Complicated post-cardiac surgery course, can lead to both prolonged ICU stay and ventilation, and may require a tracheostomy. This study represents the single-center experience with post-cardiac surgery tracheostomy. The aim of this study was to assess the timing of tracheostomy as a risk factor for early, intermediate, and late mortality. The study’s second aim was to assess the incidence of both superficial and deep sternal wound infections.
DesignRetrospective study of prospectively collected data.
SettingTertiary hospital.
PatientsPatients were divided into 3 groups, according to the timing of tracheostomy; early (4−10 days); intermediate (11−20 days) and late (≥21 days).
InterventionsNone.
Main variables of interestThe primary outcomes were early, intermediate, and long-term mortality. The secondary outcome was the incidence of sternal wound infection.
ResultsDuring the 17-year study period, 12,782 patients underwent cardiac surgery, of whom 407 (3.18%) required postoperative tracheostomy. 147 (36.1%) had early, 195 (47.9%) intermediate, and 65 (16%) had a late tracheostomy. Early, 30-day, and in-hospital mortality was similar for all groups. However, patients, who underwent early- and intermediate tracheostomy, demonstrated statistically significant lower mortality after 1- and 5-year (42.8%; 57.4%; 64.6%; and 55.8%; 68.7%; 75.4%, respectively; P < .001). Cox model demonstrated age [1.025 (1.014–1.036)] and timing of tracheostomy [0.315 (0.159−0.757)] had significant impacts on mortality.
ConclusionsThis study demonstrates a relationship between the timing of tracheostomy after cardiac surgery and mortality: early tracheostomy (within 4−10 days of mechanical ventilation) is associated with better intermediate- and long-term survival.
La evolución complicada de un postoperatorio de сirugía cardiaca puede dar lugar tanto a una estancia prolongada en UCI como a ventilación mecánica prolongada y puede requerir de una traqueotomía. Este estudio presenta la experiencia acumulada sobre traqueostomía en el postoperatorio de cirugía cardiaca en un único hospital.El objetivo era evaluar el momento de la realización de la traqueotomía como factor de riesgo de mortalidad temprana, intermedia y tardía.
DiseñoEstudio retrospectivo.
ÁmbitoHospital terciario.
PacientesPacientes fueron divididos en 3 grupos según el momento de la traqueotomía; temprano (4−10 días); intermedio (11−20 días); tardío (≥21 días).
IntervencionesNo.
Variables de interés principalsLos resultados primarios fueron la mortalidad en cada grupo.
ResultadosDurante los 17 años de duración del estudio, de los 12.782 pacientes sometidos a cirugía cardíaca, 407 (3,18%) requirieron traqueotomía postoperatoria. Se practicaron 147 (36,1%) traqueotomías tempranas, 195 (47,9%) intermedias y 65 (16%) tardías. La mortalidad temprana, a los 30 días dentro del marco hospitalario, fue similar en todos los grupos. Sin embargo, las traqueotomía temprana e intermedia demostraron una mortalidad inferior estadísticamente significativa a 1 y 5 años (42,8%; 57,4%; 64,6%; y 55,8%; 68,7%; 75,4%, respectivamente; P < ,001). El modelo de Cox demostró que la edad [1,025 (1,014–1,036)] y el momento [0,315 (0,159–0,757)] impacta significativamente la mortalidad.
ConclusionesLa traqueotomía temprana (dentro de los 4−10 días de ventilación mecánica) en el postoperatorio de cirugía cardíaca se asoció con una mejor supervivencia a medio/largo plazo.
Most patients undergoing cardiac surgery have a postoperative course characterized by a short period of postoperative mechanical ventilation and a short stay in the cardiac surgery intensive care unit (CSICU). A subset of patients experiences a complicated postoperative course that requires both a prolonged ICU stay and a period of mechanical ventilation. These patients often require tracheostomy to optimize their care. As both our patient population and surgical procedures have become more complex in recent years, the number of patients requiring prolonged mechanical ventilation after cardiac surgery has increased.1,2
Because of this trend and an increased need for tracheostomy in the critical care patient population, many high-volume centers have implemented specialized tracheostomy teams, which have proven to be the safest and most cost-effective model to meet this growing demand.3
Tracheostomy may reduce mortality in the subgroup of patients requiring long-term mechanical ventilation.4 Despite the growing experience in the management of these patients, there is no data identifying an ideal time for tracheotomy.5 Several studies have investigated the outcomes of early versus late tracheostomy. However, early tracheostomy may increase the risk of sternal wound infection.6 Nevertheless, mortality data associated with early versus late tracheostomy is controversial. The first aim of this study was to assess the timing of tracheostomy as a risk factor for early, intermediate, and late mortality. The study’s second aim was to assess the incidence of both superficial and deep sternal wound infection (SWI).
Patients and methodsThis is a retrospective, observational study of prospectively collected data of patients undergoing cardiac surgery at a large tertiary care university hospital. The Sheba Medical Center Institutional Ethics Committee (Protocol No 4257) approved the study and written informed consent was waived due to the retrospective nature of the study. Data were collected and entered into a computerized departmental database. All patients undergoing cardiac surgery were included in the cohort. The indications for tracheostomy were as follows: first, prolonged mechanical ventilation, defined as ventilation for more than 4 days. Second, failure of extubation is defined as the inability to pass spontaneous breathing trial or reintubation. We did not use a specific protocol, and the time for tracheostomy was determined jointly by the surgeon and the intensive care specialist, according to the clinical condition of the patient.
Patients were grouped according to the time that elapsed between surgery and tracheostomy. Based on the previous studies,7 patients were divided into three groups based on the timing of tracheostomy; Group I, early (4−10 days); Group II, intermediate (11−20 days) and Group III, late (≥21 days). The groups were compared for preoperative demographic data, medical comorbidities, and operative data. Demographic data included sex and age. Perioperative parameters included chronic obstructive pulmonary disease (COPD), smoking history, congestive heart failure (CHF), cardiac arrhythmia, diabetes mellitus (DM), dialysis-dependent renal failure, peripheral vascular disease (PVD), left ventricular ejection fraction (LVEF), previous myocardial infarction (MI), previous cerebrovascular accident (CVA)/transient ischemic attack (TIA), obesity, systemic and pulmonary hypertension. In the postoperative period, we also studied low cardiac output (LCO) syndrome, ventilator-associated pneumonia (VAP), and surgical re-intervention for excessive bleeding or cardiac tamponade.
COPD was diagnosed in presence of chronic bronchitis and/or evidence of emphysema. Congestive heart failure (CHF) was defined according to New York Heart Association [NYHA] classification. The presence of DM is defined as the chronic administration of antidiabetic oral medication and/or insulin use, or preoperative glycated hemoglobin level greater than 6.5%. Cardiac arrhythmia was defined as atrial fibrillation and included both chronic and paroxysmal atrial fibrillation that were detected preoperatively. Patients undergoing renal replacement therapy were defined as chronic hemo- or peritoneal dialysis for end-stage renal failure. PVD was diagnosed according to an angiogram study or Doppler ultrasound flow study. Cerebrovascular accident (CVA) was defined as the presence of a lesion in the brain shown by computed tomography (CT) or magnetic resonance imaging and/or an episode of neurologic dysfunction caused by focal ischemia without acute infarction. Obesity was defined as a BMI greater than or equal to 30.8 Hypertension was defined as any preoperative episode of medical treatment for hypertension. According to the previous research,9 pulmonary hypertension was defined as systolic pulmonary arterial pressure of 50 mm Hg or more, measured by echocardiography.
Low cardiac output (LCO) is defined as the duration of inotropic support for >24 h, or a cardiac index <2.0 L/min/M2 or/and metabolic acidosis (a lactate >2.0 or base difference >4.0) with clinical signs of hypoperfusion (tachycardia >90/min or oliguria 0.5 ml/kg/h) or using assist.10 Postoperative ventilator-associated pneumonia (VAP) was identified according to the National Healthcare Safety Network (NHSN) definition.11
Relevant data relating to surgery type included previous cardiac surgery, the priority of surgery (elective, urgent, or emergent), and logistic and standard EuroSCORE. The surgical type was divided into simple (isolated valve or coronary artery bypass graft (CABG) surgery) versus complex surgery (combined procedures or aortic procedures).
Study endpointsWe compared early (30-day and in-hospital), intermediate (1-year, 2-year), and late (5-year) mortality after tracheostomy as the primary outcome of the study. In addition, we compared the mortality of patients who did or did not undergo tracheostomy. Also, we assess the incidence of both superficial and deep sternal wound infection (SWI).
During the initial period of the study, from 01.09.2004 to 31.12.2006, the CSICU functioned according to an open model, under the supervision of a cardiac surgeon. On 01.01.2007, the CSICU was converted to a semi-closed model, and since then until the end of this study was supervised by a board-certified intensivist. In the period between 09.2004 and 01.2007 tracheostomies were performed by open approach by ENT surgeons. From 02.2007, all tracheostomies were performed at the bedside by an experienced thoracic surgical team, which includes 5–6 surgeons and 2–3 anesthesiologists, using the percutaneous dilatation technique (Portex® Griggs™ Forceps Percutaneous Dilation Tracheostomy Kits, Smith Medical, St. Paul, MN). During the study, no other major changes in hospital policy, or surgical or anesthesiological techniques were introduced.
Statistical analysisData are presented as mean ± standard deviation if normally distributed or as median values and interquartile range. Continuous variables were tested with the Kolmogorov-Smirnov test for comparison of normal distribution. Categorical variables are given as frequencies and percentages. A chi-square test was used for the comparison of categorical variables between the groups. Student's t-test and one-way ANOVA were performed for the comparison of normally distributed continuous variables, followed by post hoc tests for multiple comparisons (Bonferroni’s test).
For non-normal distribution variables, we used the Mann-Whitney U test and Kruskal-Wallis. 1‒5-year mortality compared as binary data.
We used the multivariable and univariable analysis Cox Proportional hazards model with time-dependent covariates to explore the independent association of tracheostomy timing on mortality. In the univariable analysis, we included age, gender, previous MI, COPD, smoking, morbid obesity, combined surgery, tracheostomy timing, re-intubation, method of tracheostomy (open or percutaneous), organization model of the ICU (open vs. semi-closed), preoperative mechanical ventilation, postoperative ECMO and/or IABP.
In the multivariable analysis, we included covariates that differed significantly from the univariable. Multivariable analysis for mortality was only performed in patients who had a tracheostomy. Therefore, the Cox Proportional hazards model included the following covariates: age, sex, morbid obesity, smoking, and tracheostomy timing. In addition, Kaplan-Meier survival analysis was performed to compare mortality among the groups, with statistical differences tested by the log-rank test. Statistical significance was assumed when the null hypothesis could be rejected at P < .05. All P-values are the results of two-sided tests. Statistical analyses were conducted using SPSS (version 27). (IBM SPSS Statistics, Chicago, Illinois, USA).
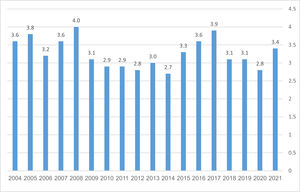
ResultsDuring the 17-year study period, between 09.2004 and 08.2021, 12,782 patients underwent cardiac surgery at our institution, of whom 407 (3.18%) required postoperative tracheostomy (see Table 1). One hundred and forty-seven (36.1%) had early (Group I); 195 (47.9%) intermediate (Group II) and 65 (16%) late (Group III) tracheostomies. The relative incidence of tracheostomies did not change throughout the study (see Fig. 1). Most baseline and operative parameters were similar for all groups, with the following exceptions: the number of men was greater in Group I than in Groups II and III. The incidence of COPD, smoking, and dialysis was higher in Group I than in Group II. In addition, the difference between the groups was regarding the frequency of MI and ECMO. 47 patients (11,5%) underwent open, surgical procedures, and 360 (88.5%) – percutaneous procedures. 70 patients (17.2%) underwent tracheostomy when the ICU functioned as an open unit and 337 (82.8%) as a semi-closed unit. From a surgical procedure perspective, no significant differences between the groups were noted concerning frequency and type of procedure, duration of cardiopulmonary bypass time, and the cross-clamp time (see Table 2).
Perioperative patient’s data.
| Tracheostomy | No tracheostomy | P Value | |
|---|---|---|---|
| N | 407 | 12,610 | |
| Age (y) | 67.8 ± 12.6 | 63.9 ± 13.4 | <.001 |
| Male | 244 (60.1%) | 8720 (69.2%) | <.001 |
| Elective | 164 (40.4%) | 6938 (55.0%) | <.001 |
| Open ICU/Semi-closed ICU | 70/337 | 3410/9200 | .36 |
| FC III | 143(41.9%) | 3043 (29.0%) | <.001 |
| FC IV | 63 (18.5%) | 583 (5.5%) | |
| NYHA III-IV | 206 (60.4%) | 3626 (34.5%) | <.001 |
| Previous MI | 87 (21.4%) | 2709 (21.5%) | .996 |
| PVD | 69 (18.0%) | 930 (7.7%) | <.001 |
| Hypertension | 268 (68.4%) | 7652 (61.5%) | .006 |
| Diabetes | 163 (41.2%) | 3722 (29.9%) | <.001 |
| Hypercholesterolemia | 229 (57.8%) | 7014 (56.5%) | .606 |
| COPD | 56 (14.1%) | 751 (6.1%) | <.001 |
| Active smoking | 76 (19.7%) | 2129 (17.4%) | .248 |
| Previous CVA | 63 (16.7%) | 1086 (8.9%) | <.001 |
| Obesity | 146 (36.4%) | 3723 (29.7%) | .004 |
| Dialysis | 13 (4.7%) | 131 (1.2%) | <.001 |
| Arrhythmia | 229 (82.7%) | 6434 (74.7%) | .002 |
| Previous operation | 112 (27.6%) | 1783 (14.1%) | <.001 |
| Pulmonary pressure (mm Hg)* | 50.5 (39.0−62.0) | 37.0 (30.0−49.0) | <.001 |
| EF (%)* | 50.0 (30.0−60.0) | 58.0 (47.0−60.0) | <.001 |
| Preoperative mechanical ventilation | 21 (5.2%) | 227 (1.8%) | <.001 |
| Postoperative ECMO | 18 (4.5%) | 76 (0.6%) | <.001 |
| Postoperative IABP | 14 (3.5%) | 214 (1.7%) | <.001 |
| Ventilator-associated pneumonia (VAP) | 72 (17.7%) | 857 (6,8%) | <.001 |
| Low cardiac output (LCO) | 143 (35.1%) | 1917 (15.2%) | <.001 |
| Surgical reintervention | 32 (0.8%) | 820 (0.65%) | .12 |
| Standard EuroSCORE I* | 9.0 (7.0−12.0) | 5.0 (3.0−7.0) | <.001 |
| Logistic EuroSCORE I* | 16.8 (8.5−30.8) | 4.4 (2.1−9.1) | <.001 |
| Isolated CABG surgery | 55 (13.5%) | 5939 (47.1%) | <.001 |
| Isolated valve surgery | 57 (14%) | 4262 (33.8%) | <.001 |
| Combined surgery | 295 (72.5%) | 2409 (19.1%) | <.001 |
Abbreviations: FC: functional class; NIHA: New York Heart Association; MI: myocardial infarction PVD: peripheral vascular disease; COPD: chronic obstructive lung disease; CVA: cerebrovascular accident; EF: ejection fraction.
Perioperative patient's parameters and mortality according to groups.
| Group I (Early, 4−10 days) | Group II (Intermediate, 11−20 days) | Group III (Late, ≥21 days) | P | |
|---|---|---|---|---|
| N | 147 | 195 | 65 | |
| Age (y) | 66 ± 12 | 69 ± 12 | 67 ± 15 | .177 |
| Male | 101 (68.7%)† | 110 (56.4%) | 33 (50.8%)† | .020 |
| Elective | 58 (39.5%) | 79 (40.5%) | 27 (41.5%) | .952 |
| FC III-IV | 79 (53.7%) | 84 (43.1%) | 35 (53.8%) | .853 |
| NYHA III-IV | 81 (55.1%) | 94 (48.2.1%) | 33 (50.8%) | .389 |
| Previous MI | 22 (15.0%)† | 46 (23.6%) | 19 (29.2%)† | .037 |
| PVD | 25 (17.0%) | 31 (15.9%) | 13 (20%) | .830 |
| Hypertension | 99 (67.3%) | 126 (64.6%) | 43 (66.2%) | .758 |
| Diabetes | 57 (38.8%) | 77 (39.5%) | 29 (44.6%) | .809 |
| Hypercholesterolemia | 84 (57.1%) | 108 (53.8%) | 37 (56.9%) | .923 |
| COPD | 28 (19%)‡ | 18 (9.2%)‡ | 10 (15.4%) | .033 |
| Smoking | 36 (24.5%)‡ | 29 (14.9%)‡ | 11 (16.9%) | .059 |
| Previous CVA | 21 (14.3%) | 30 (15.4%) | 12 (18.5%) | .852 |
| Obesity | 61 (41.4%) | 63 (32.3%) | 22 (33.8%) | .234 |
| Dialysis | 1 (0.7%)‡ | 10 (5.1)‡ | 2 (3.1%) | .087 |
| Arrhythmia | 96 (65.3%) | 103 (52.8%) | 30 (46.2%) | .483 |
| Previous operation | 37 (25.2%) | 58 (29.7%) | 17 (26.2%) | .602 |
| Pulmonary pressure (mm Hg)* | 50.0 (33.0−60.0) | 45.0 (30.0−60.0) | 50.0 (30.0−60.0) | .919 |
| EF (%)* | 54 (25−60) | 51 (27−52) | 52 (21−52) | .268 |
| Standard EuroSCORE I* | 9.0 (7.0−12.0) | 10.0 (7.0−12.0) | 9.0 (7.0−11.0) | .774 |
| Logistic EuroSCORE I* | 18.3 (7.7−32.3) | 16.6 (8.9−31.7) | 14.9 (8.1−27.2) | .879 |
| Bypass time (min)* | 134.5 (93.3−176.3) | 134.0 (94.0−187.0) | 108.0 (81.8−171.8) | .496 |
| Cross-clamp time (min)* | 85.0 (60.3−129.3) | 90.0(64.5−121.5) | 71.5(55.0−98.3) | .237 |
| Preoperative mechanical ventilation | 7 (4.8%) | 10 (5.1%) | 4 (6.2%) | .289 |
| Re-intubation | 8 (5.4%) | 8 (4.1%) | 5 (7.7.%) | .278 |
| Postoperative ECMO | 2 (1.4%)# | 12 (6.2%)# | 4 (6.2%)# | .002 |
| Postoperative IABP | 4 (2.7%) | 8 (4.1%) | 2 (3.1%) | .434 |
| Ventilator-associated pneumonia (VAP) | 23 (15.6%)† | 32 (16.4%) | 17 (26.1%)† | .235 |
| Low cardiac output (LCO) | 48 (32.6%) | 69 (35.4%) | 26 (40%) | .76 |
| Resternotomy | 31 (7.5%) | 16 (8.2%) | 5 (7,7%) | .87 |
| Open surgical tracheostomy | 19 (12.9%) | 22 (11.3%) | 6 (9.2%) | .34 |
| Open ICU/Semi-closed ICU | 25/122 | 31/164 | 13/52 | .256 |
| Isolated CABG surgery | 20 (13.6%) | 18 (9.3%) | 11 (16.9%) | .207 |
| Isolated valve surgery | 16 (10.9%) | 31 (16%) | 10 (15.3%) | .569 |
| Combined surgery | 111 (75.5%) | 145 (74.7%) | 44 (67.7%) | .457 |
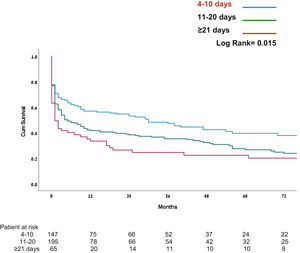
| 30 days mortality | 34 (23.1%) | 45 (23.1%) | 24 (36.9%) | .065 |
| 1 year mortality | 63 (42.9%) | 112 (57.4%) | 42 (64.6%) | .003 |
| 2 years mortality | 66 (44.9%) | 118 (60.5%) | 47 (72.3%) | <.001 |
| 5 years mortality | 82 (55.8%) | 134 (68.7%) | 49 (75.4%) | .007 |
| Superficial sternal wound infection | 7 (4.8%) | 8 (4.1%) | 1 (1.5%) | .104 |
| Deep sternal wound infection | 3 (2%)× | 7 (3.6%)× | 11 (16.9%)× | <.001 |
Abbreviations: FC: functional class; NIHA: New York Heart Association; MI: myocardial infarction PVD: peripheral vascular disease; COPD: chronic obstructive lung disease; CVA: cerebrovascular accident; EF: ejection fraction.
We observed a higher rate of deep sternal wound infection in the late tracheostomy group (18.4%) than in early (6.8%) or intermediate groups (7.7%) (P < .001), however, most of the patients in this group had known, ongoing sternal or other infection at the time of tracheostomy (see Table 2). All sternal wound infections were treated by the opening of the wound at the bedside and/or application of a vacuum device and defined accordingly as surgical complication Grade I according to Dindo classification.12 Only a small number of patients were extubated and follow re-intubated: 5.3% in early the tracheostomy group, 3.9% in the intermediate group, and 8% in the late group. The difference between the groups was not statistically significant (P = .278). Re-intubation itself, method of tracheostomy (open or percutaneous), organization model of the ICU (open vs. semi-closed), preoperative mechanical ventilation, postoperative ECMO, and/or IABP did not influence mortality according to univariable analysis.
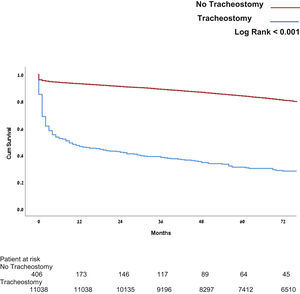
In this study 30-day, mortality was similar in all three groups. However, patients who underwent early- and intermediate tracheostomy insertion, demonstrated statistically significant decreases in mortality at 1, 2, and 5 years (55.8%; 68.7%; and 75.4%, respectively; P < .001) (see Fig. 2). The Cox model shows covariates as time from operation to tracheostomy and age as a significant factor for mortality (see Table 3). Mortality in patients who underwent tracheostomy was significantly higher in patients without tracheostomy at all measured time points (see Fig. 3).
Cox Regression for mortality with tracheostomy as a time-varying covariate.
| B | Sig | HR | 95.0% CI for HR | ||
|---|---|---|---|---|---|
| Lover | Upper | ||||
| Age (year) | .025 | 0.002 | 0.025 | 0.013 | 0.037 |
| Gender | −.156 | 0.237 | 0.856 | 0.661 | 1.108 |
| Time to tracheostomy (days) | −.154 | 0.008 | 0.135 | 0.135 | 0.138 |
| Smoking | −.197 | 0.321 | 0.821 | 0.556 | 1.213 |
| Morbid Obesity | −.131 | 0.365 | 0.877 | 0.652 | 1.165 |
In the general ICU population, up to 12% of the 800,000 mechanically ventilated patients in the United States, each year require tracheostomy.13 In trauma patients, the incidence of tracheostomy is as high as 24.7%,14 and in patients suffering from acute myocardial infarction with cardiogenic shock, the incidence is 5.7%.15 In the post-cardiac surgical population, tracheostomy rates vary between 1.4% and 6.2%.2,16,17 In our study tracheostomy rate is 3.2%. Tracheostomy offers advantages over orotracheal intubation. Tracheostomy reduces dead space and airway resistance, thereby decreasing the work of breathing and possibly allowing earlier liberation from mechanical ventilation.5,18 In addition, tracheostomy potentially reduces the need for sedation, which allows for patient mobilization, communication, and nutrition and lowers the incidence of ventilator-associated pneumonia.18–21
Tracheostomy after cardiac surgery was first described in 1964 by Robertson.22 The percutaneous tracheostomy technique was introduced in 1985 by Ciaglia, and today has proven to be safer as standard surgical tracheostomy.23 Over the last two decades, the utilization of tracheostomy for cardiac surgical patients requiring prolonged mechanical ventilation has become more frequent. The reasons for this trend are multifactorial, including an aging surgical population, increased prevalence of serious comorbidities in the surgical population and associated increased operative risk, increased number of patients undergoing redo surgery, increased utilization of ECMO, and broader surgical indications.1,2,20
The optimal timing for tracheostomy for post-cardiac surgical patients is controversial. In the current literature, the division of tracheostomy into early, intermediate, and late does not have clear time boundaries. In addition, this division changes with the acquisition of new knowledge. In the 1960s, recognition of damage to the oropharynx and larynx led to the performance of early tracheotomy within 48−72 h.24,25 With the advent of fewer traumatic tubes and ventilatory equipment, a tracheotomy was delayed. So, in 1989 the Consensus Conference on Artificial Airways in Patients Receiving Mechanical Ventilation, issued the following statement: “If the need for an artificial airway is anticipated to be greater than 21 days, a tracheotomy is preferred”.26 The need for tracheotomy should be anticipated in ventilator-dependent patients, with the procedure performed when benefits appear to outweigh the disadvantages for the specific patient at hand. The flexibility and individualization of this decision-making process discourage the “calendar watching” that routinely dictates tracheotomy after patients pass through an arbitrary gauntlet of 14–21 days.27 Since there is no generally accepted classification, we decided to divide patients into three groups, according to the large systematic review of Adly et al.7 In this review all studies were divided into three groups, by the timing of tracheostomy: within 7, 14, or 21 days of endotracheal intubation. They found significant differences favoring early tracheostomy in the duration of mechanical ventilation, hospital-acquired pneumonia, mortality rate, and duration of stay in the ICU. Studies that defined early tracheostomy as one performed within 7 days of intubation had more positive results than those that defined early tracheostomy as one performed within 14 or 21 days of intubation. A recent large national analysis of 33,765 patients undergoing tracheostomy by Sareh et al.,2 demonstrated similar early postoperative outcomes (sternal wound infection, in-hospital mortality) in both early (<14 days) and late (14–30 days) tracheostomy groups. A smaller study of 112 patients by Affronti et al.,28 demonstrated decreased time of ventilation and CSICU stay in their early tracheostomy group, but no differences in short or long-term mortality. Devarian et al.29 concluded that early tracheostomy after cardiac surgery is associated with reduced cardiac morbidity (14% vs. 33%) and lower in-hospital mortality (21.1% vs. 40.4%), but unlike this study, the authors included only isolated CABG or isolated valve procedures. In a single-center study, Ben-Avi et al.1 did not report any differences in ventilation time, ICU stays, hospital stay, and 30-day mortality, but found lower mid-term mortality for the early tracheostomy group.
Contrary to the findings of this study, a randomized trial by Trouillet et al.17 compared immediate early percutaneous tracheotomy to prolonged intubation with tracheostomy 15 days after randomization and concluded that early tracheostomy provided no benefit in terms of mortality or infectious complications.
Overall, the survival rate for patients undergoing tracheostomy after cardiac surgery remains low. Ben-Avi et al.1 described 1- and 2-year survival rates of 34% and 32%, respectively. Similar results were reported by Ballotta et al.30 Krebs et al.16 reported that tracheostomy patients had high short-term and long-term mortality, with a median survival of 6 months, 1-year survival of 41%, and 5-year survival of 29.1%. Affronti et al.28 reported 1- and 2-year survival of 43.8% and 35.7% respectively, and Walts et al.31 described 30-day and 2-year survival of 75% and 31% respectively. In our study, the in-hospital mortality rate was 49%. The survival rate for patients discharged from the hospital was 61% at 1 year, 49% at 2 years, 45% at 3 years, and 34% at 5 years.
However, early tracheostomy may be associated with an increased risk of sternal wound infection, possibly due to the proximity of respiratory secretions to a healing sternotomy incision. Several studies have demonstrated an increased risk of sternal wound infection and mediastinitis in patients undergoing tracheostomy after median sternotomy.32,33 Pilarczyk et al.34 reported a significantly higher incidence of deep sternal wound infection when a tracheostomy is performed within 48 h after surgery. More recent studies, however, have called this association into question. In their series, Rahmanian et al.35 showed no correlation between early tracheostomy and deep sternal infection. Ben-Avi et al.1 reported a high incidence of SWI in the late group (more than 15 days). In a meta-analysis, Toeg et al.36 found that patients who had undergone early or late tracheostomy after cardiac surgery had comparable rates of sternal wound infection, and tracheostomy itself may not be a risk factor for SWI, but an indicator of patient critical illness. In our study incidence of SWI, both superficial and deep was higher in the late group (18.4%) compared to the early (6.8%) and intermediate (7.7%) groups, and infection in most cases developed before tracheostomy.
The 407 patients in this study represent the largest single-center cohort of post-cardiac surgical patients undergoing tracheostomy in the literature. The short-term mortality results reported here correlate with previously documented data. However, unlike previous studies, this study demonstrates a significant mid and long-term survival benefit associated with an early tracheostomy. These results, in conjunction with the presumed advantages of early tracheostomy, suggest that earlier is better when a tracheostomy is required.
The 407 patients in the present study represent the single-center cohort of post-cardiac surgical patients undergoing tracheostomy. The short-term mortality results reported here correlate with previously documented data. However, unlike previous studies, this study demonstrates a significant mid- and long-term survival benefit associated with an earlier tracheostomy. These results, in conjunction with the other advantageous aspects of early tracheostomy, suggest that earlier is better when a tracheostomy is required.
There are several potential limitations to this study. First, the definition of the timing of tracheostomy may not be accurate if the patient had re-intubation. A more accurate definition should be based on the endotracheal intubation duration, not the surgery duration. However, in all previous works,1–4,7,14–26,28–35 the timing of tracheostomy was defined as the time between surgery and tracheostomy, and therefore we also used this definition. Second, the weight imbalance between the groups and the retrospective non-randomized nature of the trial may influence the presented results. Third, during the study period, the CSICU was converted from an open care model to a semi-closed one, which may have impacted outcomes. Fourth, in the initial period, tracheostomies were performed by open approach by ENT surgeons, and follow tracheostomies were performed at the bedside using the percutaneous dilatation technique. Five, we did not investigate secondary outcomes resulting from prolonged sedation, rate of VAP, length of hospital stay, the number of ventilator-free days, and cause of death. It was beyond scope of the study. Sixth, the authors have no data on the number of patients who were discharged from home/nursing home/rehabilitation facility or remained in the hospital. Also, we do not have information on the number of decannulated patients and the timing of decannulation. And finally: we agree that it is difficult to assess the causality of the timing of tracheostomy and long-term survival. It is possible that those who received a later tracheostomy were not initially indicated for a tracheostomy at all.
ConclusionThis study demonstrates a relationship between the timing of tracheostomy after cardiac surgery and mid and late-term mortality: early postoperative tracheostomy within 4−10 days of mechanical ventilation is associated with better long-term survival. Short-term mortality does not seem to be affected by the timing of the tracheostomy. Based on our findings, we believe that early, within 4−10 days of tracheostomy should be considered for post-cardiac surgical patients who require prolonged mechanical ventilation. In conclusion, the authors want to rephrase the rule from Marino's ICU Book37: “The indication for intubation and mechanical ventilation is thinking of it”, e.g., the indication for tracheostomy is thinking of it. If you do a tracheostomy - sooner rather than later.
Availability of dataThe data underlying this article will be shared on reasonable request to the corresponding author.
Author's contributionsStudy conception/design: Alexander Kogan, Eitan Keizman.
Data collection: Eitan Keizman, David Volvovitch, Tamer Jamal.
Statistical analysis: Shany Levin, Eilon Ram.
Drafting of paper: Eitan Keizman, Jonathan K. Frogel, Alexander Kogan.
Contribution to the writing of paper: all authors.
Critical revisions: all authors.
Approval of final version: all authors.
Conflict of interestNone.
FundingNone.