Medicina Intensiva was created in 1975 with the double aim of constituting the organ of expression for professionals in Critical Care Medicine and of publication of the best available evidence in any setting of critical care. It is the Official Journal of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva y Unidades Coronarias [SEMICYUC]) and the organ of expression of the Pan-American and Iberian Federation of Societies of Critical Care Medicine and Intensive Care (Federación Panamericana e Ibérica de las Sociedades de Medicina Crítica y Terapia Intensiva [FEPIMCTI]). This warrants the journal an important role representing international societies of professionals dedicated to the care of critical patients.
Medicina Intensiva is widely read throughout the world, particularly in Spanish speaking countries, though thanks to the simultaneous publication of its articles in English, the journal also aims to reach a broader audience. The journal is indexed in PubMed, and since 2010 possesses a Journal Citation report impact factor (ISI WEB of knowledge). Its latest IFI was 1.19 (year 2015). In ScimagoJR the index is 0.334 and has been gradually increasing in the last few years. Medicina Intensiva holds a relevant position among the Spanish journals (28 of 152).
ManuscriptsThe journal accepts manuscripts submitted in Spanish and English. The published manuscripts can be accessed free of charge at (www.medintensiva.org) from 3 months after the time of publication (open access), or immediately after publication through pay per article. The SEMICYUC members have open access at any time.
Medicina Intensiva publishes Original papers, Scientific letters, Letters to the Editor, Points of view, Images in Intensive Care Medicine, Reviews, Special articles and Editorials. Some submitted manuscripts are changed to a different section of the journal after due consultation and approval from the authors. As examples, original papers that fail to meet the established standards can be published as scientific letters, and manuscripts with imaging contents originally submitted as scientific letters but not accepted as such can be changed to the Images in Intensive Care Medicine section of the journal.
The journal receives a number of scientific letters that greatly exceeds its publication capacity. Scientific letters should mainly constitute case series describing truly novel aspects referred to the form of presentation, physiopathology, diagnosis or treatment of clinical conditions affecting the critically ill. Consequently, the Editorial Committee rejects scientific letters that simply describe a clinical case, even if very well documented, but fail to contribute anything truly new. Scientific letters are not conceived to offer a review of the literature.
Points of view, reviews, editorials and special articles are almost always requested by the Editorial Committee from authors of established prestige in a given area of Intensive Care Medicine. In points of view,1 the authors state their opinion about some current and controversial issue in Intensive Care Medicine. Reviews offer a systematic evaluation of topics of interest in critical care. In the special articles section the journal publishes consensus conferences and clinical practice guides directed or conducted by work groups.2 Updates in turn constitute a series of several reviews published in successive numbers of the journal and addressing current topics of interest.
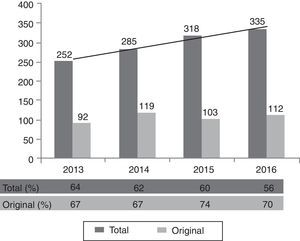
Editorial processMedicina Intensiva receives a growing number of articles each year. In 2016 it received 335 submissions, of which 35% were original papers (see Fig. 1). Acceptance for publication in Medicina Intensiva is conditioned to the quality of the article and adherence to the editorial standards.
All manuscripts must be submitted online (www.medintensiva.org/es/envio-manuscritos) and are received by Elsevier, which applies a first filter to confirm strict abidance with the publication standards of the journal - including conflicts of interest and ethical particulars. Those authors that do not meet the publication standards are immediately returned to the authors for due correction. The publication standards have recently been updated (http://www.medintensiva.org/es/guia-autores/) and should be checked by the authors before submitting the manuscript.
Once the manuscript has been seen to comply with the publication standards, it is forwarded to the Editorial Committee (Editor-in-Chief, who in turn forwards it to the Co-editors as considered opportune) for the second filter in the publication process. The Editorial Committee assesses the originality, design, quality (drafting, tables and figures) and methodological soundness—including statistical design—of the manuscript, and decides whether or not to send it for external review. Sixty percent of the manuscripts that reach the Editorial Committee are sent to reviewers. The rest are rejected in under 10 days from the date of initial submission.
Peer review constitutes the third filter in the publication process. It is important to underscore that the reviewers and the Editorial Committee seek to improve the quality of the article, advising the authors on how to produce a more optimum final version of the manuscript. Medicina Intensiva has over 400 qualified national and international reviewers that collaborate with the journal on a non-profit basis. It would not be possible to maintain the quality of the journal without their crucial contribution.
Two or three reviewers are carefully selected by the Editor according to the topic section to which the manuscript has been assigned on the basis of the indications of the authors at the time of online submission. The journal offers the authors the possibility to choose and/or reject reviewers during the online submission process, though the author reviewer preferences may or may not be fully accepted by the Editor.
The reviewers issue minor and major criticisms of the manuscript and advise the Editor on publication of the article. The Editor makes a decision based on the opinions of the reviewers, and after careful pondering with the rest of the Editorial Committee. The article is accepted, rejected or returned to the authors for modification, along with the comments of the reviewers. In the case of manuscripts that are returned for modification, it is important for the authors to answer the issues raised by the reviewers on a point-by-point basis. The revised version of the manuscript should then be returned to the journal within the time limit specified by the Editor.
The journal has made considerable efforts to shorten the review and publication process of those original papers that reach the third filter. In this regard, an average of 23 days elapse from submission to first peer review of the manuscript. If the article is accepted, the publication process takes an average of 40 days from manuscript submission to online publication in PubMed (Table 1).
Typical editorial process of an original paper (see text).
| % Manuscripts in process | Time (days) | Editorial process | Causes of rejection |
|---|---|---|---|
| 100 | Manuscript submitted | First filter • Failure to comply with editorial standards. Second and third filter • Failure to comply with ethical standards. • Retrospective studies, short single-center series, with no impact upon clinical practice. • Long introduction and/or discussion, without contextualized objectives and results. • Non-established or non-original hypotheses and/or objectives. • Lack of solid methodology, including statistical design. • Redundant results with unclear tables and figures. • Insufficiently defined limitations, conclusions or contributions of the study. • Outdated or excessive citations, or references incongruent with the study objectives. | |
| 90 | 2 | Elsevier (first filter) | |
| 60 | 7 | Editorial Committee (second filter) | |
| 30 | 23 | Peer review (third filter) | |
| Manuscripts accepted (40 days from first submission to online publication) | |||
Due to the increasing number and quality of the articles received, and in view of the constant number of articles that can be published, 56% of all manuscripts and 70% of all the original papers received are rejected for publication (year 2016; see Fig. 1).
The publication of original papers is the primary objective of the Editorial Committee. The Editorial Committee (second filter) and the reviewers (third filter) select articles based on the following criteria:
- 1.
The paper should be original and is expected to contribute relevant knowledge that may modify clinical practice. In this regard, retrospective studies comprising a limited number of patients from a single center, and which address clinical or laboratory issues and have already been published in the context of larger patient series, are rejected at the second or third filter stage.
- 2.
A sound scientific basis is expected, with clear working hypotheses and objectives, an adequate design, clear definition of the variables, and reproducible methodology—including statistical analysis consistent with the objectives of the study. The results preferably should be presented with tables and clarifying figures, avoiding redundancies in the text, with adequate use of abbreviations, and reporting of the study variables with the required international units. The Discussion is to be strictly based on the results obtained, defining the limitations of the study, and drawing the opportune conclusions. The literature references are to be current, adequate in number, and compliant with the style specified by the journal.
The journal has recently incorporated an expert in scientific methodology and statistics 3 who thoroughly reviews the methodological aspects of the studies.
- 3.
Strict compliance with the ethical standards is required, as established by the instructions of the journal, in application to all manuscripts submitted to Medicina Intensiva—including retrospective studies and scientific letters. As a rule, the journal does not accept human interventional studies submitted without approval from the Research Ethics Committee of the institution where the study was carried out.4,5
Approval of the study by the Research Ethics Committee of the institution where it was carried out, and confirmation of the obtainment of informed consent from the participants (where pertinent) must be stated at the time of online submission of the manuscript and in the Material and Methods section. Failure to do so is a clear cause for rejection of the article. Table 2 summarizes the ethical requirements referred to the different manuscripts, and which have been adopted by the Editorial Committee.
Ethical requirements according to type of manuscript.
| Type of study | Informed consent | Ethics committee approval | |
|---|---|---|---|
| Observational, with patient data | Retrospective (case series, cohorts, case-control) | No | Yes, for patient data processing |
| Prospective (cohorts, case-control) | To be considered according to type of data | Yes, for patient data processing | |
| Observational, with anonymous already existing information | Without biological samples | No | Not necessary |
| With biological samples | Yes, unless surplus samples | Yes | |
| Interventional (clinical trial) | Yes | Yes | |
| Diagnostic tests | Standard technique in GCP | Not for investigation | Yes, for patient data processing |
| Comparison of techniques in GCP | Yes | Yes | |
| Technique under study | Yes (if there is patient risk, sensitive information is produced, or biological samples are handled) | Yes | |
| Questionnaire | Yes, except where anonymous, voluntary, blinded and without especially sensitive items | Yes, except where anonymous, voluntary, blinded and without especially sensitive items | |
| Experimental | Animals | No | Yes |
| Systematic review | No | Not necessary | |
Medicina Intensiva has improved its editorial process and expanded its international projection, intensifying its presence in the social networks through the official accounts of the SEMICYUC, where important diffusion activities are being carried out, including Facebook (https://www.facebook.com/SEMICYUC-126800840666619/?fref=ts), twitter (https://twitter.com/semicyuc?lang=es), and the creation of an APP of its own. The journal favors the submission of manuscripts in English, the collaboration of prestigious authors, and shortening of the steps involved in the editorial process.
Medicina Intensiva requires the contribution of all those involved: the reviewers, Editorial Committee, and particularly the authors. The journal is open to all those who ask themselves questions about critical care and seek a means of prestige to publish their answers.6
Conflicts of interest and fundingAll the authors state that they have no conflicts of interest or funding in relation to the present article.
Please cite this article as: Leal-Noval SR, Amaya-Villar R, García-Garmendia JL, Gordo-Vidal F, Garnacho-Montero J. Política editorial de medicina intensiva. Med Intensiva. 2017;41:63–66.