for echocardiography. (5) Vancomycin (alternative daptomycin; see text for specific recommendations of this agent) plus antibiotic therapy to cover Gram-negative bacilli plus an antifungal agent in patients with septic shock or in other patients if: total parenteral nutrition, prolonged use of broad-spectrum antibiotics, malignancy, femoral catheterization, colonization due to Candida species at multiple sites or intense previous anti-anaerobic therapy. Echinocandins, or liposomal amphotericin B as an alternative should be used only in patients with septic shock. Fluconazole is the drug of choice for the remainder of situations, except in patients colonized by fluconazole-resistant Candida spp. Patients with suppuration at the insertion site but without the other conditions should not receive antibiotic therapy active against Gram-negative bacilli and antifungal agents. DTP: differential time to positivity; QBC: quantitative blood culture.
Patients with S. aureus CRBSI are at high risk for hematogenous metastasis, especially when the catheter cannot be removed and/or antibiotic treatment is not appropriate.135 As most CoNS are methicillin-resistant, the choice of empirical therapy should include antibiotics with activity against these strains. Vancomycin is the most commonly prescribed antimicrobial for CoNS and methicillin-resistant S. aureus (MRSA) bacteremia in recent decades. Studies comparing the efficacy and safety of glycopeptides (i.e., vancomycin vs. teicoplanin) for Staphylococcus spp. (including MRSA) bacteremia have not observed significant differences,136,137 although clinical isolates of Staphylococcus epidermidis and Staphylococcus haemolyticus have been reported with reduced susceptibility to teicoplanin.138
Vancomycin is associated with lower clinical success rates for MRSA bacteremia with MICs ≥1.5mg/l (measured by E-test)139,140. In a case–control study focusing on cases of MRSA bloodstream infection with a vancomycin MIC ≥1.5mg/l (measured by E-test), a higher survival rate was observed in the patient group treated with daptomycin.141 Multivariate analysis confirmed that renal impairment and previous therapy with vancomycin were associated with significantly higher clinical failure. The impact on the outcome of bacteremia caused by CoNS with vancomycin MIC ≥1.5mg/l (measured by E-test) is an unresolved issue.
Previous studies have indicated that vancomycin is inferior to beta-lactams (i.e., cefazolin or oxacillin) for the treatment of methicillin-susceptible Staphylococcus aureus (MSSA) bloodstream infections.142–144 This would justify the inclusion of a beta-lactam in the empirical treatment of any suspected case of CRBSI. A recent study compared beta-lactams and vancomycin for empirical and definitive therapy of MSSA bloodstream infections among 5787 patients from 122 hospitals.145 Patients who received definitive therapy with a beta-lactam had a 35% lower mortality compared with patients who received vancomycin (HR=0.65; 95% CI=0.52–0.80) after controlling for other factors.145
Daptomycin is a lipopeptide antibiotic with in vitro activity against Gram-positive bacteria and is also more bactericidal than vancomycin.146,147 The only randomized trial that has compared daptomycin with vancomycin or a β-lactam concluded that daptomycin was noninferior to vancomycin.148 In a recent cohort study including 579 episodes of bacteremia caused by MRSA, no significant differences were observed in the mortality of patients treated with vancomycin or daptomycin (OR=1.42 [95% CI=0.83–2.44]).149 However, a recent study analyzing the efficacy of daptomycin in 40 cancer patients treated for Gram-positive CRBSI (including S. aureus) compared with a historical control group of 40 patients treated with vancomycin confirmed faster bacteriological eradication and clinical resolution in the daptomycin group.150
In a randomized clinical trial of skin-structure infection and CRBSI with S. aureus, including MRSA, linezolid and its comparators showed similar efficacy for CRBSI.151 A meta-analysis of 5 randomized controlled trials of MRSA bacteremia observed that linezolid was noninferior to vancomycin.152
RECOMMENDATIONS
- 1.
If CRBSI is suspected, antimicrobial therapy should be started as soon as possible with a bactericidal agent active against S. aureus and CoNS, especially if associated with sepsis or septic shock (B-II).
- 2.
Vancomycin is recommended for empirical therapy in patients with suspected CRBSI (B-II). Teicoplanin is not recommended as empirical therapy, given the existence of coagulase-negative staphylococci with reduced susceptibility to teicoplanin (C-III).
- 3.
Daptomycin can be administered for cases of CRBSI with septic shock (C-III), acute kidney injury (B-III), to patients with recent exposure to vancomycin (>1 week in the past 3 months) (C-III) or if the local prevalence of S. aureus isolates with vancomycin MIC ≥1.5μg/ml is high (C-III). The local prevalence of S. aureus isolates with vancomycin MIC ≥1.5μg/ml supporting routine empirical use of daptomycin remains undefined.
- 4.
Linezolid should only be used in patients with contraindications for the previous agents (B-II).
When should empirical coverage of Gram-negative bacilli or fungi be added?
The incidence of Gram-negative bacilli (GN)-CRBSI is reported to be 17–25% of all episodes of CRBSI.153,154 GN-CRBSI is particularly relevant during outbreaks and in patients with special conditions, such as spinal cord injuries, femoral catheters, neutropenia and hematologic malignancy, gastrointestinal colonization, prolonged ICU stay, post-operative status or diabetes.155–157 In some centers, the predominance of GN-CRBSI has been related to an increase in transplants (solid organ or hematologic bone marrow)157 and the implementation of bundled strategies for the prevention of CRBSI including the use of chlorhexidine/silver sulphadiazine-impregnated catheters, which preferentially prevent Gram-positive CRBSI.158 In a recent report, solid organ transplant, prior use of penicillin and hospital stays of more than 11 days were independently associated with a significantly higher risk of GN-CRBSI, whereas, cirrhosis, diabetes and use of quinolones were associated with a higher risk of Gram-positive CRBSI.154 Femoral catheterization is associated with a higher incidence of CRBSI due to Gram-negative bacilli than at other anatomic sites, so that empirical antibiotic coverage for Gram-negative bacilii has been suggested when CRBSI is suspected in patients with femoral access.159 No clinical trial has validated the benefits of specific drugs for the management of GN-CRBSI; empirical coverage should be based on local antimicrobial susceptibility data and disease severity.158
A prospective study of risk factors for yeast bacteremia found that the rate of Candida spp. CRBSI was significantly higher in femoral catheters than at other catheter sites (16.67% vs. 1.92%; p=0.035).159 A recent study, however, identified only solid tumors (OR=3.11; 95% CI=1.75–5.53), total parenteral nutrition (OR=2.65; 95% CI=1.39–5.06) and administration of anti-anaerobic agents (OR=2.22; 95% CI=1.03–4.79) as independent variables for Candida CRBSI. In that study, the (1,3)-β-D-glucan (BDG) test was positive in 94.6% (35/37) of Candida spp.-CRBSI patients and 9.4% (10/106) of non-candidal CRBSI cases.160 For ICU patients, multivariate logistic regression analysis identified severity of illness on the day of candidemia (as measured by the SOFA score) as the only potential risk factor for CRBSI caused by Candida spp.161
RECOMMENDATIONS
- 1.
Patients with suspected CRBSI should receive empirical antibiotic therapy (in addition to coverage for Gram-positive pathogens) to cover Gram-negative bacilli under any of the following circumstances: hemodynamic instability (septic shock), neutropenia or hematologic malignancy, solid organ or bone marrow transplant, femoral catheter in place, a high index of colonization with Gram-negative bacilli or prolonged ICU admission (C-III).
- 2.
Antimicrobial therapy should be adapted to local epidemiology and must include an antipseudomonal agent (i.e., piperacillin-tazobactam, carbapenems, a fourth-generation cephalosporin, aztreonam, quinolones or aminoglycosides) (A-II). Aztreonam and cephalosporins should be avoided in patients with colonization or at risk for extended-spectrum β-lactamase infections (A-I).
- 3.
The need for empirical antifungal therapy in a patient with suspected catheter-related candidemia should be evaluated along with the possibility of catheter removal (A-III).
- 4.
Empirical therapy for suspected catheter-related candidemia should be considered in patients who are hemodynamically unstable with one or more of the following conditions: total parenteral nutrition, prolonged use of broad-spectrum antibiotics, malignancy, femoral catheterization, colonization due to Candida spp. at multiple sites or intense previous anti-anaerobic therapy (C-III).
- 5.
The use of biomarkers (such as 1,3-β-D-glucan) may be useful when considering initiation of empirical antifungal treatment (B-III).
What particular aspects should be considered in the empirical treatment of CRBSI in patients on hemodialysis?
Vascular catheters are the leading source of bacteremia in HD patients.162,163 Bacteremia usually develops when the catheter is in use. Catheter salvage should be a priority in these patients.
Conservative management is associated with a higher success rate when a combination of systemic antibiotics and catheter antibiotic lock protocol is used.164–167
The microorganisms that cause CRBSI in hemodialysis patients are similar to those observed in other patient populations, although usually with a higher proportion of S. aureus in most series.168–171S. aureus CRBSI is one of the most difficult microorganisms to treat while maintaining a catheter in place due to its propensity to cause septic complications, treatment failure and relapses.172,173S. epidermidis CRBSI, however, has shown excellent results when treated conservatively by combining systemic and local antibiotics during the interdialytic period.166
Alternatively, if retaining the catheter is not possible, catheter exchange over a guidewire has been shown to be safe. This approach could lead to higher cure rates for S. aureus infections than treatment based on antibiotic lock therapy.166 Systemic antibiotics should be administered taking into consideration the PK/PD characteristics of each particular drug for patients with end-stage renal disease or undergoing hemodialysis.
RECOMMENDATIONS
- 1.
Conservative management of CRBSI should be attempted with hemodialysis patients. Combining systemic and local intracatheter antibiotics is associated with better results when compared to systemic antibiotics alone (A-I).
- 2.
In patients with a tunneled hemodialysis catheter, guidewire exchange is an alternative, especially when catheter removal is not feasible (C-III).
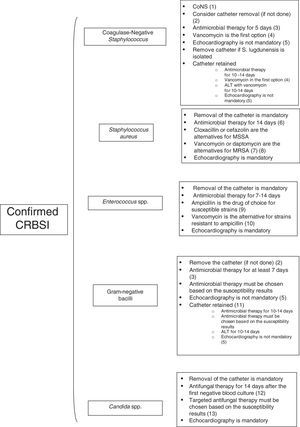
Fig. 2 summarizes the pathogen-directed management of confirmed CRBSI.
Approach to the treatment of a patient with confirmed CRBSI. (1) With the exception of Staphylococcus lugdunensis, which should be managed as for Staphylococcus aureus. (2) Catheter must be removed in patients with septic shock secondary to CRBSI or in patients with intravascular devices. (3) In patients with intravascular devices, foreign bodies (such as articular prostheses) or in whom markers of inflammation persist after catheter removal therapy, antibiotic therapy for 10–14 days is recommended. (4) Cloxacillin or cefazolin are the alternatives for methicillin-susceptible strains. Optimal trough levels of vancomycin for CoNS are not defined. (5) Echocardiography should be done in patients with valvular diseases or in case of persistent bacteremia despite appropriate therapy. (6) Complicated episodes require longer courses of treatment (4–6 weeks). (7) Trough levels of vancomycin should be15–20mg/l. (8) Daptomycin is preferred for isolates with MIC for vancomycin >1.5mg/l. (9) Combined therapy with an aminoglycoside is discouraged for Enterococcus spp. CRBSI. (10) Optimal trough levels of vancomycin for Enterococcus spp. CRBSI are not defined. (11) Only in immunocompetent patients without septic shock and when the isolate is susceptible to antibiotics that are available for ALT. (12) If metastatic complications have been ruled out. (13) De-escalation from an echinocandin or a lipid formulation of amphotericin B to fluconazole is highly recommended in patients with isolates susceptible to fluconazole, are clinically stable and the catheter has been removed.
What is the recommended directed therapy and optimal duration of treatment for CRBSI due to Staphylococcus aureus?
Methicillin-susceptible S. aureus (MSSA) CRBSI. The treatment of choice is high-dose intravenous isoxazolyl penicillin, (i.e., cloxacillin). Cefazolin is an adequate alternative.174–176 Treatment with other beta-lactams, including second- and third-generation cephalosporins, has been associated with increased mortality.176 Likewise, the in vitro activity and clinical results of vancomycin therapy for MSSA have been repeatedly shown to be significantly worse.142–144,177 In patients allergic to beta-lactams, the use of intravenous daptomycin yields comparable results to cloxacillin.148 Infections caused by methicillin-susceptible S. aureus (MSSA) strains with reduced susceptibility to vancomycin (MIC ≥1.5mg/l, measured by E-test) have been associated with worse outcomes, even when treated with cloxacillin.178
Duration of uncomplicated MSSA CRBSI treatment is 14 days, including for patients with intravenous prosthetic devices and negative transesophageal echocardiographic (TEE) findings.179 Blood cultures should be obtained after 72h of antibiotic therapy.180 The management of patients with persistent positive blood cultures and/or no clinical improvement after catheter removal is outlined elsewhere.179 Duration of treatment for these episodes of complicated CRBSI is 4–6 weeks.
Methicillin-resistant S. aureus (MRSA) CRBSI. Vancomycin is the treatment of choice for MRSA-CRBSI.179 The vancomycin dose should be adjusted to maintain trough levels of 15–20mg/l in order to achieve the best predictor of efficacy for this antibiotic in MRSA bacteremia (i.e., AUC/MIC >400).181 Teicoplanin is a suitable alternative to vancomycin, probably associated with fewer side effects, although serum level concentrations cannot be measured in clinical practice and the optimal dose is not well defined.182 If the vancomycin MIC is ≥1.5mg/l,183,184 alternative antibiotics such as daptomycin should be considered, although there are no randomized studies available. Combination therapies for complicated MRSA bacteremia have been reported, such as daptomycin with a beta-lactam (i.e., cloxacillin), daptomycin with fosfomycin, and imipenem with fosfomycin. For further information, this panel recommends a guideline recently released by the SEIMC.179 Duration of treatment for uncomplicated and complicated MRSA CRBSI is the same as for MSSA.
RECOMMENDATIONS
- 1.
The treatment of choice for an episode of MSSA CRBSI is cloxacillin or cefazoline (B-I).
- 2.
Patients allergic to beta-lactams should be treated with daptomycin (A-I) or a glycopeptide (B-II).
- 3.
The best antimicrobial treatment for episodes caused by MSSA strains with reduced susceptibility to vancomycin (MIC ≥1.5mg/l measured by E-test) has not been elucidated. This panel suggests using a combination of cloxacillin and daptomycin when blood cultures remain positive and/or there is no obvious clinical improvement after catheter removal (C-III).
- 4.
Vancomycin is the treatment of choice for CRBSI caused by MRSA (B-II). Teicoplanin may be a valid alternative, especially in cases of serious side effects associated with the use of vancomycin (C-III).
- 5.
Alternatively, patients may be treated with daptomycin, specifically if the MIC measured by E-test is ≥1.5mg/l (A-I).
- 6.
Linezolid should only be used in patients when the previous agents are contraindicated (C-III).
- 7.
For both MSSA and MRSA CRBSI, blood cultures should be obtained after 72h of antibiotic therapy (C-III).
What is the recommended directed therapy and optimal duration of treatment for CRBSI due to coagulase-negative Staphylococcus (CoNS)?
CoNS-CRBSI is associated with a significant increase in duration of hospital stay, although without attributable mortality.185–187 As these infections may resolve simply by removing the catheter, some authors suggest that antibiotic therapy is not necessary in immunocompetent patients with no signs of infection and no foreign bodies. If the catheter is removed, uncomplicated CRBSI can be treated with a short course of 5–7 days of antibiotics. In the infrequent case of a strain that is susceptible to methicillin, the recommended antibiotics are a penicillinase-resistant penicillin (i.e., cloxacillin 2g/4h) or cefazolin. Vancomycin is the treatment of choice for MR-CoNS CRBSI. Teicoplanin is also a suitable alternative for directed therapy.188
10–14 days of antibiotic therapy is recommended for patients with intravascular devices, biomedical devices, or persistent markers of inflammation after catheter removal, although this issue has not been addressed in clinical studies. If for some reason the catheter needs to be retained, antibiotic lock therapy is a further reasonable alternative.189
Staphylococcus lugdunensis can cause severe infection, with an aggressive clinical course similar to Staphylococcus aureus infection. For this reason, S. lugdunensis CRBSI should be managed as for S. aureus bloodstream infection.190
RECOMMENDATIONS
- 1.
Cloxacillin or cefazolin are the treatments of choice for episodes of CRBSI caused by CoNS susceptible to methicillin (B-I).
- 2.
For CoNS resistant to methicillin, a glycopeptide is the treatment of choice for directed therapy (B-II). Teicoplanin is recommended in the case of serious side effects associated with vancomycin. (C-III).
- 3.
The optimal trough concentration of vancomycin for the treatment of CoNS CRBSI is an unresolved issue and this panel cannot issue a specific recommendation (C-III).
- 4.
S. lugdunensis CRBSI should be managed as for S. aureus CRBSI (C-III).
What is the recommended directed therapy and its optimal duration for CRBSI due to Enterococcus spp.?
Enterococcus spp. are becoming an increasingly common cause of CRBSI and represent the fourth leading cause of these infections.191 For susceptible isolates, ampicillin is the drug of choice. After adjusting for confounders, glycopeptide use is associated with increased mortality in patients with Enterococcus faecalis bacteremia, compared with β-lactam therapy.192 There is no information to support the superiority of combination therapy (a beta-lactam plus an aminoglycoside) over β-lactam monotherapy for uncomplicated CRBSI.189 For other species of Enterococcus, particularly E. faecium, with a high rate of resistance to ampicillin, vancomycin is the drug of choice. For Enterococcus faecium isolates resistant to vancomycin, linezolid seems to be superior to daptomycin.193,194 Duration of treatment is an unresolved issue, but is within the range of 7–14 days.
It is worth mentioning that a recent retrospective cohort study of adults with enterococcal CRBSI showed a lower in-hospital mortality rate for patients whose CVCs had been removed (18.3% vs. 37.9%; p=0.03). In the multivariate analysis, catheter retention was an independent predictor of mortality (OR=3.34 [95% CI=1.21–9.26]).195
RECOMMENDATIONS
- 1.
Enterococcal CRBSI should be treated with catheter withdrawal and one active antimicrobial (A-III).
- 2.
Ampicillin is the drug of choice for susceptible isolates (A-II). Vancomycin should be reserved for isolates resistant to ampicillin or cases of beta-lactam allergy. For vancomycin-resistant isolates or severe adverse effects, linezolid is preferred to daptomycin (B-III).
- 3.
There is no evidence that combination therapy is necessary if IE has been properly ruled out (A-III).
- 4.
Despite data suggesting that duration of treatment may be shorter, the standard 7–14 days regimen continues to be recommended (A-III).
What is the recommended directed therapy and its optimal duration for CRBSI due to Gram-negative bacilli?
As stated in the section on empirical therapy, no clinical trials have assessed specific antibiotic drugs in the management of GN-CRBSI. For targeted therapy, the choice should be based on susceptibility results and directed at the narrowest spectrum antibiotic. In this clinical scenario, the principles of antimicrobial stewardship should be applied wisely.196 There are no studies evaluating the length of antimicrobial therapy for patients with GN-CRBSI. Duration of therapy should be individualized, taking into account clinical factors such as resolution of symptoms or immunological status. Recommended length of treatment is usually no less than 7 days.
RECOMMENDATIONS
- 1.
Directed therapy for GN-CRBSI should be chosen on the basis of the susceptibility results (C-III).
- 2.
The appropriate length of antimicrobial therapy has not been elucidated, although it is recommended to continue therapy for at least 7 days (C-II).
What is the recommended directed therapy and its optimal duration for CRBSI due to Candida spp.?
Echinocandins are currently recommended for empirical therapy in candidemic patients with severe infections.197,198 The decision of whether to continue with an echinocandin or use a step-down therapy to an agent with a narrower spectrum (i.e., fluconazole) is based on several factors: (a) catheter removal; (b) the strain is fluconazole-susceptible; (c) the patient has a good clinical response and is hemodynamically stable; (d) blood cultures have become negative. An open-label, non-comparative study documented de-escalation from anidulafungin to fluconazole as a safe strategy for patients with candidemia.199 In critically ill patients with invasive candidiasis, an observational study confirmed that de-escalation within 5 days is not related to increased day-28 mortality.200 No study has specifically assessed the impact of de-escalation of antifungal treatments in CRBSI caused by Candida spp. Combination therapy is not recommended for Candida-CRBSI.197,198 Removal of an intravenous catheter is an independent determinant of survival in patients with candidemia, especially when the catheter is the source of Candida bloodstream infection or associated with septic shock.120,201–203
Biofilm formation is an important factor in the pathogenesis of CRBSI and the choice of the most appropriate treatment should be guided by differences in the activity of antifungals against Candida biofilms. Liposomal amphotericin B and echinocandins are active against Candida cells in biofilm, while the activity of amphotericin B deoxycholate and azoles is poor.204 In the possible situation with certain types of patient that the catheter cannot be removed for some reason and must remain in place, it is wise to use an antifungal agent with high activity against the biofilm.205–208
Based on the study protocol of relevant clinical trials, the recommended duration of treatment is two weeks (14 days) after the first negative blood culture, so that follow-up blood cultures every other day until blood cultures become negative are helpful to establish the appropriate duration of antifungal therapy.
RECOMMENDATIONS
- 1.
In patients with Candida spp. CRBSI, this panel advocates de-escalation from an echinocandin or a lipid formulation of amphotericin B to fluconazole for susceptible isolates in clinically stable patients who have undergone catheter removal (B-II).
- 2.
The recommended duration of therapy for candidemia without obvious metastatic complications is two weeks after the first set of negative blood cultures (B-III).
- 3.
In candidemia, all intravascular catheters should be removed if at all feasible (B-II), particularly in patients with septic shock and when Candida CRBSI is suspected (B-III).
- 4.
If a catheter that is the source of a Candida bloodstream infection cannot be removed for any reason and remains in place, an antifungal agent with high activity against biofilms should be used (i.e., an echinocandin or liposomal amphotericin B) (A-II).
What is the recommended directed therapy and its optimal duration for CRBSI due to nontuberculous mycobacteria (NTM)?
CRBSI and/or sepsis are the most common healthcare-associated types of infection due to pathogenic rapidly growing mycobacteria (RGM) in both immunosuppressed and immunocompetent patients. The organisms may not only cause mycobacteremia, but can also present as local wound exudate from an exit site or tunnel infection. The most commonly recovered RGM species or groups include M. fortuitum, M. abscessus, and the M. mucogenicum group.209–211 Both short- and long-term catheters should be removed in CRBSI due to mycobacteria.
The duration of treatment for NTM CRBSI varies, but is usually at least 6–12 weeks to prevent relapse.212,213 In leukemic children, recent studies suggest that systemic infections due to mycobacteria may require up to 2 years of therapy, even if the catheter is removed. The prognosis is excellent if the catheter is pulled in addition to systemic antibiotic therapy over an extended period.
RECOMMENDATIONS
- 1.
The treatment for CRBSI caused by NTM involves removal of the infected catheter (B-II) followed by combination antimicrobial treatment appropriate for the species involved (B-III).
- 2.
The duration of treatment for NTM CRBSI should be 6 to 12 weeks to prevent recurrence of infection and the development of septic metastases (B-III).
Should antimicrobials for CRSBI be administered intravenously for the entire course of treatment?
The efficacy of treatment for CRBSI depends on the following factors: (a) early or prompt removal of the catheter; (b) documentation of bacteremia, identification of the causative organism and its susceptibility pattern; (c) clinical response during the first 48–72h of empiric therapy; and (d) development of complications. All patients with CRBSI require initial intravenous antimicrobial therapy. The above factors should determine duration of treatment and whether to use a sequential treatment or switch to the oral route. A randomized open trial compared oral combination therapy with a fluoroquinolone plus rifampicin (iv for 24h, but switched to the oral route as soon as possible) with standard parenteral therapy (flucloxacillin or vancomycin) for bacteremia or deep-seated infections caused by S. aureus or catheter-related bacteremia due to drug-susceptible CoNS. Approximately 40% of infections were CRBSI: two-thirds due to S. aureus and the rest to CoNS. Clinical and bacteriological cure rates were similar in both groups, although the median length of hospital stay was significantly shorter in the oral group.214 A recent study demonstrated that oral linezolid as monotherapy or combination therapy, mostly with rifampicin, is a valid alternative to intravenous therapy for patients with Gram-positive infections, although the number of CRBSI cases was low. Interestingly, none of the patients with CRBSI needed to be readmitted to hospital due to infection or to revert to intravenous antibiotic treatment.215
Clinical trials evaluating echinocandins allowed a swift change to oral fluconazole after 7–10 days of intravenous therapy, although specific analyses of the outcome of the Candida CRBSI subgroup are not available.216–218 A recent non-comparative trial of candidemia, in which approximately 50% of the episodes were CRBSI, showed that an early step-down strategy from intravenous anidulafungin to oral azole therapy after 5 days was effective and safe and reduced the length of intravenous treatment.199
No specific information is available about the use of oral therapy for Gram-negative CRBSI. Sequential oral therapy can be considered for clinically stable patients without metastatic complications and with negative blood cultures after onset of treatment and removal of the intravenous line.
RECOMMENDATIONS
- 1.
Sequential oral therapy can be considered in clinically stable patients without metastatic complications and with negative blood cultures after onset of treatment and removal of the intravenous line, if a therapeutic option with high oral bioavailability is available (A-II).
- 2.
In uncomplicated CRBSI caused by fluoroquinolone-susceptible staphylococci, initial intravenous antibiotic treatment may be switched to high-dose oral fluoroquinolones plus rifampicin in order to complete the course of antibiotic therapy if the patient is clinically stable and clearance of bacteremia is documented. Linezolid could be an option if the microorganism involved is fluorquinolone-resistant (A-II).
- 3.
In uncomplicated CRBSI caused by fluoroquinolone-susceptible Gram-negative bacilli, initial intravenous antibiotic treatment may be switched to high-dose oral fluoroquinolones in order to complete the course of antibiotic therapy if the patient is clinically stable and clearance of bacteremia is documented (A-II).
- 4.
A step-down from an echinocandin or lipid formulation of amphotericin B to oral fluconazole is safe and effective (C-III).
When is conservative management with antibiotic lock therapy recommended?
Whenever a conservative treatment is chosen, antibiotic lock therapy should be combined with a systemic antimicrobial. The patient should also be in a stable condition and the causative microorganism considered of low virulence, i.e., CoNS. Metastasis or local septic complications should be excluded before initiating conservative treatment. Table 4 summarizes the indications for catheter removal that make antibiotic lock therapy impossible. Lock therapy involves filling the catheter lumen with a mixture of an anticoagulant and a highly concentrated antibiotic or antiseptic, and temporarily stopping the catheter from flushing. There is no complete agreement at present about the choice of drugs, the duration of each lock period or local treatment.219 The first randomized, placebo-controlled trial220 included only CRBSI from long-term VADs, whether tunneled or totally implanted, and compared an antibiotic lock solution containing vancomycin and ceftazidime with placebo, in addition to parenteral antimicrobial treatment in both arms. 174 patients developed bacteremia, 85 of which were catheter-related and 44 patients met the criteria for the modified intention-to-treat analysis. Failure to cure CRBSI occurred in 33% of patients in the antibiotic lock arm and 57% in the placebo group (HR=0.55, p=0.10). The study failed to show statistically significant differences and had to be prematurely stopped due to enrolment difficulties. An open, retrospective and prospective non-comparative study of antibiotic lock therapy with vancomycin plus ciprofloxacin or amikacin for 7–16 days showed an 82% cure rate.172 A prospective non-comparative study of tunneled hemodialysis catheters causing bacteremia combined systemic antimicrobial therapy with lock therapy and cured 40 of 79 patients.221 When compared with the author's own historical series of patients treated with systemic antibiotics and immediate catheter withdrawal, salvage therapy was not associated with increased complications or long-term differences in survival.
Indications for catheter removal in patients with CRBSI.
| CRBSI presenting with septic shock |
| CRBSI caused by certain pathogens: S. aureus, non-fermenting Gram-negative bacilli, Candida spp. or Mycobacterium |
| Metastatic complications (endocarditis, thrombophlebitis or septic pulmonary embolism) |
| Bacteremia (or candidemia) persisting after 72h of adequate treatment |
| Pus is observed at the insertion site |
| Signs of infection at the subcutaneous tunnel |
| No possibility of antibiotic lock therapy |
RECOMMENDATIONS
- 1.
Conservative treatment should not be prescribed for patients with metastatic or local septic complications (A-II).
- 2.
The use of lock therapy added to systemic antimicrobial agents is systematically recommended for infected catheters that fulfill the criteria for catheter retention: the patient is stable, and the microorganism involved is considered to be of low virulence (i.e., CoNS) (A-I).
- 3.
In stable patients without local or systemic complications, conservative treatment may also be attempted for enterococci, corynebacterium (except Corynebacterium jeikeium) and Gram-negatives (consultation with an ID expert is suggested in such cases) (C-III).
- 4.
The use of an antibiotic lock does not preclude the need for systemic antimicrobial therapy (A-I).
What antibiotics and concentrations of antibiotic lock solutions are recommended?
The ideal antibiotic for the conservative treatment of CRBSI should have the following characteristics: (1) high activity against biofilms (ability to penetrate and disrupt the biofilm); (2) able to achieve high concentrations (100–1000 times the MIC of planktonic cells); (3) prolonged stability at room temperature over several days (enables prepared solutions to be stored and the antibiotic lock to be replaced every 24–72h); (4) compatibility with anticoagulants; (5) safety; (6) low potential for resistance; and (7) cost-effective.222–224
There are no randomized studies comparing the effectiveness of different antibiotics used for antimicrobial lock therapy (ALT). The data derive from very heterogeneous observational studies. This is a summary of the most commonly used published evidence.
Vancomycin is probably the most widely used antibiotic for ALT at concentrations ranging from 2000 to 20,000mg/l, with 2000mg/l being the most commonly used222,225 since the drug precipitates at 10,000mg/l. Vancomycin at 2000mg/l is stable at 37°C,172 and can be combined with heparin at 20–100IU/ml and 4% sodium citrate,226,227 as well as with other antibiotics such as ciprofloxacin, gentamicin, amikacin and ceftazidime, which facilitates the treatment of polymicrobial infections. In terms of efficacy, 2000mg/l vancomycin has been shown to cure 77–93% of infections caused by CoNS.172,225,228
Teicoplanin has been used at concentrations between 5000 and 20,000mg/l, the most commonly used being 10,000mg/l.172 It remains stable for 96h, with and without associated heparin.229 It can combine with 100IU/ml heparin,225 and with amikacin and gentamicin for polymicrobial infections.230 Teicoplanin 10,000mg/l has shown superior efficacy to vancomycin 2000mg/l.225
Daptomycin has been used at concentrations of between 3500 and 5000mg/l.225,231 Ringer's lactate should be added to the solution. The solution remains stable with and without heparin for 96h,229 and can be combined with heparin 100, 400 and 5000IU/ml and 4% sodium citrate (daptomycin 5000mg/l), as well as with 25% ethanol.232 In a study of 13 cases, daptomycin 5000mg/l achieved an 85% clinical cure rate.233
Ciprofloxacin at 2000mg/l has been used for the treatment of infections caused by Gram-negative bacilli, including Pseudomonas spp.,172,228,234 reaching success rates of 95% in selected populations.223 The solution remains stable for 10 days at 37°C. It precipitates with heparin,230 but maintains its efficacy.172
Amikacin has been widely used at concentrations of between 1500 and 60,000mg/l, the most frequently used being 2000mg/l.222 It can be administered with heparin and its efficacy is high, above 90%.222
Other antibiotics used as ALT for the conservative treatment of CRBSI are gentamicin (2000–5000mg/l), cefazolin (5000–10,000mg/l), and ceftazidime (500–10,000mg/l).222,223
RECOMMENDATION
- 1.
The most frequently used antibiotics for conservative treatment of CRBSI using ALT are vancomycin 2000mg/l, teicoplanin 10,000mg/l, daptomycin 5000mg/l, ciprofloxacin 2000mg/l, and amikacin 2000mg/l (B-I).
How should antibiotic lock therapy be performed?
Lock solutions reported in the literature with potential use in clinical practice are described in Table 5. Although many published studies on the effectiveness of ALT are available, few describe the technique in detail.104,222–224
Lock solutions described in the literature with potential use in clinical practice.
| Microorganism | Antimicrobial | Concentration | Notes |
|---|---|---|---|
| Staphylococcia | Daptomycin | 5mg/ml | Dilute in Ringer's lactate solution (with calcium) |
| Vancomycin | 2mg/ml | Incompatible with heparin >5mg/ml | |
| Teicoplanin | 10mg/ml | ||
| Enterococcib | Vancomycin+Gentamycin | Both 2mg/ml | |
| Gram-negative bacillic | Levofloxacin | 5mg/ml | Precipitates with heparin |
| Ciprofloxacin | 2mg/ml | Precipitates with heparin | |
| Amikacin | 2–10mg/ml | ||
| Piperacillin-tazobactan | 10mg/ml | ||
| Candida speciesd | Echinocandins | 5mg/ml | |
| Liposomal amphotericin B | 1–5mg/ml | ||
This table is not intended to be an exhaustive list. Since there are no clinical trials using levels of evidence, it reflects only the opinion of experts. Although there is no scientific evidence to make recommendations regarding optimal time duration and replacement of lock solutions, we recommend extending it for 14 days, and also drawing a blood culture through all catheter lumens 72h after completion of therapy. We also remind users that antimicrobial lock therapy is necessary but not sufficient. Any antimicrobial lock therapy must be accompanied by a systemic antibiotic treatment that will last over time, depending on the pathogen involved.
A conservative treatment is recommended only in the case of coagulase-negative staphylococci. Catheter removal is recommended if S. aureus is involved.
There is insufficient experience to recommend conservative treatment. However, if the patient is stable and bacteremia is uncomplicated, a conservative treatment may be considered.
ALT preparation and storage. The solution should be prepared under sterile conditions, ideally in the Pharmacy Service. These solutions have prolonged stability and can be prepared every 3–7 days and stored at 4°C until required for use (Table 6).
Preparation of the most common antibiotic solutions for lock therapy.
| Vancomycin 2000mg/l plus sodium heparin 20IU/ml | 250ml of 0.9% saline or 5% glucose+500mg of vancomycin+5ml of 1% sodium heparin (1ml heparin=1000IU) |
| Teicoplanin 10,000mg/l plus sodium heparin 125IU/ml | Reconstitute 400mg of teicoplanin with 3ml sterile water for injection Remove 18ml from a bag of 50ml of 0.9% saline Add 3ml of reconstituted teicoplanin to saline bag Add 5ml of 1% sodium heparin to saline bag |
| Daptomycin 5000mg/l plus sodium heparin 100IU/ml | Reconstitute 350mg of daptomycin with 7ml of sterile water for injection With a 1ml syringe, take 1ml of reconstituted daptomycin With the same syringe, take 1ml of 1% sodium heparin With the same syringe, take 8ml of Ringer lactate |
| Ciprofloxacin 2000mg/l plus sodium heparin 20IU/ml | Add 4ml of 1% sodium heparin in a bag of 400mg of ciprofloxacin Stir for a minute before taking the required amount of solution |
| Amikacin 2000mg/l plus sodium heparin 20IU/ml | 250ml of 0.9% saline or 5% glucose+500mg of amikacin+5ml of 1% sodium heparin |
Volume of the lock solution. Most studies use between 2 and 3ml in tunneled catheters and 3–5ml in totally implantable ports.172,234–239 However, considering the great variability of catheters used, the exact catheter volume specified in the instructions provided by the manufacturer should be instilled.234,236,240
Replacement of ALT solutions. Before using the catheter or replacing the ALT solution, the previous ALT should be removed172,221,237,240–243 to prevent the risk of adverse events associated with the rapid infusion of antibiotics at high concentrations and the cleaning of the catheter lumen occurring by entrainment.
Length of ALT. The optimal duration of ALT is not known. In most recent studies, ALT was given for 10–14 days,172,220,228,233,234,238,242–244 although a shorter treatment duration may be efficacious, especially for Gram-negative infections.172,244
Frequency of ALT. The frequency of ALT replacement has not been established. It is usually performed every 24–72h and adapted to the use and needs of the infected line. In hemodialysis patients, ALT is replaced after each hemodialysis session.172,233–235,240,245 If more frequent use of the catheter is needed, the lock is replaced every 24h.220,228,244
Catheter use. Ideally, the catheter should not be used while the ALT solution is in place. However, for patients receiving parenteral nutrition or those with few or no other venous access options, ALT and catheter may be alternated. In such cases, a minimum of 8–12h a day is recommended.220,228,234,244,246 If the catheter has more than one lumen, all should be treated.
Systemic treatment. Bacteremic patients should be treated with systemic antibiotics for a period of 7–14 days.172,228,234,236,242,244,246 This period may be shorter for CoNS infections.25
RECOMMENDATION
- 1.
An ALT solution should be prepared under sterile conditions. It should be infused after removing the previous dose and the exact volume of the catheter lumen should be infused. The recommended duration of ALT is 10–14 days. The ALT solution must remain in the catheter lumen for a minimum of 12h a day and should be replaced every 24–72h (B-I).
What non-antibiotic substances could be used for lock therapy?
Apart from the antibiotics described above, other non-antibiotic substances have been used for lock therapy.
Ethanol (with activity against bacteria and fungi) has been used for the prevention of CRBSI in long-term CVCs. In several therapeutic randomized trials, a 70% ethanol lock showed a significant decrease in the rate of CRBSI compared with saline or heparin solutions.167,247–250 It is important to note that these studies also reported severe adverse events, such as flushes, dizziness, doubling of liver enzymes, catheter rupture or thrombosis, leading to interruption of therapy in some patients.250 In two retrospective studies and one randomized study including more than 100 patients that used 70% ethanol lock therapy for the treatment of CRBSI, cure rates were reported in 62–91% of cases with no significant adverse events.167,251,252
Taurolidine, like 70% ethanol, has been evaluated in several large randomized studies of the prevention of CRBSI. Taurolidine, mostly compared with heparin, was associated with significant reductions in the rate of bloodstream infections. In a retrospective study of 11 cancer patients treated for CRBSI with a taurolidine lock, only three relapsed, but were eventually cured with another taurolidine lock.253
EDTA and citrate. These two chelators are able to disrupt biofilm, thus increasing antimicrobial activity. Several in vitro studies have demonstrated the proven anti-biofilm effect of EDTA alone or in combination with gentamicin or minocycline plus 25% ethanol.104,222 Further clinical studies are needed to establish the role of these two substances.254
RECOMMENDATION
- 1.
70% ethanol and taurolidine locks might be used for the conservative treatment of CRBSI. However, there is no evidence to advocate for their routine use (B-I).
What are the criteria for failure of conservative management?
The criteria for failure of conservative treatment of CRBSI are based on the patient's worsening clinical condition, persistence of infection, and catheter dysfunction or removal.85,166,172,228,255–257
It does not seem to be ethical to perform a randomized clinical trial about retaining infected catheters for certain critical clinical conditions. Catheter dysfunction requiring replacement is also considered failure of conservative treatment. In most reports, catheters were removed for ongoing sepsis, defined as persistent fever or bacteremia after 48–72h of adequate therapy, or if metastatic septic complications like endocarditis or osteomyelitis, or local complications, such as venous thrombosis, septic phlebitis or tunnelitis, occurred. Conservative management is contraindicated for some of these complications, which should be followed by sequential blood cultures drawn both from a peripheral vein and through the catheter to monitor the clinical course of CRBSI.228,255
Definitions of efficacy or failure of conservative management in clinical studies or clinical practice sometimes include late relapse of infection.257
RECOMMENDATION
- 1.
Any clinical condition or catheter dysfunction prompting catheter removal should be considered failure of conservative management (A-I).
How should insertion site infection be managed?
Short-term catheters (peripheral venous, non-tunneled CVCs and arterial catheters) with erythema, pain, warmth, induration and/or purulent drainage within 2cm of the catheter exit site should be removed in spite of absence of concomitant bacteremia.25,258 Any exudate at the insertion site should be submitted for Gram staining, routine culture, and fungal culture when assessing immunocompromised patients.25
In uncomplicated infections involving long-term catheters (tunneled CVCs, hemodialysis), defined as absence of fever, positive blood cultures or purulence, cultures of any drainage from the exit site should be obtained, together with peripheral blood cultures.259 Under these circumstances, topical application of an antibiotic ointment at the insertion site may be considered, based on exit-site culture results. If the infection does not resolve or purulent exudate develops, systemic antibiotics should be administered. If clinical signs of infection persist after 48–72h of appropriate antimicrobial therapy, the catheter should be removed.25,259 The topical application of antibiotic ointment to the insertion site following catheter removal is not recommended.260
RECOMMENDATIONS
- 1.
For peripheral venous catheters, catheter removal is mandatory if there is local pain, induration, erythema or exudate (A-I).
- 2.
For non-tunneled CVCs, the presence of erythema or purulence at the catheter insertion site requires immediate catheter removal (B-II).
- 3.
For uncomplicated exit site infections with long-term catheters, a conservative approach with topical antimicrobial agents should first be attempted. In cases of topical treatment failure, systemic antibiotics should be administered (B-III).
- 4.
Persistence of clinical signs of infection beyond 72h of conservative management requires removal of the catheter (B-II).
How should tunnelitis be managed?
Tunnel infection in a long-term catheter, other than a hemodialysis catheter, should be managed with catheter removal, drainage and incision, if indicated, and 7–10 days of systemic antibiotic therapy in the absence of concomitant bacteremia or candidemia.25,261 If systemic antibiotics fail, the catheter should be removed. In the setting of tunnel infection with fever, catheter removal is the first option, together with adequate antibiotic therapy.68,262
Taking a conservative approach, failure rates of more than 50% have been reported and, in this case, are associated with increased cost and workload.222,263
RECOMMENDATIONS
- 1.
Patients with tunnelitis not associated with a hemodialysis catheter require catheter removal, incision and drainage, if indicated, and 7–10 days of systemic antimicrobial therapy in the absence of concomitant bacteremia or candidemia (A-II).
- 2.
For tunnelitis without fever in hemodialysis catheters, systemic antibiotic therapy may be attempted first (A-II). In tunnel infection with fever, catheter removal is the first therapeutic option together with systemic antimicrobial therapy (A-II).
- 3.
Tunnelitis conservative management is associated with higher failure rates (B-II).
How should a local infection associated with a port reservoir be managed?
A complicated local infection of a venous access device is defined as infection of the tunnel or port pocket with erythema or induration (more than 2cm), purulent collection, skin necrosis and spontaneous rupture and drainage. A stitch abscess is a focal area of purulence or redness around a suture. The single offending stitch can usually be removed without further consequences and should not be confused with a port infection.263 Management of a port reservoir infection requires removal of the port, drainage of affected tissues and administration of antibiotic therapy for 7–10 days in the absence of concomitant bacteremia or fungemia.25,222,224 Depending on the severity of the infection, the insertion wound may be sutured following removal of the port, or, if there is significant drainage, exudate or pus, the wound should be left open and packed with iodoform gauze to heal by secondary intention.263 Surgical removal of a venous access port is frequently a challenge to management and is initially avoided. Alternately, it may be possible to salvage the port with a conservative treatment by stopping use of the device and initiating a combination of antibiotic lock therapy and systemic antibiotic treatment.104 Most infections are associated with intraluminal colonization and it is necessary therefore to administer a high concentration of antimicrobial solution to try and sterilize the device.264
RECOMMENDATIONS
- 1.
In the presence of signs of local inflammation at the reservoir pocket, the port must be removed, the affected tissue drained, and systemic antibiotic therapy started (A-II).
- 2.
If a conservative strategy is the only option, a combination of systemic antibiotics and antibiotic lock therapy should be prescribed, bearing in mind that this approach is associated with a high failure rate (B-II).
In which patients and when should a follow-up blood culture be taken?
Persistent bloodstream infection is defined as the presence of viable pathogens in the blood after 3 days of appropriate antimicrobial treatment. Persistent bacteremia with certain pathogens has been associated with the development of complications and worse outcomes.265 Patients with persistent bacteremia due to Staphylococcus aureus present higher relapse rates and related mortality within 12 weeks of a bacteremia episode.266 The most robust predictor of complicated S. aureus bacteremia was positive follow-up blood cultures at 48–96h after the first positive blood culture.267 In a study in which blood cultures were taken every 3 days following a positive blood culture for S. aureus, the rate of septic metastasis for bacteremia lasting <3 days was 5%, increasing to 25% in patients with ≥10 days of documented bacteremia.268
Persistent candidemia has also been associated with a high mortality rate. Kim et al. reported that persistent candidemia increased the risk of mortality, with adjusted hazard ratio of 2.5 (95% CI=1.33–4.72). As antifungal therapy should be continued until 14 days after the first negative blood culture, follow-up blood cultures should be obtained daily until the first negative blood culture.269
RECOMMENDATIONS
- 1.
Follow-up blood cultures should be taken from all patients with S. aureus or Candida spp. CRBSI (A-II).
- 2.
In patients with S. aureus CRBSI, we recommend that follow-up blood cultures should be obtained every 72h until the first negative result (A-II).
- 3.
Control blood cultures in CRBSI due to Candida spp. should be obtained every 48h until the first negative blood culture (A-II).
- 4.
For other causative microorganisms of CRBSI and if catheter salvage is attempted, follow-up blood cultures should be obtained 72h after starting appropriate antibiotic therapy. If persistent bacteremia is documented, catheter removal is required (B-II).
- 5.
It is not necessary to routinely perform follow-up blood cultures in patients with CRBSI due to microorganisms other than S. aureus or Candida spp. if the catheter has been withdrawn (A-II).
When should echocardiography be performed?
The risk of underlying infective endocarditis in bacteremic patients depends mainly on the etiologic agent causing the bacteremia and the predisposing conditions of the patient. Patients with S. aureus bacteremia are at high risk for IE, which is frequently not clinically evident or suspected.
The absence of valvular risk (no valvular disease, either previously or diagnosed at the moment of SAB), together with a clinical and microbiological response (negative blood cultures) to therapy within the first 72h of catheter removal and initiation of adequate antibiotic therapy were associated with a favorable outcome (absence of complications or relapse) in more than 95% of patients who received treatment for at least 14 days after negative blood cultures. A recent systematic review270 of 9 observational studies with sample sizes ranging from 98 to 877 patients271,272 reported an incidence of 2% to 14% detected by transthoracic echocardiography (TTE) and 14% to 25% by transesophageal echocardiography (TEE). Clinical findings and TTE were poorly predictive of subsequent TEE findings. In a high proportion of cases, IE was not suspected on clinical grounds and 15% of cases were reclassified by TEE.273
Currently, 6 studies274–279 suggest that, due to the very low risk for IE, TEE can safely be avoided in patients without any of the following risk factors: prolonged bacteremia, hemodialysis, community-acquisition, metastatic foci of infection, immunologic or embolic phenomena, intravenous drug abuse (IVDA), implantable CVC, intracardiac device), prosthetic valve, previous IE or cardiac structural abnormality.
In patients with proven enterococcal CRBSI, the requirement to systematically rule out endocarditis is currently under discussion. Estimates of endocardial involvement vary and are not well addressed in the medical literature. In a recent study of 1515 patients with enterococcal BSI (E-BSI), 65 (4.29%) had enterococcal endocarditis, representing 16.7% of patients with E-BSI who underwent TTE and 35.5% with E-BSI who underwent TEE. A bedside score totalling 12 points for predicting enterococcal endocarditis was developed, the NOVA score, based on the number of positive blood cultures, origin of the bacteremia, prior valve disease and the auscultation of a heart murmur. A NOVA score of less than 5 points, which corresponded to 14 to 27% of patients with enterococcal bacteremia, identified a subgroup at very low risk for enterococcal endocarditis who could avoid TEE.280
The incidence of endocarditis in patients with candidemia has been assessed less frequently. In a recent study, endocarditis was detected in 2.9% of patients with candidemia using TTE and in 11.5% undergoing TEE.281
RECOMMENDATIONS
- 1.
TEE should be performed in the vast majority of patients with Staphylococcus aureus bacteremia. TEE is not necessary or can be delayed in patients without the following risk factors: prolonged bacteremia, hemodialysis, metastatic foci of infection, IVDA, implantable CVC, intracardiac device, prosthetic valve, previous IE or cardiac structural abnormality (A-II).
- 2.
The need for TEE in episodes of CRBSI caused by other pathogens should be individualized. This panel considers that IE should be ruled out in all patients with persistent bacteremia (or fungemia) (C-III). Enterococcus spp. and Candida spp. pathogens are associated with a high risk of developing endocarditis.
What is the diagnosis and management for suppurative thrombophlebitis?
Suppurative thrombophlebitis refers to venous thrombosis associated with infection and bacteremia. The pathogenesis of catheter-related thrombosis results from the activation of coagulation pathways by the foreign material in the bloodstream, vascular endothelial damage and endothelial cell activation.25,282 Infection may also stimulate thrombus formation by aggravating coagulation abnormalities. The presence of a thrombus mass around the catheter increases the risk for microbial colonization and bacteremia.283 CRBSI and thrombosis appear therefore to have a bidirectional relationship.
Suppurative thrombophlebitis combines the signs and symptoms of infection from the thrombosis with the dysfunction of the involved catheter. Microbiological and radiologic tests are necessary to confirm the diagnosis. Thrombosis should be confirmed with ultrasonography (70–100% sensitivity and 93% specificity), high-resolution computed tomography or phlebography. Limited experience with magnetic resonance imaging suggests that it may also be useful in the diagnosis of thrombophlebitis.25,284 Recent data indicate that a proactive search for thrombosis in the setting of suspected CRBSI is a safe and effective strategy that enables the catheter to be preserved in neutropenic patients if thrombosis is ruled out.284
Management of thrombophlebitis requires catheter removal, prolonged antimicrobial treatment of at least 4–6 weeks, surgery (drainage of abscess and/or venous resection) if a collection is detected or the clinical response is not achieved, and thrombus treatment (anticoagulation or even thrombolytic therapy).283 Venous resection has not been shown to be superior to conservative management (including involvement of superficial veins). There is insufficient clinical evidence available to support the use of systemic anticoagulation, while systemic thrombolysis has only been used in specific cases.285–287
Follow-up of thrombophlebitis should include clinical data, sequential ultrasonography and possibly biomarkers. Procalcitonin (PCT) will probably be more effective for detecting non-responding CRBSI potentially complicated by the associated thrombophlebitis, in which case urgent catheter removal would be required.288
RECOMMENDATIONS
- 1.
Suppurative thrombophlebitis should be ruled out in all episodes of CRBSI with persistent bacteremia (A-II).
- 2.
Confirmed diagnosis, mainly by ultrasonography, should be followed by catheter withdrawal, prolonged antibiotic treatment and an individualized assessment of the need for anticoagulation (A-II).
When can a new catheter be inserted?
There is no scientific evidence indicating how long the patient should wait before a new catheter can be safely inserted after an episode of CRBSI. The placement of a new catheter will obviously be determined by the need for vascular access. Patients with short-term catheters for vital continuous infusion medications usually require immediate insertion of a new catheter. If it is feasible to wait, PCT may be useful for monitoring the response to therapy. In a small prospective study including 26 patients with CRBSI, a serum PCT concentration of >1.5ng/ml on day 3 of therapy was associated with lack of response to therapy (sensitivity 70%, specificity 68.7%; p=0.028), while a decrease in serum PCT concentrations of at least 1.00ng/ml from day 1 to day 2 and of 0.30ng/ml from day 2 to day 3 indicated response to therapy (p=0.037 and 0.017, respectively).288
The clinical situation of patients with long-term catheters, implantable venous access catheters (IVAC) or tunneled catheters may allow for a time interval before a new catheter is placed. Experts recommend waiting for resolution of clinical signs or even microbiological eradication (negative blood cultures). The only available study is a small case-control evaluation, which showed no differences between removal with simultaneous reimplantation in 13 patients and delayed reimplantation (mean 14 days) in 21. There were two cases of re-infection in the simultaneous reimplantation group (15.4%) and one case in the delayed reimplantation group (4.8%).289 Non-randomized studies of hemodialysis-associated CRBSI have shown heterogeneous results.166
RECOMMENDATIONS
- 1.
Although there is a clear lack of scientific evidence, it seems advisable to wait, if feasible, before placing a new catheter after an episode of CRBSI. The waiting period should be determined by the resolution of signs and symptoms. If a patient urgently needs vascular access, a catheter should be inserted without delay (C-III).
- 2.
Insertion of a new catheter after a diagnosis of CRBSI is always possible if the patient's clinical condition dictates the need for a new vascular access (A-III).
Jaime Esteban has participated in counseling and lectures at meetings sponsored by Laboratorios Leti SL in the last year, as well as received grants for research and teaching from laboratories Pfizer, Sysmex, Angelini and bioMérieux.
Paula Ramírez has participated in counseling and lectures at meetings sponsored by Pfizer, MSD, Astellas, Gilead and Otsuka in the last year, as well as received grants for research and teaching at Otsuka laboratories.
José Luis del Pozo has lectured at meetings sponsored by Pfizer, MSD and Angelini Laboratories.
Miguel Salavert has participated in counseling and lectures at meetings sponsored by MSD, Pfizer, Gilead, Astellas Ph and Angelini in the last year, as well as received grants for research and teaching from Janssen and ViiV laboratories
José Ramón Paño has participated in counseling and lectures at meetings sponsored by Janssen, Gilead and MSD in the last year.
José Garnacho-Montero has participated in conferences sponsored by MSD and Astellas.
Emilio Bouza has participated in advisory services and lectures sponsored by MSD, Pfizer and Astellas in the last year.
The rest of the authors declare that they have no conflicts of interest.
The complete consensus statement has also been published in: Enferm Infecc Microbiol Clin. 2017. http://dx.doi.org/10.1016/j.eimc.2017.10.019